Summary
The actin cytoskeleton plays essential roles in modulating T-cell activation. Most models of T-cell receptor (TCR) triggering, signalosome assembl, y and immune synapse formation invoke actin-dependent mechanisms. As T cells are constitutively motile cells, TCR triggering and signaling occur against a cytoskeletal backdrop that is constantly remodeling. While the interplay between actin dynamics and TCR signaling have been the focus of research for many years, much of the work in T cells has considered actin largely for its ‘scaffolding’ function. We examine the roles of the actin cytoskeleton in TCR signaling and immune synapse formation with an emphasis on how poroelasticity, an ensemble feature of actin dynamics with the cytosol, relates to how T cells respond to stimulation.
Keywords: T cells, T-cell receptors, signal transduction
TCR triggering and the actin cytoskeleton
Triggering mechanisms and actin
T cells are activated through the interaction of T-cell recpetors (TCRs) with agonist-peptide bound to major histocompatibility complexes (pMHCs) on the surface of various antigen-presenting cells (APCs). Sustained TCR signaling is dependent on the integrity of the actin cytoskeleton (1, 2). Given the essential role that the actin cytoskeleton plays in T-cell activation, it is natural to hypothesize that actin contributes directly to TCR triggering. In two-dimensional settings (2D), TCRs show high on rates for agonist pMHCs, as well as high off rates (3, 4). The high off rates depend on F-actin (3), indicating that the actin cytoskeleton facilitates binding and unbinding sequences to serially trigger many TCRs with limited pMHC agonists (5). Rapid binding and unbinding implies that TCRs must rapidly translate transient binding into signalosome formation. A number of groups have proposed that the TCR can act as a mechanosensor when bound to pMHC (6, 7). This is suggestive of a conformational change induced mechanism (8), in which force applied to the receptor complex is converted into a biochemical change, such as through exposure of a phosphorylation site (9, 10), to trigger signalosome formation. This would allow TCRs to couple signal generation to the mechanical energy of the actin cytoskeleton.
In the kinetic segregation mechanism of triggering, TCRs are continuously phosphorylated and dephosphorylated, and the transition to signalosome formation entails physical segregation of TCRs from inhibitory phosphatases (11). This exclusion is based on the relatively bulky extracellular domain of CD45 versus the short interaction distance of TCR-pMHC (12, 13). Obviously, such a mechanism lends itself to actin-based mechanisms through the ability of the cytoskeleton to generate force, squeezing the cell membranes together (1). This mechanism is appealing, as it is nicely aligns with general synapse architecture and with the exclusion of CD45 phosphatase from microclusters and the signaling regions of the interface (14, 15). Recent work from James and Vale supports an actin-independent kinetic segregation mechanism of TCR triggering (16). Their work further indicated that TCRs can trigger in the absence of conformational changes, implying that mechanically driven mechanisms of conformational receptor changes are not important for TCR triggering. However, these experiments rely on a reconstituted system driving high expression of Lck and Zap70, as well as highly abundant agonist pMHCs on the engineered APCs. In this regard, it is not clear how well kinetic segregation ‘scales’ to limited agonist doses presented against a background of nonstimulatory pMHCs. In a nascent T cell-APC contact that is not yet stable, mechanical triggering might dominate, allowing small signaling islands to form, which grow through segregation into stable signaling domains.
TCR signaling complexes and actin scaffolds
Interactions with actin are important for TCR signaling beyond the initial triggering event. As TCR signaling begins, TCRs coalesce into signaling microclusters containing hundreds of TCRs (17). These signaling microclusters require an intact actin cytoskeleton to form and dynamically recruit factors that can scaffold signaling complexes onto the actin cytoskeleton (2, 18–20). Linker of activated T cells (LAT), a critical TCR signaling adapter (21), is rapidly incorporated into signaling microclusters (22). In turn, LAT facilitates recruitment of SLP-76, which can mediate interaction with cytoskeletal regulatory proteins, such as Vav and WASp (23, 24).
As the size scale of TCR signaling microclusters is amenable to conventional fluorescence microscopy, their general composition and dynamics have been studied by microscopy for years. However, the submicroscopic scale has only recently been accessible via microscopy through the development of super resolution microscopy, such as photoactivation localization microscopy (PALM) (25). Using PALM, two groups have analyzed the nanoscale organization of TCRs and how that organization changes in response to agonists (26, 27). TCRs were found to pre-cluster into islands—approximately 50 nm radius probability peaks consisting of 7–20 TCRs (26). Upon stimulation, TCRs islands linked together into microclusters containing hundreds of TCRs. LAT was also shown to reside in nanoclusters independent of stimulation. Like TCRs, LAT clustering was increased on stimulating surfaces. Following stimulation, domains of TCR and LAT clusters were found to concatenate—the domains mixed, but did not fully merge (26). These regions of mixing or overlap were suggested to be ‘hotspots’ of LAT phosphorylation (27). These new super-resolution analyses brings to mind previous work demonstrating that a T cell’s TCRs appear to undergo ‘avidity maturation’ upon encounter with antigen; this maturation corresponds to an increase in TCR oligimerization (28, 29). Not surprisingly, this effect appeared to depend on the actin cytoskeleton and lipid rafts, in agreement with the recent super-resolution analyses that indicated that nanocluster distributions were dependent on the actin cytoskeleton for maintenance (26, 27). More recent work has produced a formal model of the nanoscale organization of cell surface molecules that suggests that actin ‘asters’ drive dynamic nanoclustering (30).
The scaffolding of TCRs into pre-clusters that can be rapidly brought together likely contributes to the sensitivity of the TCR; a limited number of agonist pMHCs can quickly trigger a number of closely associated TCRs (5, 31). By increasing the number of TCRs per nanocluster, the T cell can gain sensitivity. By pre-clustering signaling factors, the T cell can then rapidly deliver a collection effectors to TCRs in response to triggering and assemble nascent signaling complexes. The concatenation, rather than complete mixing, of the TCR and LAT clusters suggests that interactions between receptors and their signaling adapters are transient, rather than long-lived interactions. This view is supported by observations that molecules such as SLP-76 and Vav1 do not constitutively interact with TCRs microclusters in synapses, and enter independent microclusters that traffic autonomously of TCRs (19, 22, 32). Zap-70, likewise, appears to continuously cycle among signaling complexes (22). This indicates that triggered TCRs are stabilized or semi-stabilized, perhaps needing only occasional interaction with signaling factors to remain triggered. This might also allow the cell to rapidly deactivate signaling as TCRs reach the end of their signaling lifecycle.
