Abstract
Acetaldehyde is a toxic compound produced by Saccharomyces cerevisiae cells under several growth conditions. The adverse effects of this molecule are important, as significant amounts accumulate inside the cells. By means of global gene expression analyses, we have detected the effects of acetaldehyde addition in the expression of about 400 genes. Repressed genes include many genes involved in cell cycle control, cell polarity, and the mitochondrial protein biosynthesis machinery. Increased expression is displayed in many stress response genes, as well as other families of genes, such as those encoding vitamin B1 biosynthesis machinery and proteins for aryl alcohol metabolism. The induction of genes involved in sulfur metabolism is dependent on Met4p and other well-known factors involved in the transcription of MET genes under nonrepressing conditions of sulfur metabolism. Moreover, the deletion of MET4 leads to increased acetaldehyde sensitivity. TPO genes encoding polyamine transporters are also induced by acetaldehyde; in this case, the regulation is dependent on the Haa1p transcription factor. In this paper, we discuss the connections between acetaldehyde and the processes affected by this compound in yeast cells with reference to the microarray data.
Acetaldehyde is produced during Saccharomyces cerevisiae metabolism under several natural conditions, such as alcoholic fermentation or the biological aging of some wines, such as sherry. In the latter case, concentrations of 0.3 to 0.4 g/liter are commonly reached, but values can even reach 1 g/liter in some cases (23). Acetaldehyde diffuses poorly across the plasma membrane compared to ethanol, leading to its intracellular accumulation in fermenting yeasts, reaching concentrations of up to 10 times the prevailing extracellular concentration, with values of about 0.33 g/liter (32). Acetaldehyde at high concentrations stops cell growth; however, this arrest can be relieved by the addition of exogenous ethanol (33). This observation suggests that acetaldehyde can be more toxic at the early stages of fermentation, before ethanol is produced in large amounts. Acetaldehyde is a known inhibitor of a wide range of metabolic activities and is even more toxic than ethanol (16). A few years ago several proteins were detected at a higher concentration in acetaldehyde-treated cells than in ethanol-treated cells, and it was thought likely that one of those proteins was Hsp90p (30). Recent studies carried out in our laboratory have demonstrated that the addition of acetaldehyde to exponentially growing cells provokes an induction in the transcription of stress response genes, such as HSP genes (3), and of aldehyde dehydrogenase (ALD) genes (4). The induction of all of these genes by acetaldehyde is positively regulated by Msn2/4p and Hsf1p proteins, while their expression is repressed by protein kinase A (4).
During recent years, the development of techniques for analyzing changes in global gene expression has led to a better understanding at a molecular level of the effects in yeast cells of a plethora of stress situations under laboratory conditions (14) or even during industrial processes such as must or beer fermentations (5). Ethanol stress has been studied among the conditions considered (2), but so far no information about yeast responses to acetaldehyde has been reported.
In this work, we consider the changes in gene expression in S. cerevisiae after 1 h of growing in the presence of 1 g of acetaldehyde/liter, a concentration which does not cause a significant viability loss in yeast cultures. According to the data obtained, about 400 genes show significant changes in gene expression, and among the genes activated by this compound, it is possible to find most of the genes involved in sulfur metabolism and some of the TPO genes, which are involved in polyamine transport. Moreover, the results obtained with isolates having mutations in the transcription factors involved in the regulation of these genes indicate that transcriptional activation of sulfur metabolism genes by acetaldehyde is dependent on Met4p and partially dependent on Met31/32p, while polyamine transporter induction is dependent on Haa1p.
MATERIALS AND METHODS
Yeast strains.
The yeast strains used in this work are listed in Table 1. The table includes details about their genotypes and origins.
TABLE 1.
