Abstract
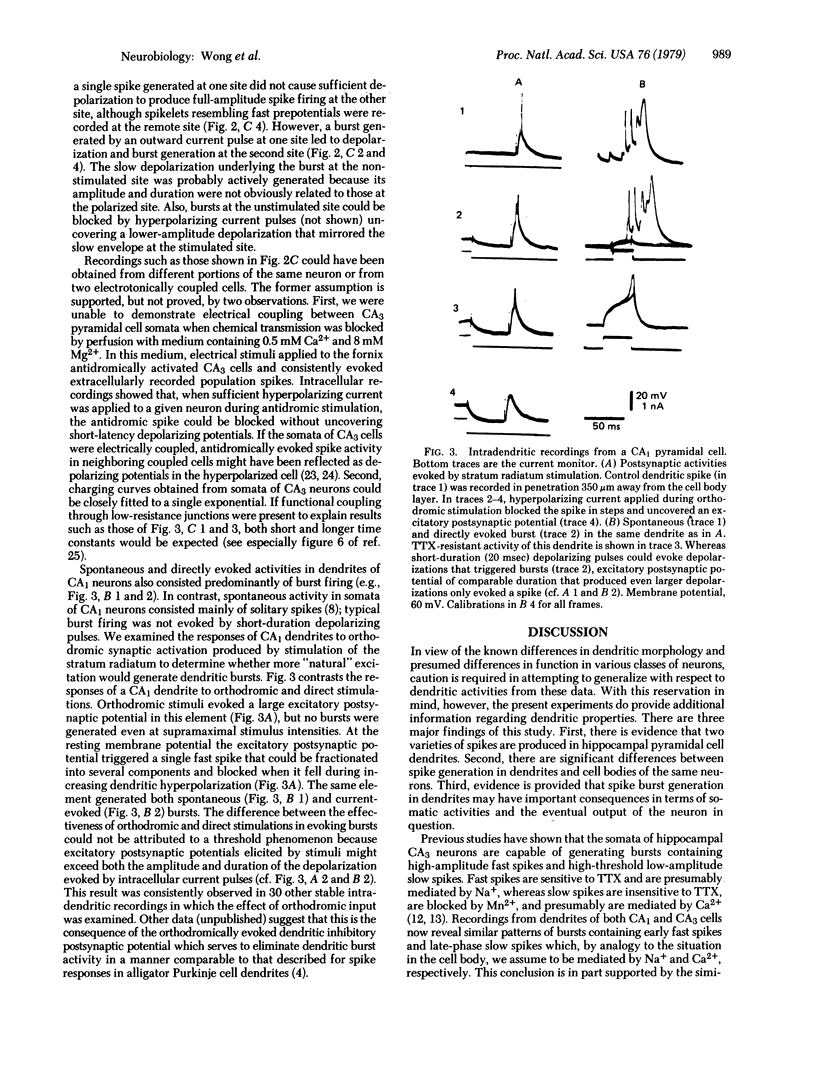
Dendritic activity in guinea pig hippocampal CA1 and CA3 pyramidal neurons was examined by using an in vitro preparation. Histologically confirmed intradendritic recordings showed that dendrites had an average input resistance of 47.0 M omega and average membrane time constant of 33.3 msec. Active spike responses could be evoked by intracellular injection of outward current or by the activation of synaptic inputs. The predominant activity was burst firing. A typical intracellularly recorded dendritic burst consisted o spikes on a slowly increasing depolarizing potential. The spike components of the burst were of two distinct types: low threshold, fast spikes; and high threshold, slow spikes. Tetrodotoxin (1 microgram/ml) blocked the fast spikes, but slow spikes could still be evoked with direct intracellular stimulation. In contrast to dendritic responses, direct depolarization of CA1 somata did not give rise to burst generation. Orthodromic stimuli evoked large-amplitude excitatory postsynaptic potentials, followed by inhibitory postsynaptic potentials in dendrites of CA1 and CA3 neurons. In two instances, simultaneous recordings were obtained from coupled pairs of elements that were presumed to be soma and dendrite of the same CA3 pyramidal neuron. Depolarization of either element led to burst generation at that site, and the underlying slow depolarization appeared to evoke a burst at the other site. This potential postsynaptic amplifying mecahnism was not ordinarily functional because even suprathreshold orthodromic activation did not normally evoke bursting in dendrites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Baker R., Llinás R. Electrotonic coupling between neurones in the rat mesencephalic nucleus. J Physiol. 1971 Jan;212(1):45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai S. T., Kocsis J. D., Preston R. J., Sugimori M. Monosynaptic inputs to caudate neurons identified by intracellular injection of horseradish peroxidase. Brain Res. 1976 Jun 18;109(3):601–606. doi: 10.1016/0006-8993(76)90039-1. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince D. A. Neurophysiology of epilepsy. Annu Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Further characteristics of hippocampal CA1 cells in vitro. Brain Res. 1977 Jun 3;128(1):53–68. doi: 10.1016/0006-8993(77)90235-9. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978 May 19;147(1):117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Westgaard R. H. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971 Dec 24;35(2):589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res. 1978 Dec 29;159(2):385–390. doi: 10.1016/0006-8993(78)90544-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Activation of hippocampal neurons by mossy fiber stimulation in thin brain sections in vitro. Exp Brain Res. 1972;14(4):423–435. doi: 10.1007/BF00235037. [DOI] [PubMed] [Google Scholar]