Coordinating TCR signaling with actin meshwork dynamics
Organizing TCR signaling through polymerization and depolymerization
TCR microclusters in immune synapses are dynamic, both in terms of their protein components and their position in the synapse. Immune synapse formation is often analyzed using supported lipid bilayers as a surrogate for an authentic APC (17). The bilayers can be loaded with Intercellular adhesion molecule (ICAM), pMHC and various other surface proteins to mimic the plasma membrane of an APC. TCR microclusters form rapidly as T cells spread onto activating bilayers. Following spreading, microclusters translate radially within the synapse face, aggregating into a central supramolecular activating cluster (cSMAC) (17). These synapses resemble the original description of the synapse, with TCRs occupying the interior of the interface, the integrin pair LFA-1-ICAM occupying the peripheral SMAC (pSMAC), and outer, CD45-enriched distal SMAC (dSMAC) (17, 33, 34). The flow of TCRs into the synapse is regulated by the actin cytoskeleton (35). As the cSMAC is associated with the termination of signaling and recycling of TCRs (36, 37), this demonstrates that the actin cytoskeleton controls both the initiation and down-regulation of TCR signaling.
Depolymerization can terminate signaling by destroying the scaffold necessary to hold signaling factors and TCRs together. Using the actin depolymerization antagonist Jasplakinolide, several groups have demonstrated the significance of actin depolymerization in guiding TCRs into cSMACs (38–40). These results are not necessarily surprising—it is known that depolymerization is critical to continuous actin retrograde flow (41, 42). Ultimately, depolymerization is necessary to recycle actin monomers and generate continuous treadmilling (42, 43). However, these results do underscore the fact that TCR signaling is integrated into actin cytoskeletal homeostasis and could be regulated by actin turnover. Cofilin activity increases actin turnover and retrograde flow rates (41). Balancing the activities of kinases and phosphatases that regulate cofilin could modulate the residence time of TCR microclusters in the signaling-supportive pSMAC (36). Given how critical cofilin activity is to all aspects of actin cytoskeletal function, cofilin knockouts and short interfering RNA (siRNA) depletion in T cells might not be tractable. A peptide-based approach can allow acute impairment of cofilin function, though, allowing its role in TCR signaling to be analyzed (44). Alternatively, proteins that regulate cofilin activation, such as Slingshot, could be targeted (45).
One recent study has suggested that actin retrograde flow was required for sustained TCR signaling. By inhibiting actin turnover in Jurkat cells interacting with anti-CD3ε-coated coverslips, Babich et al. (40) implicated actin turnover in sustaining PLCγ1 phosphorylation and calcium signaling. However, this analysis used high dose Jasplakinolide. High doses of Jasplakinolide can impair actin polymerization, as well as depolymerization, and generate gross abnormalities in the cytoskeletal architecture (46, 47). As a result, this result might not reflect a depolymerization specific defect. We found that calcium signaling in OT1 T-cell blasts on stimulating lipid bilayers appeared to be unaffected when actin depolymerization was inhibited with Jasplakinolide (39). However, because of the sensitivity of the primary mouse T cells to Jasplakinolide, we limited our analysis to a minimal effective dose. The actin cytoskeletal activities that control the interactions of TCRs with signaling effectors might have remained partially intact.
Myosin motors and TCR signaling
Questions remain about the roles that myosin motors play in TCR signaling, microcluster transport and synapse formation. Some reports indicate that myosin II affects TCR centralization into cSMACs (38, 48). Others show that myosin II is dispensable for TCR microcluster transport (39, 40, 49, 50). In Jurkat cells, myosin II-enriched actin arcs were reported to drive TCR centralization in the pSMAC (38). However, these actinomyosin arcs are not evident in primary T cells. It is not clear that the actin cytoskeletal activities in Jurkat cells, a large, nonpolarized cell line that lacks PTEN activity, are directly comparable to the actin cytoskeletal dynamics of T cells (51).
Beyond the mechanics of microcluster movements, the more significant underlying biological question is whether myosin motors affect TCR signaling outputs. Whatever accessory role myosins might play in TCR microcluster reorganization in synapses, a role for myosins in the formation of TCR signalosomes or modulating signaling would be more interesting. This too is unresolved, though. Knockouts do not clearly support a role in modulating TCR signaling outputs, whereas the use of blebbistatin, often under conditions that favor its non-specific actions (52), largely dominate the work that support this role (48, 50). To the extent that myosin II apparently increases the rate of TCR microcluster transport into the interior of synapses in some studies, the observation that signaling is largely extinguished in those zones would lead one to expect that loss of myosin II would enhance TCR signaling (35, 37). However, the studies that support a role for myosin II in TCR signal generation point to impaired signaling following reduction of myosin II activity (48–50).
At present, these questions remain unresolved in the literature, but genetic knockouts of myosin II have dominant motility and cell viability effects and less obvious signaling defects (39, 53). The viability effects of myosin II depletion might, in part, account for some observations of impaired signaling. It is difficult to separate specific effects on TCR signaling from effects on general cellular functions and viability. For example, it was reported that loss of myosin II resulted in increased synapse areas on antigen presenting bilayers (49). However, myosin II is required for cytokinesis (54), and cells become larger following myosin II depletion (39). Cells treated acutely with blebbistatin, an inhibitor of myosin II (55), do not show increased synapse areas on bilayers (39), suggesting that increased synapse areas in myosin II-depleted cells are not directly related to TCR signaling responses. Another confounding effect is introduced by the use of blebbistatin with the calcium-sensitive dye Fura-2 (56), which is frequently used to measure calcium influx into the cytoplasm following TCR triggering. Blebbistatin-mediated cross-linking is induced by short wavelength light (52, 57), such as that used for Fura-2 imaging. This clouds the interpretation of results that use Fura-2 and blebbistatin (48). Other than myosin II, little is known about how other myosin motors affect TCR signaling. This lack of data likely reflects the difficulty, broadly, in perturbing myosin motors in sensitive, primary T cells or partial redundancy among myosin families. Analyzing how a myosin motor might control TCR signaling and T-cell responses in vivo will be even more difficult, due to roles in motility and lymph node recirculation (53, 58).