Strains used for this work
| Strain | Genotype | Reference |
|---|---|---|
| W303-1A | MATaade2 his3 leu2 trp1 ura3 | 12 |
| Wmsn2msn4 | W303-1A msn2-Δ3::HIS3 msn4Δ::URA3 | 12 |
| CD106 | MATaade2 his3 leu2 trp1 ura3 met4::TRP1 | 19 |
| CC718-1A | MATaade2 his3 leu2 trp1 ura3 cbf1::TRP | 20 |
| CD128-6A | MATaade2 his3 leu2 trp1 ura3 met28::URA3 | 20 |
| CD845-1A | MATaade2 his3 leu2 trp1 ura3 met31::TRP1 met32::HIS3 | 6 |
| CD145 | MATaade2 his3 leu2 trp1 ura3 mup3::LEU2 | 15 |
| CA350-22C | MATaade2 his3 leu2 trp1 ura3 mup1 | 15 |
| BY4741 | MATahis3 leu2 met15 ura3 | 17 |
| BY4741ΔLPZ8 | MATahis3 leu2 met15 ura3 haa1::kanMX | 17 |
| MCY1389 | MATaura3 leu2 | 12 |
| BQS110 | MATaura3 leu2 bcy1::LEU2 | 12 |
Yeast growth and stress treatments.
Yeast cells were grown with vigorous orbital agitation at 30°C in complete growth medium (YPD, consisting of 1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone, and 2% [wt/vol] glucose). Acetaldehyde was added to exponentially growing cells (optical density = 0.3) to give a final concentration of 1 g/liter. Incubation was continued for 1 h at 30°C, without agitation, in tubes filled to the top with solution to minimize the evaporation of this compound (3). A control was carried out with a culture in YPD medium without acetaldehyde addition.
For the analysis of MET gene expression (see Fig. 4), cells were grown in synthetic growth medium (SD, consisting of 2% glucose and 0.67% [wt/vol] yeast nitrogen base without amino acids, with or without 1 mM methionine).
FIG. 4.
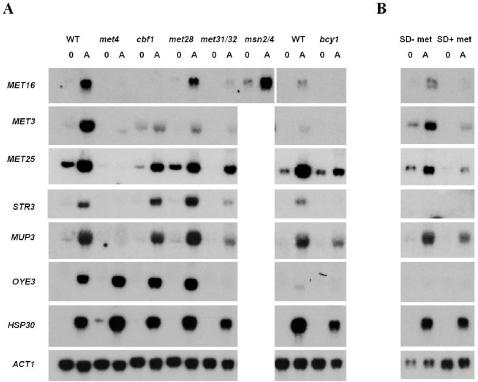
Transcriptional regulation of sulfur metabolism genes by acetaldehyde. (A) Effect of mutants in several transcription factors (mainly involved in the regulation of sulfur metabolism genes) on the expression of several genes induced by acetaldehyde. The blots show hybridization (with specific probes corresponding to the genes indicated) of RNA samples from exponentially growing cells in 2% (wt/vol) glucose (YPD medium; samples 0) and 1 h after incubation in the presence of 1 g of acetaldehyde/liter (samples A). The ACT1 gene was used as a loading control. Experiments were performed at least twice. The figure shows the results of one representative experiment. (B) Effect of methionine level in the growth medium on the expression of several acetaldehyde-induced genes. Experiments were performed as described for panel A, but cells were grown in SD medium with or without 1 mM methionine. WT, wild type.
For viability assays on plates, YPD plates containing 1 g of acetaldehyde/liter were prepared as follows. Three hundred eighteen microliters of a 10-fold dilution of acetaldehyde was spread on dried plates with 25 ml of YPD medium and was allowed to diffuse overnight at 4°C. Five-microliter serial dilutions from the exponentially growing cultures were spotted onto YPD plates, with or without acetaldehyde. Plates were incubated at 30°C until colonies appeared (approximately 2 days for YPD plates and 4 days for acetaldehyde-containing plates). These experiments were performed at least three times for each mutant strain.
RNA analysis.
Aliquots were taken from the cultures grown under the conditions described above. RNA isolation was performed as described previously (3). RNA samples (10 μg) were analyzed by electrophoresis in formaldehyde-agarose gels (29), followed by blot transfer to Hybond-N membranes and hybridization with probes corresponding to the genes of interest.
The probes used for these experiments were obtained by PCR amplification of the oligonucleotides shown in Table 2. Hybridization and quantification were carried out as described elsewhere (3). A 1-kb XhoI-HindIII fragment corresponding to the ACT1 gene was used as a loading control. Every mutant was tested at least twice.
TABLE 2.
