Abstract
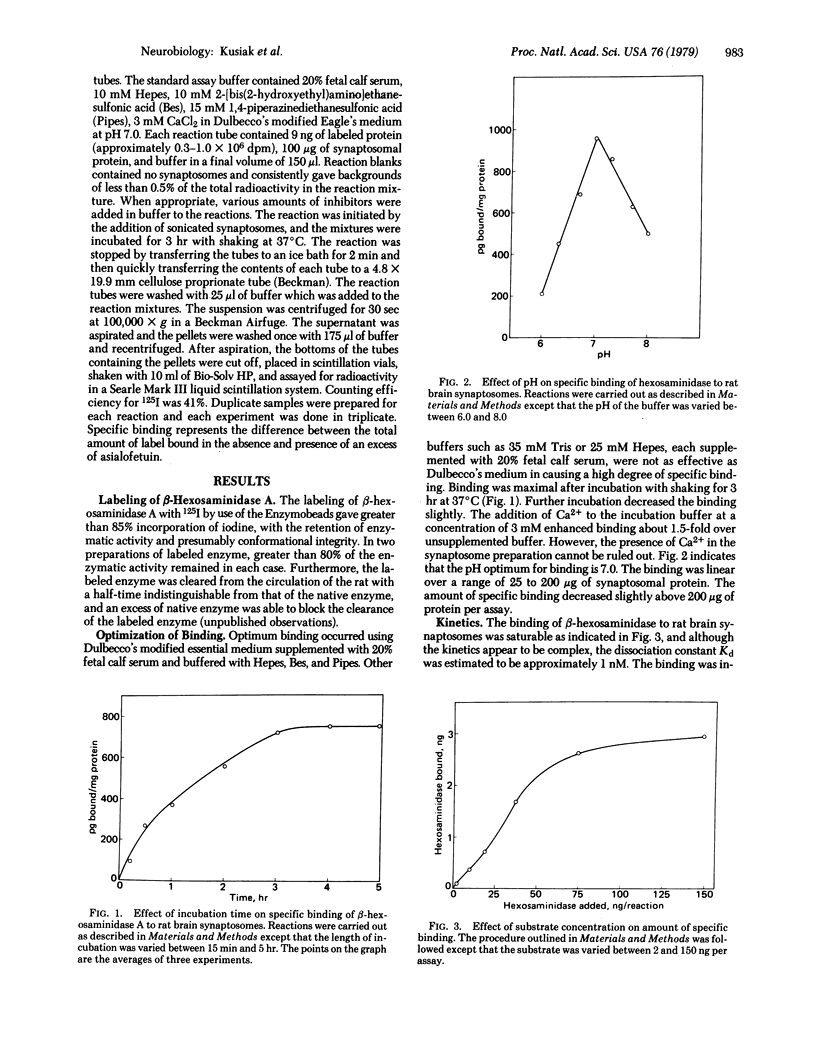
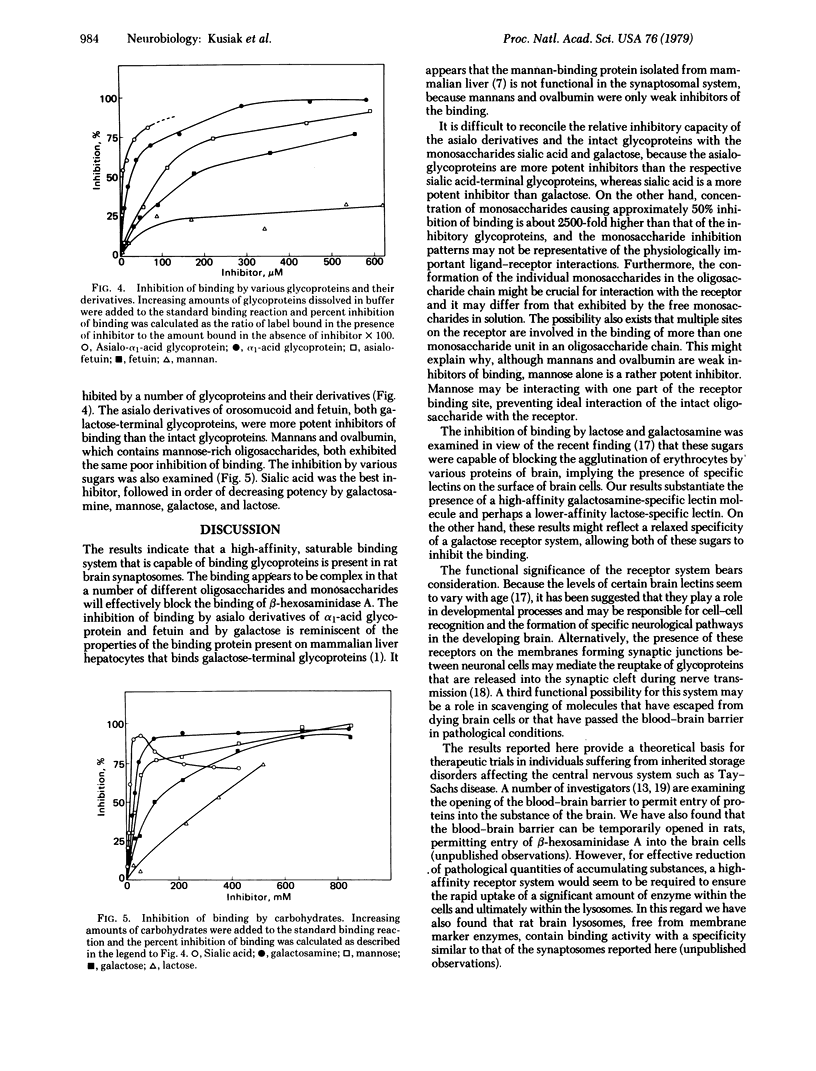
Purified human beta-hexosaminidase A (beta-N-acetylgulcosaminidase; 2-acetamido-2-deoxy-beta-D-glucoside acetamidodeoxyglucohydrolase, EC 3.2.1.30) has been labeled with 125I to high specific activity with the retention of 80% of its enzyme activity. The binding of this enzyme to sonicated synaptosomes from rat brain was shown to be a saturable and specific process. Glycoproteins containing a sialic acid-terminal oligosaccharide or a galactose-terminal oligosaccharide (i.e., alpha 1-acid glycoprotein and fetuin and their asialo derivatives) were strong inhibitors of the binding. In contrast, ovalbumin, which contains a mannose-rich oligosaccharide, and mannans were poor inhibitors of the binding. Of the monosaccharides tested, sialic acid, galactosamine, mannose, galactose, and lactose were inhibitory in decreasing potency of inhibition. Optimal binding occurred at pH 7.0 in the presence of 3 mM calcium ions. The binding was a linear function of synaptosomal protein concentration between 25 and 200 microgram of protein per assay and was directly proportional to time up to 3 hr, beyond which there was no further increase in specific binding. The data suggest a unique but complex mode of interaction of glycoproteins with receptors on synaptic membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barranger J. A., Rapoport S. I., Fredericks W. R., Pentchev P. G., MacDermot K. D., Steusing J. K., Brady R. O. Modification of the blood-brain barrier: increased concentration and fate of enzymes entering the brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):481–485. doi: 10.1073/pnas.76.1.