Calcium release from the endoplasmic reticulum typically is associated with myosin light chain kinase activation, phosphorylation of the myosin II light chain, and increased motor activity (59). However, in murine T cells, TCR triggering is accompanied by phosphorylation of MyH9 heavy chain at a C-terminal threonine, but not light chain phosphorylation (60). Phosphorylation of the MyH9 tail region is associated with unbundling of F-actin by myosin II (61). Calcium signaling has also been reported to antagonize myosin I mediated tensioning (62), raising the possibility that myosin tensioning might generally be reduced by TCR signaling. This would lead to a reduction in cortical rigidity as filament crosslinking drops. Reducing cortical rigidity might facilitate mixing of receptor and signaling factor nanoclusters.
TCR signaling and calcium influx classically have been associated with T-cell stopping, particularly a near-complete cessation of motion (63). A more nuanced view is that signaling induces a deceleration that is proportional to the magnitude of the stimulation (39, 64). Inhibition of myosin II is generally associated with lower cell speeds (42, 58, 65), suggesting that TCR signaling could reduce motility by deactivating myosin II. We have proposed that myosin motor activities in synapses are downregulated, allowing the T cell to slow and spread over the APC surface to scan for additional agonists. Myosin-independent actin dynamics would allow the T cell to continue reorganizing synapse receptors while it moves slowly over an APC. Subsequent termination of TCR signaling would lead to reactivation of myosins, contraction of the synaptic interface, release from the APC and the resumption of high speed motility that typically characterizes T cells. Myosin activities that promote release from the APC would also be consistent with a myosin II-dependent mechanism of LFA-1 de-adhesion in T-cell migration (66).
Poroelastic cytoplasm, hydraulic force, and the actin cytoskeleton
In conventional models of actinomyosin-based cell motility and transport, microfilament growth and movement generate forces that propel a cell’s leading edge forward and transports materials. Lamellipodial actin polymerization can push the lamellipod forward (67), provided that anchors, such as focal adhesions, are in place to generate traction (68, 69). Resistance from the plasma membrane can cause filaments to move backwards, termed retrograde flow (70). Myosins, through their ability to shift along actin microfilaments, can move material over the actin cytoskeleton, or move entire filaments and associated cargo (71). In addition, there is evidence that myosin II contributes to retrograde flow through actin disassembly as well (42, 72). Given the extended network of branched filaments that comprise the actin cytoskeleton, all proteins and complexes embedded in the actin meshwork will undergo retrograde flow to some extent, even if they do not specifically bind to the actin cytoskeletal factors.
This view naturally emphasizes the solid components of the cell and tends to posit the cell as a perfectly coordinated machine. Unlike man-made machines, though, in which individual parts perform limited functions and make precisely delineated connections to other parts, cellular components make multiple connections with other components. The components connect with varying affinities, and those affinities can be modulated over time or based on cellular context. In addition, the solid compartments of the cell, from single proteins to ribosomes to organelles, are bathed in the cytosol, which can exert its own pressure on cellular components. If the cytoplasm was a viscous and incompressible medium—that is, if forces were instantly transmitted across cells—the machine like view of cells would seem appropriate. However, it is now evident that cells can experience and respond to non-equilibrium hydraulic pressures at subcellular length scales and at time scales comparable to cell biological processes (73, 74). As a result, the cell is best viewed as a poroelastic media. In simple terms, poroelasticity models the cytoplasm as two phases, fluid and solid, that permeate each other, similar to water within a sponge (75). The solid phase, constituted by the cytoskeleton, organelles and macromolecular complexes, forms an elastic and porous solid bathed in the fluid, cytosol phase. Crucially, these phases are not perfectly coupled in a poroelastic model, but instead can move relative to each other, leading to hydraulic microenvironments (74, 75). As a macroscopic analogy, you can wring water out of one end of a sponge, with little or no water displacement in adjacent regions. After releasing the sponge, water will move through the pores of the sponge, equilibrating the hydrostatic pressure across the sponge. Similarly, if you wet one half of a dry sponge, the pores within the sponge will be at hydrostatic non-equilibrium, and water will flow into the dry half of the sponge. In a cell, hydrostatic pressure could be generated by the activities of ion antiporters, leading to locally hyperosmotic conditions and water influx (75). The localization of the NHE1 Na-H exchanger to the leading edge of motile cells (76), and the requirement for aquaporins and water influx for motility support this view (77, 78). Alternatively, hydraulic pressure can be generated on small spatial and temporal scales by abrupt contractility that squeezes cytosol into different regions of the cell (65). The rate of diffusion of water along an osmotic gradient would likely exceed the rate at which the dendritic actin mesh can move, allowing hydrostatic pressure to transport material at speeds greater than actin-based flow. Such a hydrostatic mechanism is hypothesized to transport actin monomers from regions of microfilament depolymerization to protrusion sites in motile cells (79).
Poroelasticity and scaffolding
Regional dehydration can decrease pore size as water leaves the dehydrating region (74, 80). This can reduce diffusion, as particles with diameters greater than the local pore size are no longer able to move through pores. Conversely, local rehydration can increase diffusion as the average pore size increases. This can facilitate interactions between molecules that were previously isolated, or allow molecules that are only weakly bound to release and separate. Coupled with fluid flow, this could also allow proteins and macromolecular complexes to be rapidly reorganized.
The observed pre-clustering of TCRs might reflect interactions between TCR complexes that weakly hold several TCR complexes in a single pore (26, 27). The basal affinity that pre-aggregates TCRs together could be indirectly regulated by actin associated factors, which could transiently bind to and corral receptors prior to triggering (30, 81), or lipid raft-like structures (28, 29). Notably, TCR oligomerization is mediated by the CD3ζ subunit (29), which is known to facilitate linkage of the TCR to the actin cytoskeleton (82, 83). However, this result was based on the association of phosphorylated CD3ζ with detergent insoluble cell fractions and coprecipitation. Similarly, many TCR-actin associations have been identified by coimmunoprecipitation or colocalization analyses. As a result, associations between TCRs and actin regulatory factor might represent general corralling through indirect or transient interactions. Corralling of TCRs is supported by evidence that diffusion of untriggered TCRs is reduced by nonspecific activation of actin polymerization (84). Nanoclusters need not be thermodynamically stable or long-lived, as cytoskeletal remodeling would regularly form, reshape, and destroy pores, allowing the receptors to continuously reshuffle among pores and different nanoclusters. The extent to which TCRs could hold together in a pore would be limited by the relatively low basal affinity of the complexes for each other, and steric considerations that limit the number of TCR complexes per pore. Sherman et al. (27) reported a continuum of LAT nanocluster sizes that ranged from a few molecules to hundreds of molecules, and that nanoclusters of all sizes participated in signaling. The smallest nanoclusters of LAT might be important for the initiation of signaling, though. As they could more easily cross cytosolic pore boundaries, smaller nanoclusters could visit TCR nanoclusters more readily.