Oligonucleotide pairs used for PCR amplification of probes corresponding to the genes under study
| Gene | Oligonucleotide pair (forward/reverse) |
|---|---|
| STR3 | GACCAACATTCAGCCTCCG/AAAGATACCGTCACAGCCC |
| MET16 | GCTGGAAACGCCACAGGAG/ATTGCGCGAATCGGATGGC |
| MET3 | TGCCTGCTCCTCACGGTGG/AGTTCTTACCTGGGCCCGC |
| MET25 | GTTCAACTACACGCCGGCC/CCGTGATATCCTTCGGCAGG |
| MUP3 | TACTGTGGAAGCGCAGGCG/ACACGGAACCAACCAGGGG |
| TPO2 | CGGGATCCTATGCAAAAACCCTTCC/GGAATTCGTAAAGAGCTCCAGCAGG |
| TPO3 | GTAGAAAGTCAGCAGCCG/ATCAGGTCCATACGTCCGG |
| TPO4 | TCAGAGCAAACGGTACCGG/ATTCAGACCAACGCCAGCC |
| YPR157W | CTAAGCGTCATACGGGCGC/GTTGATCACCCCATCCACC |
| YRO2 | TCTCGTGGTTCAGACTGGC/AGAGTCGGTGGCTACATC |
| OYE3 | CGGGATCCGATGTACGTGTGTGTGG/GGAATTCAAAGACCCCGCTATGAGG |
| HSP30 | CGTAACGAGGCTTTAGGGC/TGCTCTGCTTCAGGTTCGG |
Microarrays.
Probes for the microarrays were prepared by labeling of total RNAs, as described by Fazzio et al. (13) at the Microarray Facility (Fred Hutchinson Cancer Research Institute, Seattle, Wash.). Fifteen micrograms of total RNA was combined with 5 μg of oligo(dT) in a final volume of 15 μl and incubated at 70°C for 10 min. After being chilled on ice, the solution was mixed with 3 μl of 2.5 mM Cy3- or Cy5-conjugated dUTP (Amersham), 3 μl of 0.1 M dithiothreitol, 6 μl of first-strand buffer, 0.5 μl of deoxynucleoside triphosphates (a 25 mM concentration [each] of dATP, dCTP, and dGTP and 15 mM dTTP), and 2 μl of Superscript II (Invitrogen-Life Technologies). The reaction was incubated at 42°C for 2 h and then diluted with 500 μl of Tris-EDTA buffer. RNAs isolated from control cells were labeled with Cy3-dUTP, and RNAs from cells incubated in the presence of 1 g of acetaldehyde/liter were labeled with Cy5-dUTP. Reverse labeling of the samples with the dyes was also done.
Microarrays were hybridized and processed at the Microarray Facility, as previously described (10), and were scanned by use of a commercially available scanning laser microscope (GenePix 4000) from Axon Instruments (Foster City, Calif.). Full details on using the GenePix 4000 can be obtained from Axon. All arrays were analyzed with the program ScanAlyze (http://rana.lbl.gov/EisenSoftware.htm).
Microarray data were downloaded into a Microsoft Excel file. Spurious or anomalous results which had been flagged by the ScanAlyze program were deleted from the data set. Also, values of <1.5 times the background were not considered. Data analysis was carried out as described by Fazzio et al. (13). All calculations involving expression ratios were performed on a log2 scale by computing averages and standard errors. A negative or positive log2 value indicates a reduced or induced transcript level, respectively.
RESULTS
Gene expression changes after short-term acetaldehyde stress in S. cerevisiae.
We used cDNA microarrays for a genome-wide transcription analysis to measure the changes in the relative expression levels of yeast mRNAs 1 h after the addition of acetaldehyde to exponentially growing cells to a final concentration of 1 g/liter. Replicates of the samples (nonstressed and acetaldehyde treated) with reversed labels were also examined. Genes were considered to be down- or up-regulated if the intensity ratio changed by a factor of at least three for both labels after normalization. All data are available on our web site (www.uv.es/∼arandaa).