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn R. W., Goodwin F. K., Murphy D. L., Bunney W. E., Jr, Davis J. M. Quantitative studies of norepinephrine uptake by synaptosomes. Biochem Pharmacol. 1968 Jun;17(6):957–964. doi: 10.1016/0006-2952(68)90354-7. [DOI] [PubMed] [Google Scholar]
- Furbish F. S., Steer C. J., Barranger J. A., Jones E. A., Brady R. O. The uptake of native and desialylated glucocerebrosidase by rat hepatocytes and Kupffer cells. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1047–1053. doi: 10.1016/0006-291x(78)91456-0. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. Subcellular distribution of a mammalian hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Dec 10;251(23):7539–7544. [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R., Loh H. H. Developmentally regulated lectin in neonatal rat brain. Nature. 1977 Mar 24;266(5600):367–369. doi: 10.1038/266367a0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Furbish F. S., Barranger J. A., Brady R. O., Jones E. A. The uptake of agalacto-glucocerebrosidase by rat hepatocytes and Kupffer cells. FEBS Lett. 1978 Jul 15;91(2):202–205. doi: 10.1016/0014-5793(78)81172-7. [DOI] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Wallace E. F., Lovenberg W. Studies on the carbohydrate moiety of dopamine beta-hydroxylase: interaction of the enzyme with concanavalin A. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3217–3220. doi: 10.1073/pnas.71.8.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]