As a consensus emerges on the resting nanoscale organization of TCRs and signaling factors, the next question is how TCRs and the actin cytoskeleton interact and change at this level to support TCR signaling. Although TCR signaling and microcluster formation clearly depend on F-actin (1, 2), and TCR activation clearly leads to global increases in actin polymerization (23), the extent to which TCRs generate nanoscale-local actin polymerization and directly interact with actin is not yet clear. LAT, through interactions with SLP-76, Nck, Vav, and WASp (20, 32), provides a likely candidate to scaffold TCRs into actin microfilament supported structures. However, many cytoskeletal regulators, such as Vav, do not directly bind to F-actin, raising the possibility that TCRs activate actin polymerization non-locally through brief interactions with cytoskeletal regulators. This idea is supported by observations that Vav and SLP-76 partition into separate microclusters from TCRs (32), as well as ‘slippage’ in the coupling of TCR flow to actin retrograde flow (85, 86).
The dense mesh of the actin cortex creates a barrier to macromolecular reorganization. In the resting state, segregating TCRs and signaling factors might be important to prevent inappropriate activation. Measurements of the average pore size are on the order of tens of nanometers, which could keep macromolecular assemblies from freely diffusing (74, 80). These estimates represent coarse averages based on dehydration experiments. Also, membrane proximal F-actin densities might be lower than this average. However, it does suggest that the local mesh might need to be remodeled to allow larger aggregates of TCRs and signaling factors to interact. A poroelastic mechanism dependent on TCR-stimulated, local osmotic changes would allow the TCRs to expand the local pore size, facilitating rearrangement into concatemers of nanoclusters and provide room to incorporate signaling adapters into the pore (Fig. 1). Alternatively, signalosomes might segregate into larger cytosolic pores, allowing for larger aggregates of TCRs, while also making room to incorporate other signaling factors. Recently, evidence that TCRs generate local actin polymerization factors following triggering was reported (87). The authors make the intriguing argument that rather than actin scaffolding and stabilizing the TCR microcluster, it is the TCRs that template the actin cytoskeleton in their local environment. TCR microclusters and the actin cytoskeleton likely provide cross-stability to each other—peripheral TCR microclusters are slow to disassemble following latrunculin treatment, but ultimately do dissipate (2). However, this does suggest that TCRs can directly generate a de novo pore within the cytosol or stabilize an existing pore to ensure signal propagation.
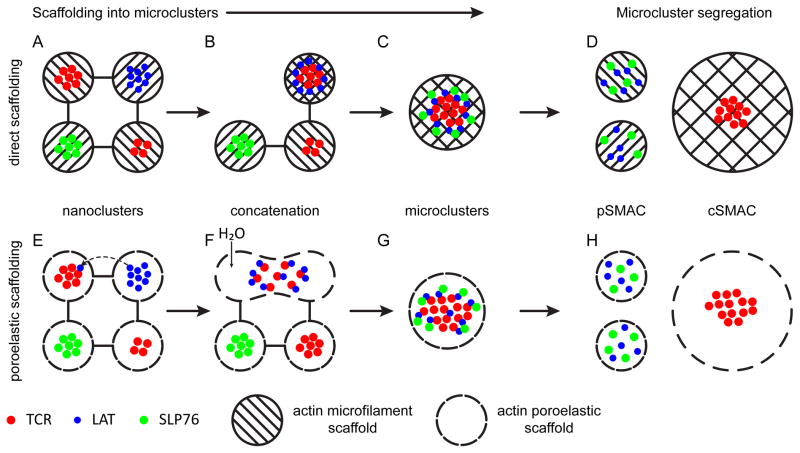
Fig. 1. Models of T-cell receptor scaffolding.
Two models of signaling microcluster scaffolding. (A–D) A direct, microfilament interaction-based model of signalosome scaffolding. (E–H) A poroelastic model of signalosome scaffolding. (A) TCRs and signaling effectors like LAT interact with the actin cytoskeleton, organizing into nanoclusters. (B) Upon triggering, actin cytoskeletal rearrangements allow protein islands of TCRs and LAT to concatenate. (C) Further concatenation and cytoskeletal remodeling leads to the aggregation of microclusters and the recruitment of other signaling effectors, such as SLP-76. (D) Association with signaling effectors is dependent on the actin cytoskeleton for scaffolding. As a result, as microclusters move into the cSMAC, signaling effectors are lost from TCR microclusters. In the pSMAC, the effectors can be rescaffolded with F-actin. (E) The actin cytoskeleton corrals TCRs into cytosolic pores. Confinement to a pore is not absolute, and diffusion into nearby pores can occur (dotted line). The rate at which molecules move into neighboring pores would be inversely proportional to the strength of the homotypic interactions holding molecules in nanoclusters and the hydrodynamic radius of the molecule. (F) As signaling is initiated, water influx, possibly induced by Na-H antiporter activity, changes the local hydrostatic pressure, causing pore deformation and swelling. This increases the mean pore size, allowing nanoclusters of TCRs and LAT to merge in growing pores. (G) Continued hydrostatic pressure facilitates the merging of TCR nanoclusters into microclusters and the incorporation of more signaling effector molecules, such as SLP-76 to sustain signaling. (H) In the low F-actin density interior of the synapse, effectors that require actin scaffolding to remain associated with TCRs release and are separated from TCRs by anterograde fluid flow. The molecules are free to diffuse back into smaller peripheral pores, terminating TCR signaling. Meanwhile, TCRs, which have become independent of the actin cytoskeleton for association in microclusters, remain trapped in the cSMAC. The TCRs cannot diffuse into the periphery of the synapse due to the fine pore size of the pSMAC.
Poroelasticity, hydrostatic pressure and receptor flow
Hydrostatic pressure simultaneously exerts pressure on both the actin cytoskeleton and the protein components embedded within it. It is tempting to speculate that this mechanism controls TCR segregation from signaling factors in cSMACs (2, 14). In signaling synapses, TCR microclusters recruit SLP-76 as they move inward from the periphery (18). Incorporation of SLP-76 is transient, though, and SLP-76 microclusters do not remain associated with TCRs as they reach the pSMAC-cSMAC border, and non-TCR microclusters dissipate (18, 32). Other factors that support and enhance TCR signaling transiently associate with TCR microclusters and are similarly restricted to the periphery of synapses (20, 88, 89). TCR microclusters, on the other hand, become autostable over time. By the time TCR reach the cSMAC, they will remain clustered after the actin cytoskeleton is dissociated with latrunculin (2).