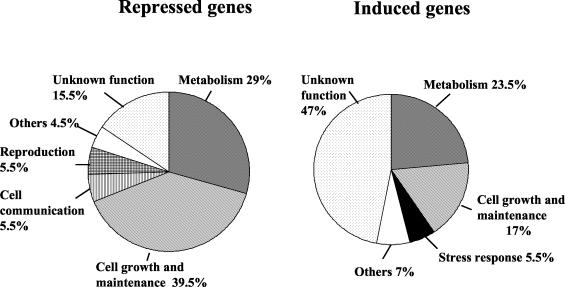
Acetaldehyde introduced changes in the expression of a significant number of genes. Of all the yeast genes, 4% (273) were up-regulated >3-fold, and of these, 130 (2%) were up-regulated >5-fold, 54 (0.9%) were up-regulated >10-fold, and 4 were up-regulated >100-fold. Conversely, 128 genes were down-regulated more than threefold after acetaldehyde treatment. Figure 1 shows the distribution of repressed and induced genes into main functional categories according to the Fatigo algorithm (http://fatigo.bioinfo.cnio.es/). Most of the repressed genes are involved in cell growth and maintenance or in metabolism. Many induced genes (47%) have an unknown function, and the metabolism and cell growth categories contain most of the genes with known functions.
FIG. 1.
Distribution of acetaldehyde-induced and -repressed genes into the most representative functional categories according to the Fatigo algorithm.
We grouped the genes that were transcriptionally affected by acetaldehyde into more specific significant functional categories than the ones shown in Fig. 1, according to the Saccharomyces Genome Database's GO Term Mapper (db.yeastgenome.org/cgi-bin/SGD/GO/goTermMapper). The results of this analysis are shown in Tables 3 and 4, which contain data for the most repressed and induced genes from each significant functional category. As shown in Table 3, many of the repressed genes participate in cell cycle regulation. Genes coding for cyclin-dependent proteins involved in the G1/S transition, the G2/M transition, or cell cycle arrest and genes involved in DNA replication, chromatin silencing, cell polarity, transport, and protein biosynthesis were found. According to this analysis, the addition of acetaldehyde to exponentially growing cells may directly or indirectly produce adverse effects on the cell cycle progression, DNA replication, protein biosynthesis, and transport of several molecules. This result could be related to the already known fact that the addition of acetaldehyde to exponentially growing cell produces growth arrest (33). We have confirmed this result in our laboratory under the conditions used for these experiments (data not shown).
TABLE 3.
Transcript levels of selected genes that are repressed by acetaldehyde
| Functional category | Open reading frame | Gene | Expression level
|
|
|---|---|---|---|---|
| Log2 valuea | Repression ratioa | |||
| Cell cycle | YNL289W | PCL1 | −3.65 | 12.97 |
| YML127w | YOX1 | −3.24 | 9.45 | |
| YDR113C | PDS1 | −2.92 | 7.56 | |
| YCR065W | HMC1 | −2.69 | 6.47 | |
| YDL179W | PCL9 | −2.55 | 5.86 | |
| YCLO24W | KCC4 | −2.54 | 5.82 | |
| YMR198W | CIK1 | −2.53 | 5.79 | |
| YER070W | RNR1 | −2.32 | 4.98 | |
| YGL021W | ALK1 | −2.24 | 4.71 | |
| YDL127W | PCL2 | −2.22 | 4.67 | |
| YPR119W | CLB2 | −2.07 | 4.21 | |
| YJL157C | FAR1 | −2.00 | 4.01 | |
| YAL040C | CLN3 | −1.97 | 3.91 | |
| YCL016C | DCC1 | −1.89 | 3.70 | |
| Cell polarity | YLR084C | RAX2 | −2.68 | 6.43 |
| YLL088C | ENT4 | −2.39 | 5.26 | |
| YGR014W | MSB2 | −2.12 | 4.35 | |
| YJR092W | BUD4 | −1.97 | 3.92 | |
| Transport | YKR039W | GAP1 | −2.77 | 6.81 |
| YPL265W | DIP5 | −2.40 | 5.28 | |
| YOL020W | TAT2 | −2.28 | 4.85 | |
| YKL120W | OAC1 | −2.01 | 4.03 | |
| Chromatin | YDL042C | SIR2 | −2.26 | 4.78 |
| silencing | YIL131C | FKH1 | −1.86 | 3.63 |
| YKL112W | ABF1 | −1.76 | 3.40 | |
| YDR310C | SUM1 | −1.68 | 3.21 | |
| Mitochondrial | YOR201C | PET56 | −2.26 | 4.78 |
| ribosomal | YBR268W | MRPL37 | −2.15 | 4.44 |
| protein | YGL236C | MTO1 | −2.06 | 4.18 |
| biosynthesis | YIL093C | RSM25 | −1.82 | 3.52 |
| YKR085C | MRPL20 | −1.76 | 3.38 | |
| Unknown function | YOR359W | VTS1 | −3.70 | 12.97 |
| YGR079W | −2.95 | 7.65 | ||
| YJR054W | −2.71 | 6.56 | ||
Data are averages obtained from fluor reversed labeling of control and acetaldehyde-treated cells.
