Abstract
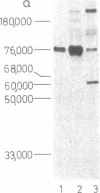
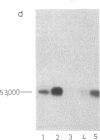
Sera from certain rabbits bearing Schmidt-Ruppin strain Rous sarcoma virus (RSV)-induced tumors precipitated p60src from chicken cells transformed by the homologous virus as well as by other strains [Prague strain RSV, Bryan high-titer strain RSV, and Bratislava 77 strain of avain sarcoma virus (ASV)], the molecular weights (Mrs) ranging from 60,000 to 64,000. The p60src immunoprecipitated from cells transformed by each of these strains incorporated [γ-32P]ATP into the Mr 53,000 subunit of IgG, though with differing activities. No such protein kinase activity (ATP:protein phosphotransferase, EC 2.7.1.37) was observed when the following immunoprecipitates were used: from uninfected cells, from untransformed cells infected by Rous-associated virus, or from cells transformed by acute leukosis viruses, avian erythroblastosis virus, or myelocytoma virus 29. The kinase reaction had a pH optimum at pH 5.9 and an apparent Km for ATP of 4.9 ± 2 μM, and was dependent on Mg2+ (Kb = 46 ± 12 mM), for which Ca2+ was no substitute. The kinase was cyclic AMP independent.
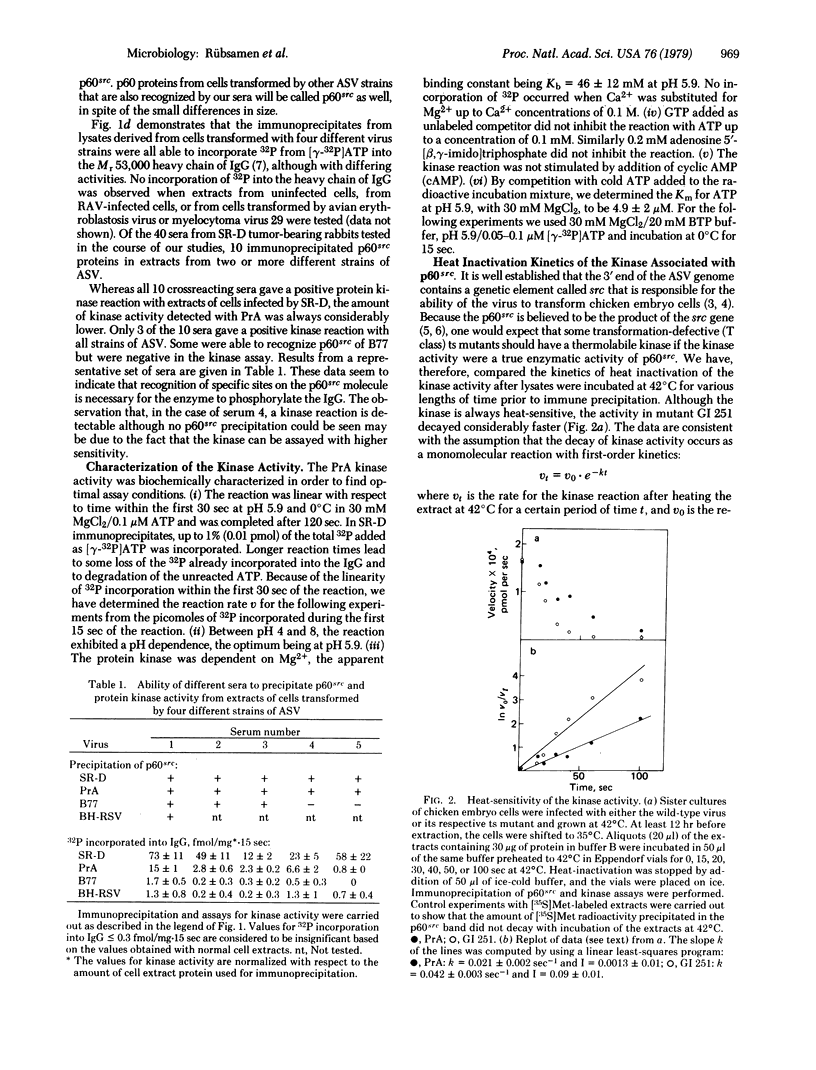
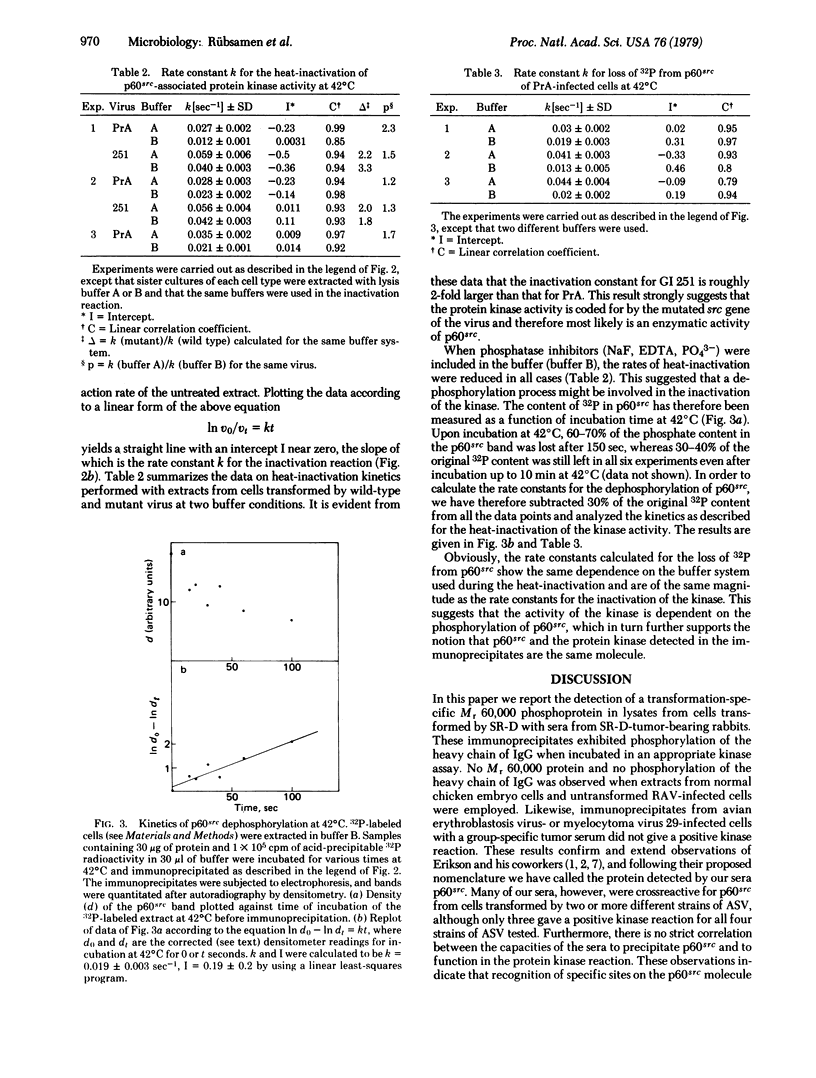
In order to test whether the protein kinase reaction is directly catalyzed by p60src, we compared the in vitro temperature sensitivities of the kinase activities from cells infected by transformation-temperature-sensitive mutant and parental wild-type virus. The first-order rate constant for