Like TCR signaling microclusters, the integrin molecules that mediate adhesion are also known to couple to retrograde flow in the synapse (86). In a clever set of experiments, Hartman and others showed that cross-linking LFA-1 integrins with an antibody increased the extent to which it centralized in synapses. Adding a cross-linking level using a secondary antibody further increased the extent to which the integrins centralized (90). In terms of poroelasticity, this would hint at a gradient of pore sizes across the synapse coupled with an anterograde hydraulic force pressing signalosomes against the pores. In the periphery of the synapse, actin polymerization would generate finely-sized pores, while actin disassembly closer to the center of the synapse would deconstruct actin filaments, expanding the pores. Hydraulic pressure, directed against actin retrograde flow would force signalosomes against this mesh, sieving the proteins by size (65).
We present some of the known and postulated features of flow and hypothesize how these may be set up in the immune synapse (Fig. 2). This starts with inward directed actin flow and putatively outward directed fluid flow. Individual proteins, which are smaller than the mean cytosolic pore size, could even move anterograde—against the flow of actin—with fluid flow. This would be consistent with the active transport of actin monomers to the leading edge of migrating cells (79) and might explain, for example, molecules, such as SLP-76, which move outward even when cells are stimulated with non-motile TCR ligands that prevent TCRs from moving inward. Ultimately, signalosomes and other cellular components would be forced to move inward with retrograde actin flow. However, hydraulic force would modulate this retrograde flow, providing a sort of positive buoyancy. Larger signaling complexes would flow inward until the local mean pore size is large enough that hydraulic force could counteract the force of actin retrograde flow. At that point, for molecular assemblies that require actin for stability, the local pore size would fail to provide adequate scaffolding and the complex would disassemble. TCRs in microclusters that have reached the cSMAC would be prevented from recycling, perhaps through a lipid-based retention mechanism (14). The hydrostatic pressure gradient and the fluid flow generated need not be monotonic. For example, the pSMAC-cSMAC border could form a high pressure ridge through the activity of myosin. Less stable complexes might not penetrate this region, while complexes that did traverse the boundary would become trapped behind it (Fig. 2). This would still allow signalosomes in the pSMAC to be segregated by size and further enforce TCR microcluster sequestration in the cSMAC. This mechanism would require a balance of actin polymerization and depolymerization to generate actin flow, but also to segregate molecular complexes, as both polymerization and depolymerization are known to be required for proper microcluster movement.
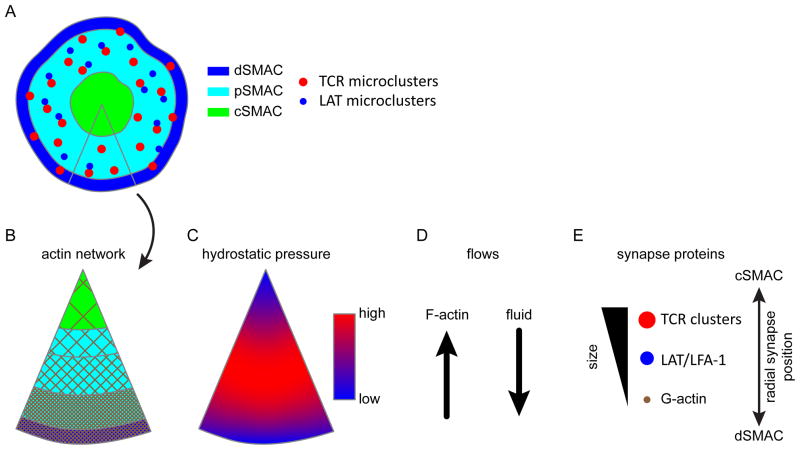
Fig. 2. Models of cellular transport.
(A) The organization of the immune synapse macromolecular domains. Microclusters are generated in the periphery in the pSMAC and dSMAC, and then flow into the cSMAC. (B) A slice through the immune synapse domains showing the branched actin network. F-actin density is highest in the peripheral SMAC zones. As a result, the cytosolic pore size is inversely proportional to the distance from the center of the synapse. Due to the small pore size in the pSMAC, signaling complexes are efficiently coupled to retrograde actin flow. (C) A proposed, simplified map of hydrostatic pressure across the synapse. (D) As signaling complexes centralize, they are simultaneously subjected to retrograde actin flow toward the cSMAC and anterograde fluid flow through the poroelastic media toward the periphery of the cell. (E) The two forces counteract each other, modulating the flow of solid material through the cell in a size-dependent manner. The balance of these two forces regulates the speed and direction of protein complex movement. Large complexes, such as TCRs microclusters, segregate into the interior region with a larger cytosolic pore size. Intermediate size complexes, such as LAT and integrin microclusters, are coupled to actin retrograde flow in the pSMAC but are unstable in the cSMAC. Very small particles, such as actin monomers, are driven by hydrostatic pressure to the edge of the synapse as in migrating cells, allowing continuous actin treadmilling.
The combination of actin-based retrograde flow and anterograde hydraulic pressure could also explain our observation that nascent microclusters formed at the edge of synapses move outward with the expanding synapse edge (39). Resisting this outward hydraulic pressure might require stabilization of actin-TCR linkages, or incorporation into a stable pore. In that case, there would be a lag until the TCR could interact with actin modifying activities and integrate into a pore within the actin meshwork. The microcluster would then be coupled to retrograde flow. We see other hints that hydraulics might affect TCR microcluster flows, as microclusters appear to squeeze together and move in the direction of motility as they reach the F-actin poor synapse interior of synapses (39).
Reconciling synapse models with transient, discontinuous signaling
Issues with in vitro cell motility and artificial synapses
Many studies of actin cytoskeletal dynamics and cell motility are conducted using large fibroblasts or keratinocytes. The mechanics of how these cells crawl are likely to differ from T cells in many ways (53, 58, 91), so care must be taken when applying insights from these cells to T cells. In particular, the small size of T cells means that hydraulic forces propagate proportionally further from back to front. Additionally, small ‘cam’ like contacts may generate significant friction for force transfer and motility. In contrast, fibroblasts are typically analyzed as they move across large, flat, homogenous glass interfaces. While this substrate might arguably be relatively appropriate for those cell types, it clearly does not reflect the context of antigen presentation to T cells. Cellular motility in three dimensions is clearly different than crawling across planar surfaces (91).