TABLE 4.
Transcript levels of selected genes that are induced by acetaldehyde
| Functional category | Open reading frame | Gene | Expression level
|
|
|---|---|---|---|---|
| Log2 valuea | Induction ratioa | |||
| Stress response | YCR021C | HSP30 | 8.15 | 284.03 |
| YBR072W | HSP26 | 7.57 | 189.94 | |
| YER150W | SP11 | 6.29 | 78.26 | |
| YER103W | SSA4 | 6.10 | 68.66 | |
| YJL144 | 5.58 | 48.00 | ||
| YDR171W | HSP42 | 4.36 | 20.58 | |
| YBR054W | YRO2 | 3.83 | 14.23 | |
| YLL026W | HSP104 | 3.67 | 12.71 | |
| YGR088W | CTT1 | 3.33 | 10.08 | |
| YBR169C | SSE2 | 3.25 | 9.49 | |
| YDR258C | HSP78 | 2.68 | 6.40 | |
| Polyamine | YGR138C | TPO2 | 6.80 | 111.65 |
| tranport | YOR273C | TPO4 | 2.70 | 6.5 |
| Sulfur metabolism | YGL184C | STR3 | 4.81 | 28.07 |
| YHL036W | MUP3 | 4.37 | 20.69 | |
| YPL274W | SAM3 | 4.09 | 16.98 | |
| YKR069W | MET1 | 3.33 | 10.06 | |
| YLR092W | SUL2 | 3.33 | 10.03 | |
| YPR167C | MET16 | 3.19 | 9.13 | |
| YGR055W | MUP1 | 3.13 | 8.76 | |
| YLL061W | MMP1 | 3.06 | 8.33 | |
| YJR010W | MET3 | 2.99 | 7.97 | |
| YLL060C | GTT2 | 2.92 | 7.56 | |
| YFL055W | AGP3 | 2.68 | 6.41 | |
| YIR017C | MET28 | 2.63 | 6.17 | |
| Vitamin B1 | YGR144W | THI4 | 4.13 | 17.56 |
| biosynthesis | YNL332W | THI12 | 2.85 | 7.20 |
| YDL244W | THI13 | 2.76 | 6.77 | |
| Aryl alcohol | YFL056C | AAD6b | 3.15 | 8.89 |
| metabolism | YFL057C | AAD16b | 3.10 | 8.57 |
| YNL331C | AAD14 | 2.91 | 7.53 | |
| YOL165C | AAD15b | 2.65 | 6.28 | |
| Unknown function | YPR157W | 6.66 | 101.01 | |
| YER067W | 5.85 | 57.80 | ||
| YPL171C | OYE3 | 5.46 | 44.07 | |
| YBR100W | 4.78 | 27.57 | ||
Data are averages obtained from fluor reversed labeling of control and acetaldehyde-treated cells.
According to Delneri et al. (9), these genes are unlikely to be functional.
The most interesting results of this study are those related to the genes induced by acetaldehyde (Table 4). The role of the corresponding proteins in the cell can give us new insights about the response mechanisms of yeast cells to the presence of this toxic agent. In Table 4, only the genes that were induced more than sixfold by this compound were included. The first category of genes induced by acetaldehyde corresponds to genes already characterized by their induction under other forms of stress (14). Among them, the HSP26 and HSP104 genes have been shown to be induced by this stress condition in several yeast strains (3). Another group of genes which are highly induced encode polyamine transport proteins. Another interesting group of genes induced by acetaldehyde are related to sulfur metabolism. Actually, almost all of the genes involved in the pathway from sulfate uptake to homocysteine synthesis were upregulated. Several genes implicated in thiamine biosynthesis and aryl alcohol metabolism were detected. In the case of AAD genes, it is worth mentioning that according to Delneri et al. (9), AAD6, AAD16, and AAD15 are unlikely to be functional genes. Finally, the OYE3 gene is one of the genes induced by acetaldehyde whose function is unknown. Little is known about the physiological functions of Oye3p and related proteins in the yeast S. cerevisiae. However, based on studies with Oye3p homologues in Saccharomyces carlbergensis and Hansenula polymorpha (18, 28), it has been suggested that Oye-like proteins may have a general function in several organisms in the detoxification of dangerous α,β-unsaturated ketones and aldehydes (18).