the inactivation of the kinase from extracts of cells infected by the mutant virus was 2-fold greater than that from cells infected by wild-type virus. This result implicates the protein kinase as an enzymatic activity of the src gene product, the p60src. Concomitant with the loss of the kinase activity by heat inactivation, p60src loses 60-70% of its phosphate content. The kinetics of dephosphorylation exactly parallel those for the inactivation of the kinase activity, suggesting that the p60src kinase is itself dependent on phosphorylation for its activity.
Keywords: src gene product p60src, immunological group specificity, phosphorylation of protein kinase
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J., Erikson E., Collett M. S., Erikson R. I. Peptide analysis of the transformation-specific antigen from avian sarcoma virus-transformed cells. J Virol. 1978 Jun;26(3):773–782. doi: 10.1128/jvi.26.3.773-782.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R., Mason W. S., Chen Y. C., Halpern M. S. A replication defective mutant of Rous sarcoma virus which fails to make a functional reverse transcriptase. Virology. 1975 Mar;64(1):49–62. doi: 10.1016/0042-6822(75)90078-1. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Struck C. J., Ahnert G., Glossmann H., Schaeg W. Solid phase radioimmunoassay for cyclic AMP using staphylococcal protein A-antibody adsorbent. Naunyn Schmiedebergs Arch Pharmacol. 1977 May;298(1):67–73. doi: 10.1007/BF00510989. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]