Much of our understanding of cytoskeletal roles in synapse and motility dynamics is derived from supported lipid bilayers. These have been enormously informative and have generated considerable data about synapse formation, but these APC surrogates suffer from a range of issues. In a typical experiment, the bilayer formation regimen is optimized to maximize the lateral mobility of the adsorbed protein ligands. The bilayer itself is mounted on an absolutely rigid substrate with no topological variation. In some ways, this is ideal for testing treatments hypothesized to affect T cells’ ability to reorganize receptors and membrane domains—any impairment of T cell functions will be fully reflected in the morphology of the synapse and the T cell response. However, it is also clear that APCs can influence the organization of immune synapses (92, 93), and that synapses can take on diverse conformations (94, 95). When coupling with other cells to form synapses, T cells must adapt to varying contours and stiffness as they move across the surface of their synaptic partner. In addition, the APC might generate resistance to receptor reorganization.
An additional concern with lipid bilayer-based experiments is that cells are usually transferred from suspension, where they are nonpolarized, to the bilayer and allowed to directly adhere. In vivo, T cells encounter APCs as they constitutively move through an environment presenting various chemokine and adhesive signals. TCR signaling is initiated in brief, serial encounters with agonist bearing dendritic cells (96). During the early phases of antigen responses, T cells establish and break contact with one or more dendritic cells, allowing them to ‘integrate’ the agonist abundance, which controls incorporation of T cells into the immune response (97, 98). In an in vitro bilayer experiment, though, the T cell is typically activated with a relatively massive, coordinated stimulus—many hundreds to thousands of pMHC molecules are presented under the T cells as it spreads onto the bilayer over the first few minutes of contact. As a result, T cells activated with bilayers generally bypass the early stages of activation in which interaction with agonist is intermittent and response thresholds are reached over long time periods. Even if the bilayer is loaded with super low doses of agonist, the T cell is also unable to break contact with the surrogate APC. If T cells really do sum the TCR signaling generated over successive contacts, then the bilayer will ultimately present an activating dose, no matter the local concentration, as the many square millimeters of bilayer provide an effectively infinite antigen dose. As a result, it is clear that synapses formed on supported lipid bilayers are not capable of recapitulating all of the dynamics of authentic cell- cell synapses.
Steady state actin dynamics and discontinuous TCR signaling
As T cells cease crawling for only brief periods during their lifecycle, it is important to understand how the factors that regulate TCR activation integrate into the continuously remodeling cortical actin meshwork. In the early stages of T-cell responses to antigen, T cells make sequential, relatively brief contacts with agonist bearing dendritic cells (96, 99, 100). Over time, T-cell motility declines and cells form longer duration contacts with dendritic cells. However, it is clear that TCR signaling is initiated during the early, transient interactions, and that these interactions shape the T-cell response (97). The transient interfaces between T cells and dendritic cells formed in the early immune response are unlikely to resemble the stable, highly organized immune synapses seen on bilayers. A complete model of the immune synapse will describe the organization of TCRs and signaling domains in both short-lived contacts and stable, longer duration interactions.
Maintenance of symmetry has been proposed as an important factor in production of a stable, productive synapse (49, 101). It is difficult to reconcile this idea with the short-lived, dynamic contacts of early T-cell activation. It is possible that the requirement for symmetry is not absolute but rather context dependent and that some synapses do not need to maintain symmetry to be productive. This is an attractive idea, and would indicate that T cells can bias the types of synapses they generate based on their differentiation state (94), as well as the nature of the information exchange occurring at the synapse (102). For example, in early T-cell encounters with antigen, the information exchanged at the synapse is limited and largely one-way—a relatively limited amount of information (agonist) is presented by the dendritic cell to the T cell. Perhaps in other synapses, such as T-helper cell-B-cell synapses where cytokines are transmitted across the synapse, stability and symmetry are paramount to ensure that sufficient, bidirectional information exchange occurs. Conversely, cytotoxic T cells, which are exquisitely sensitive (103), need only receive a very limited amount of information before committing to cytolysis of their target. By adapting to more flexible, unstable synapses, cytotoxic T cells would then be able to rapidly kill a series of targets (104, 105). This could be reflected in our analyses of motile synapses formed by antigen-experienced CD8+ T cells (39). We found that CD8+ T cell blasts could initiate migration on bilayers even as synapse formation began. In these motile synapses, TCR microcluster movements did not appear to focus on a single location. Instead, TCR microclusters flowed independent of the location of the cSMAC into F-actin poor regions of the synapse and passively merged into larger aggregates.
If T cells are able to adapt various synapse conformations based on context, the actin cytoskeleton will surely be an important factor in shaping those context-specific synapses. Modulating the relative activity levels of proteins like WASp and PKC Θ could enhance the ability of cytotoxic T cells to form kinapses or motile synapses (101, 106). Synapses are also often viewed as binary partnerships, and much work has been done analyzing T cell polarization toward an exclusive APC partner (107, 108). However, rather than stably polarizing, the MTOC polarization can oscillate and can rapidly flop between multiple associated target cells (109). Cytotoxic T cells can uncouple effector functions from synapse formation to simultaneously kill multiple targets, and T cells can rapidly switch polarization towards different partners in synapses (105, 110). Therefore, T cells manage multiple independent signaling interfaces, possibly on opposite sides of the cell. By coupling organelle movements to hydrostatic pressures, T cells could quickly squeeze organelles related to synapse effector activities, such as degranulation, between distal signaling interfaces. In this regard, the F-actin poor interior of the synapse might facilitate secretion into the synaptic interface, but might also represent a low hydrostatic pressure region toward which organelles can move. In the case of a multifaceted synapse, multiple F-actin poor regions could form multiple low hydrostatic pressure zones, which organelles would cycle through by following fluctuating hydrostatic pressure gradients.
Conclusion
Not all of the observations we discuss require poroelastic mechanisms to be explained, and some can instead be modeled in terms of mechanical pushing and pulling by the actin cytoskeleton. However, given that the poroelastic model of the cytoplasm can explain features of animal cell dynamics that have gone unappreciated, it is important to incorporate it into models of how cells respond to stimuli. Moreover, poroelasticity and non-hydraulic mechanisms are not mutually exclusive, and hydraulic and filament-based mechanical forces likely interact. Binding interactions between signaling factors and the actin cytoskeleton can scaffold factors into complexes and exert force on those complexes, leading to or cooperating with hydraulic-driven mechanisms that squeeze factors into different porosity regimes; these pore-related dynamics could change not just diffusion behaviors but also the binding partners available for interactions, feeding back into signaling activities. In this way, poroelastic mechanics can reinforce weak or transient interactions between signaling factors that might not survive the relatively massive shear forces that the cytoskeleton can apply to receptor complexes as cells crawl over an APC surface.