Northern blot analyses using specific probes for many of the genes induced by acetaldehyde that are listed in Table 4 have indicated a concordance with the microarray data (Fig. 2 and data not shown; see also Fig. 4).
FIG. 2.
Transcriptional regulation of TPO genes by acetaldehyde. The blots show hybridization (with specific probes corresponding to the genes indicated) of RNA samples from exponentially growing cells in 2% (wt/vol) glucose (YPD medium; samples 0) and 1 h after incubation in the presence of 1 g of acetaldehyde/liter (samples A). The ACT1 gene was used as a loading control. Experiments were performed at least twice. The figure shows the results of one representative experiment.
Induction of TPO2 and TPO3 genes by acetaldehyde is Haa1p dependent.
The TPO1 to -4 genes encode a group of proton-motive-force-dependent multidrug transporters called the major facilitator superfamily (27) and are classified as members of the drug-H+ antiporter DHA12 (with 12 predicted transmembrane-spanning segments) drug efflux family (26). The proteins were originally described as being involved in polyamine transport on the yeast vacuolar membrane (38, 39), but they have recently been located in the plasma membrane (1). Tpo1p is the best characterized member of the family and shows typical multidrug transporter behavior, being able to transport eight different compounds, including polyamines, quinidine, cycloheximide, and nystatin (see reference 27 and references therein). Our microarray analyses indicated a significant induction of the TPO2 gene by acetaldehyde, a more moderate increase in TPO4 mRNA levels, and no significant induction of the TPO1 and TPO3 genes. Northern blot analyses with specific probes showed a clear induction of the TPO2, TPO3, and TPO4 genes by this compound (Fig. 2). We do not know the reasons for the discrepancy between the microarray and Northern blot data for TPO3. However, YPR157w, a gene of unknown function, which shares a promoter with TPO3 (YPR156c), is highly induced according to both kinds of analysis, which suggests that TPO3 is indeed activated by acetaldehyde.
It was recently found that the copper-activated transcription factor Ace1p homologue Haa1p is a transcription activator. The function of Haa1p is unaffected by the copper status of the cell. Comparisons of gene expression between wild-type and Δhaa1 cells indicated that this transcription factor controls the expression of a set of yeast genes, many of which encode membrane proteins (17). Most of those genes were up-regulated in the presence of acetaldehyde. Among them, TPO2, TPO3, YPR157w, and YRO2 (a gene similar to HSP30 that was induced 14-fold in our array experiments) are included. In fact, in Δhaa1 mutant cells, the expression of these genes is repressed about 10, 3, 11, and 8 times, respectively (17). In Δhaa1 mutant cells, acetaldehyde-induced expression of the TPO2, TPO3, and YPR157w genes was not found. TPO4 was not described as being Haa1p dependent and its induction by acetaldehyde was maintained in the mutant strain (Fig. 2). The induction of YRO2 was highly reduced. This indicates that the Haa1p transcription factor is involved in regulating the expression of many genes in the presence of acetaldehyde, although its expression is not significantly affected by this compound. Previous data indicated that Haa1p is a transcriptional activator of a set of genes encoding membrane stress proteins (17), but our results give new clues regarding the biological function of this protein. However, the contribution of this transcription factor is not the same for all the acetaldehyde-regulated genes. For instance, in the case of the HSP30 gene, Haa1p is not involved in acetaldehyde induction (Fig. 2). The same result was obtained for STR3 (Fig. 2) and OYE3 (data not shown). These two genes were also induced by acetaldehyde (Table 4) and will be considered in more detail below.
Acetaldehyde induction of genes involved in sulfur metabolism in S. cerevisiae depends on the transcription factor Met4p and partially depends on Met31p and Met32p.