Acknowledgments
The authors thank Jordan Jacobelli and Audrey Gerard for helpful discussion sand acknowledge the NIH (R01 AI052116 and P01 HL024136) for support.
Footnotes
The authors declare that they have no financial conflict of interest.
References
- 1.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huppa JB, Axmann M, Mörtelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, Klein LO, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 6.Kim ST, Takeuchi K, Sun Z-YJ, Touma M, Castro CE, Fahmy A, Lang MJ, et al. The αβ T Cell Receptor Is an Anisotropic Mechanosensor. J Biol Chem. 2009;284:31028–31037. doi: 10.1074/jbc.M109.052712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y-C, Chen B-M, Wu P-C, Cheng T-L, Kao L-S, Tao M-H, Lieber A, et al. Cutting Edge: Mechanical Forces Acting on T Cells Immobilized via the TCR Complex Can Trigger TCR Signaling. J Immunol. 2010;184:5959–5963. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-Anchored Monomeric Agonist pMHCs Alone Trigger TCR with High Sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil D, Schamel WWA, Montoya M, Sánchez-Madrid F, Alarcón B. Recruitment of Nck by CD3_ Reveals a Ligand-Induced Conformational Change Essential for T Cell Receptor Signaling and Synapse Formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 11.Burroughs NJ, Lazic Z, van der Merwe PA. Ligand Detection and Discrimination by Spatial Relocalization: A Kinase-Phosphatase Segregation Model of TCR Activation. Biophys J. 2006;91:1619–1629. doi: 10.1529/biophysj.105.080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irles C, Symons A, Michel F, Bakker TR, van derMerwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 13.Choudhuri K, Parker M, Milicic A, Cole DK, Shaw MK, Sewell AK, Stewart-Jones G, et al. Peptide-Major Histocompatibility Complex Dimensions Control Proximal Kinase-Phosphatase Balance during T Cell Activation. J Biol Chem. 2009;284:26096–26105. doi: 10.1074/jbc.M109.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leupin O, Zaru R, Laroche T, Müller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–280. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 16.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 18.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 19.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario M-C, Barr VA, et al. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 2006;26:7155–7166. doi: 10.1128/MCB.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 Tyrosine Kinase Substrate that Links T Cell Receptor to Cellular Activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 22.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic Actin Polymerization Drives T Cell Receptor–Induced Spreading: A Role for the Signal Transduction Adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. SLP-76 Coordinates Nck-Dependent Wiskott-Aldrich Syndrome Protein Recruitment with Vav-1/Cdc42-Dependent Wiskott-Aldrich Syndrome Protein Activation at the T Cell-APC Contact Site. J Immunol. 2003;171:1360–1368. doi: 10.4049/jimmunol.171.3.1360. [DOI] [PubMed] [Google Scholar]
- 25.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, et al. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 26.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman E, Barr V, Manley S, Patterson G, Balagopalan L, Akpan I, Regan CK, et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity. 2011;35:705–720. doi: 10.1016/j.immuni.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR Avidity after T Cell Activation: A Mechanism for Sensing Low-Density Antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- 29.Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, Schamel WWA, et al. Increased Sensitivity of Antigen-Experienced T Cells through the Enrichment of Oligomeric T Cell Receptor Complexes. Immunity. 2011;35:375–387. doi: 10.1016/j.immuni.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Gowrishankar K, Ghosh S, Saha SCR, Mayor S, Rao M. Active Remodeling of Cortical Actin Regulates Spatiotemporal Organization of Cell Surface Molecules. Cell. 2012;149:1353–1367. doi: 10.1016/j.cell.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Schamel WWA, Alarcón B. Organization of the resting TCR in nanoscale oligomers. Immunol Rev. 2013;251:13–20. doi: 10.1111/imr.12019. [DOI] [PubMed] [Google Scholar]
- 32.Sylvain NR, Nguyen K, Bunnell SC. Vav1-Mediated Scaffolding Interactions Stabilize SLP- 76 Microclusters and Contribute to Antigen-Dependent T Cell Responses. Sci Signal. 2011;4:ra14. doi: 10.1126/scisignal.2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 34.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 35.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T Cell Receptor Microcluster Transport through Molecular Mazes Reveals Mechanism of Translocation. Biophys J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K-H, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T Cell Receptor Signaling Precedes Immunological Synapse Formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 37.Lee K-H, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, et al. The Immunological Synapse Balances T Cell Receptor Signaling and Degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 38.Yi J, Wu XS, Crites T, Hammer JA. Actin Retrograde Flow and Acto-Myosin II Arc Contraction Drive Receptor Cluster Dynamics at the Immunological Synapse in Jurkat T-Cells. Mol Biol Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012;13:787–795. doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babich A, Li S, O’Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCγ1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delorme V, Machacek M, DerMardirossian C, Anderson KL, Wittmann T, Hanein D, Waterman-Storer C, et al. Cofilin activity downstream of Pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev Cell. 2007;13:646–662. doi: 10.1016/j.devcel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollard TD, Borisy GG. Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 44.Eibert SM, Lee K-H, Pipkorn R, Sester U, Wabnitz GH, Giese T, Meuer SC, et al. Cofilin peptide homologs interfere with immunological synapse formation and T cell activation. Proc Natl Acad Sci USA. 2004;101:1957–1962. doi: 10.1073/pnas.0308282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of Actin Reorganization by Slingshot, a Family of Phosphatases that Dephosphorylate ADF/Cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 46.Bubb M, Senderowicz A, Sausville E, Duncan K, Korn E. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 47.Bubb M, Spector I, Beyer BB, Fosen KM. Effects of Jasplakinolide on the Kinetics of Actin Polymerization. An explanation for certain in vivo observations. J Biol Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 48.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumari S, Vardhana S, Cammer M, Curado S, Santos L, Sheetz MP, Dustin ML. T Lymphocyte Myosin IIA is Required for Maturation of the Immunological Synapse. Front Immunol. 2012;3:230. doi: 10.3389/fimmu.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Fay NC, Smoligovets AA, Wu H-J, Groves JT. Myosin IIA modulates T cell receptor transport and CasL phosphorylation during early immunological synapse formation. PLoS ONE. 2012;7:e30704. doi: 10.1371/journal.pone.0030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan X, Czar MJ, Bunnell SC, Liu P, Liu Y, Schwartzberg PL, Wange RL. Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation. Mol Cell Biol. 2000;20:6945–6957. doi: 10.1128/mcb.20.18.6945-6957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320:1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 53.Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil A-M, Piel M, Sorensen CM, Adelstein RS, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11:953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 55.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 56.Grynkiewicz G, Poenie M, Tsien R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 57.Sakamoto T, Limouze J, Combs CA, Straight AF, Sellers JR. Blebbistatin, a myosin II inhibitor, is photoinactivated by blue light. Biochemistry. 2005;44:584–588. doi: 10.1021/bi0483357. [DOI] [PubMed] [Google Scholar]
- 58.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 Regulate the Interchange between Two Distinct Modes of T Cell Migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 59.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 60.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 61.Egelhoff TT, Lee RJ, Spudich JA. Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell. 1993;75:363–371. doi: 10.1016/0092-8674(93)80077-r. [DOI] [PubMed] [Google Scholar]
- 62.Lewis JH, Greenberg MJ, Laakso JM, Shuman H, Ostap EM. Calcium Regulation of Myosin-I Tension Sensing. Biophys J. 2012;102:2799–2807. doi: 10.1016/j.bpj.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 65.Keren K, Yam PT, Kinkhabwala A, Mogilner A, Theriot JA. Intracellular fluid flow in rapidly moving cells. Nat Cell Biol. 2009;11:1219–1224. doi: 10.1038/ncb1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morin NA, Oakes PW, Hyun Y-M, Lee D, Chin YE, Chin EY, King MR, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theriot JA, Mitchison TJ. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 68.Beningo KA, Dembo M, Kaverina I, Small JV, Wang Y-l. Nascent Focal Adhesions Are Responsible for the Generation of Strong Propulsive Forces in Migrating Fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–599. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 71.Cramer L. Organelle transport: dynamic actin tracks for myosin motors. Curr Biol. 2008;18:R1066–R1068. doi: 10.1016/j.cub.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 72.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- 73.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Charras GT, Mitchison TJ, Mahadevan L. Animal cell hydraulics. J Cell Sci. 2009;122:3233–3241. doi: 10.1242/jcs.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchison TJ, Charras GT, Mahadevan L. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol. 2008;19:215–223. doi: 10.1016/j.semcdb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 78.Loitto V-M, Forslund T, Sundqvist T, Magnusson K-E, Gustafsson M. Neutrophil leukocyte motility requires directed water influx. J Leukoc Biol. 2002;71:212–222. [PubMed] [Google Scholar]
- 79.Zicha D, Dobbie IM, Holt MR, Monypenny J, Soong DYH, Gray C, Dunn GA. Rapid actin transport during cell protrusion. Science. 2003;300:142–145. doi: 10.1126/science.1082026. [DOI] [PubMed] [Google Scholar]
- 80.Moeendarbary E, Valon L, Fritzsche M, Harris AR, Moulding DA, Thrasher AJ, Stride E, et al. The cytoplasm of living cells behaves as a poroelastic material. Nature Mater. 2013;12:253–261. doi: 10.1038/nmat3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA. 2006;103:18992–18997. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozdzial MM, Pleiman CM, Cambier JC, Finkel TH. pp56Lck Mediates TCR ζ-Chain Binding to the Microfilament Cytoskeleton. J Immunol. 1998;161:5491–5499. [PubMed] [Google Scholar]
- 83.Rozdzial MM, Malissen B, Finkel TH. Tyrosine-phosphorylated T cell receptor zeta chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3:623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 84.Dushek O, Mueller S, Soubies S, Depoil D, Caramalho I, Coombs D, Valitutti S. Effects of Intracellular Calcium and Actin Cytoskeleton on TCR Mobility Measured by Fluorescence Recovery. PLoS ONE. 2008;3:e3913. doi: 10.1371/journal.pone.0003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 86.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smoligovets AA, Smith AW, Wu H-J, Petit RS, Groves JT. Characterization of dynamic actin associations with T-cell receptor microclusters in primary T cells. J Cell Sci. 2012;125:735–742. doi: 10.1242/jcs.092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, et al. Spatiotemporal Regulation of T Cell Costimulation by TCR-CD28 Microclusters and Protein Kinase C θTranslocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaizuka Y, Douglass AD, Vardhana S, Dustin ML, Vale RD. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J Cell Biol. 2009;185:521–534. doi: 10.1083/jcb.200809136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci USA. 2009;106:12729–12734. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, Keller M, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 92.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, Trautmann A. Multifocal structure of the T cell – dendritic cell synapse. J Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 93.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 94.Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, Parker DC. Th1 and Th2 Cells Form Morphologically Distinct Immunological Synapses. J Immunol. 2008;181:393–399. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thauland TJ, Parker DC. Diversity in immunological synapse structure. Immunology. 2010;131:466–472. doi: 10.1111/j.1365-2567.2010.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 97.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. Cutting Edge: T Lymphocyte Activation by Repeated Immunological Synapse Formation and Intermittent Signaling. J Immunol. 2003;171:1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- 99.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-Photon Imaging of Lymphocyte Motility and Antigen Response in Intact Lymph Node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 100.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the Single Cell Dynamics of CD4+ T Cell Activation by Dendritic Cells in Lymph Nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler BJ, Hofman JM, Waite JC, Cameron TO, et al. Opposing Effects of PKCθ and WASp on Symmetry Breaking and Relocation of the Immunological Synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 102.Krummel MF. Illuminating emergent activity in the immune system by real-time imaging. Nat Immunol. 2010;11:554–557. doi: 10.1038/ni0710-554. [DOI] [PubMed] [Google Scholar]
- 103.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a Single Peptide MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 104.Lee S-JE, Hori Y, Groves JT, Dustin ML, Chakraborty AK. Correlation of a dynamic model for immunological synapse formation with effector functions: two pathways to synapse formation. Trends Immunol. 2002;23:492–499. doi: 10.1016/s1471-4906(02)02285-8. [DOI] [PubMed] [Google Scholar]
- 105.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci USA. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvez R, Lafouresse F, Demeester J, Galy A, Valitutti S, Dupre L. The Wiskott-Aldrich syndrome protein permits the assembly of a focused immunological synapse enabling sustained TCR signalling. Haematologica. 2011;96:1415–1423. doi: 10.3324/haematol.2011.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martín-Cófreces NB, Robles-Valero J, Cabrero JR, Mittelbrunn M, Gordón-Alonso M, Sung C-H, Alarcón B, et al. MTOC translocation modulates IS formation and controls sustained T cell signaling. J Cell Biol. 2008;182:951–962. doi: 10.1083/jcb.200801014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 109.Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- 110.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]