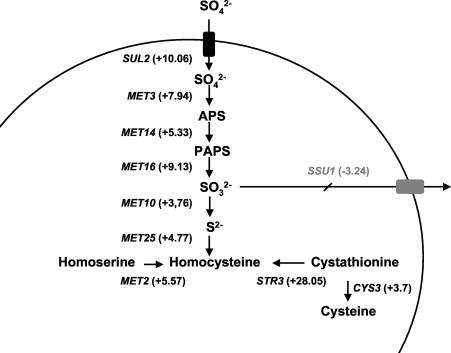
A close inspection of Table 4 and all the data obtained from the microarrays indicates that many of the genes involved in sulfur metabolism are induced by acetaldehyde. Figure 3 shows that all genes in the pathway from sulfate uptake to homocysteine synthesis (35) were induced by acetaldehyde. The same happened with MET1 (and also MET8, with an induction of 3.45-fold by acetaldehyde), which is involved in the synthesis of siroheme, a molecule that is required for a functional sulfite reductase (encoded by the MET10 gene). Moreover, genes encoding transporters of sulfur compounds, such as MUP1 and MUP3 (encoding highly related products corresponding to the high- and very-low-affinity methionine permeases [15]), SAM3 and MMP1 (encoding the S-adenosylmethionine and S-methylmethionine transporters, respectively), and AGP3 (involved in general amino acid transport) were also activated. In addition, the MET28 gene, encoding a transcription factor involved in sulfur metabolism regulation, was also activated about sixfold. Of the genes involved in glutathione metabolism, only two were also induced (GTT2, with a 7.5-fold induction, and GSH1, with a 3.6-fold induction).
FIG. 3.
Scheme of the steps of the sulfate assimilation pathway affected by the presence of acetaldehyde, according to microarray analysis results. APS, 5′-adenylylsulfate; PAPS, 3′-phospho-5′-adenylylsulfate.
The regulation of sulfur metabolism in yeast is quite complicated and depends on the transcription factors Cbf1p, Met4p, Met28p, Met31p, and Met32p (for a review, see reference 35 and references therein). Met4p constitutes the main transcriptional activator of the sulfate assimilation pathway; transcriptional activation of MET2, -3, -5, -10, -14, -16, and -25 does not occur in met4 mutants. Cbf1p appears to function by tethering Met4p to the promoter; MET10, MET14, and MET16 gene expression was shown to depend strictly on the presence of active Cbf1p under nonrepressive conditions, while MET3 and MET25 transcription is partially dependent. The full induction of the MET genes requires Met28p; a complex of Cbf1p, Met4p, and Met28p has actually been identified. Met31p and Met32p appear to act along with this complex to promote the coordinated expression of the structural genes from the sulfur amino acid biosynthesis pathway. The role of these proteins in the expression of genes containing this kind of element in the promoter depends on the case. In the absence of both Met31p and Met32p, neither MET3 nor MET14 is expressed, whereas MET25 gene transcription appears to occur in a constitutive manner, albeit at a lower level than in wild-type cells.
In order to understand the contribution of the transcription factors Met4p, Cbf1p, Met28p, and Met31/32p to the regulation of gene expression by acetaldehyde, we performed Northern blot analyses using probes specific for acetaldehyde-induced genes involved in sulfur metabolism or other processes. These analyses confirmed the induction by acetaldehyde of genes involved in sulfur metabolism, as shown in Fig. 4A. This is the case for at least STR3, MET3, MET16, and MET25. We also checked the expression of MUP3 (encoding a low-affinity methionine transporter) as an example of transporters, due to its high induction. Regarding the expression regulation of these genes by the transcription activators involved in sulfur metabolism, there are some common aspects, although the identity is not certain. STR3, MUP3, MET3, MET16, and MET25 induction by acetaldehyde was shown to be strictly dependent on the Met4p activator and partially dependent on Met31/32p. MET16 induction is completely dependent on Cbf1p, and MET3 induction is hightly reduced in this mutant.
It is worth mentioning that the protein kinase A pathway is also involved in the regulation by acetaldehyde of sulfur metabolism genes. For instance, the induction of STR3, MET3, and MET16 does not occur in the bcy1 mutant with constitutive protein kinase A activity (Fig. 4A). In the case of MUP3, there is a partial effect of the mutation in the regulatory subunit of protein kinase A. There was no previous indication regarding the involvement of this pathway in the regulation of the expression of these genes.
Repression of MET gene expression occurs when high concentrations of methionine are added to the growth medium (35). However, the transcription induction of sulfur metabolism genes by acetaldehyde seems to be a process that is independent of this repression. As shown in Fig. 4B, there is an induction of most of the genes tested when methionine is present at repressive concentrations (1 mM), although this induction is lower than the one that occurs during nonrepressive conditions, and there are even significant MET3 and MUP3 mRNA steady-state levels under basal conditions. This suggests that the presence of a high acetaldehyde concentration causes a certain depletion in sulfur sources that can be at least partially compensated for by the addition of sulfur-containing organic compounds.
The effect of these transcription factors on other genes induced by acetaldehyde was also analyzed. The OYE3 gene showed a significant induction by acetaldehyde according to microarray data (45-fold induction) and our Northern blot analyses (Fig. 4A). OYE3 induction was shown to be strictly dependent on the Met31/32p transcription factors, which indicates that these transcription factors are involved in the regulation of different genes from those involved in the sulfate assimilation pathway. In the case of the Met31/32p transcription factors, this is not new, because they have been associated with organic sulfur metabolism pathways (6) and with cadmium-mediated regulation of GSH1 gene expression (11). Other genes induced by acetaldehyde, such as HSP30 (Fig. 4), TPO2, and HSP26 (data not shown), were not regulated by these transcription factors.
Expression of several acetaldehyde-induced genes contributes to resistance to this toxic compound.
Once the molecular response to acetaldehyde and its regulation were analyzed, it was important to consider to what extent the deletion of genes induced by 1 g of acetaldehyde/liter affected the viability of yeast cells grown in a medium containing this concentration of the toxic compound.
For this purpose, viability assays were performed with several mutant strains. It is worth mentioning that the acetaldehyde concentration used in these assays affected the cell growth (data not shown). However, the viability of the cells was not significantly affected for wild-type yeast strains after a 1-h treatment, although differences were found between strains (Fig. 5) (3).
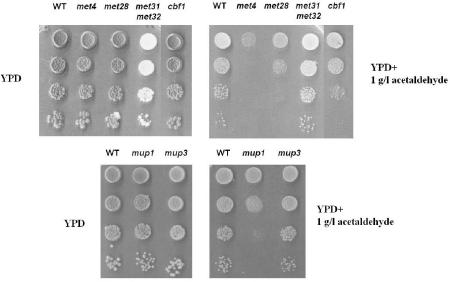
FIG. 5.
Effect of mutations in several sulfur metabolism genes on resistance to acetaldehyde. Five-microliter aliquots of serial dilutions from exponential cultures in YPD medium (2% [wt/vol] glucose) were spotted onto YPD or YPD plus acetaldehyde (1 g/liter) plates. Plates were incubated for several days until colonies appeared. Experiments were performed at least three times. The figure shows the results of one representative experiment.
Figure 5 shows the results of these experiments. For met4 mutant cells, the viability was significantly lower than that for wild-type cells when grown in rich medium in the presence of acetaldehyde. The same happened with cbf1 and met28 mutants, although to a minor extent. Deletion of the MET31/32 genes did not affect viability under these conditions. Several sulfur transporters were tested. Only a mutation in the high-affinity methionine transporter gene MUP1 caused a decreased viability. Neither the low-affinity transporter gene MUP3 (Fig. 5) nor the S-adenosylmethionine (SAM3) or S-methylmethionine (MMP1; data not shown) mutant showed a hypersensitive phenotype. It is worth mentioning that mutations in enzymes of sulfur metabolism, such as str3 and met16, did not cause significant growth differences in wild-type cells on YPD plates containing 1 g of acetaldehyde/liter (data not shown). This suggests that in rich medium, different sulfur sources can be used for yeast cells as a protective mechanism against acetaldehyde. Taken together, these results indicate that S. cerevisiae needs some degree of sulfur metabolism and sulfur compound uptake to deal with acetaldehyde excess.
Neither individual tpo mutants nor the haa1 mutant showed any effect in this kind of assay (data not shown). This may indicate a redundancy in the function of these genes.
DISCUSSION
This paper constitutes the first global analysis of the effect of acetaldehyde on yeast gene expression and allows an initial understanding of the changes in yeast gene expression and physiology related to the presence of this toxic compound and of the underlying regulatory mechanisms. According to the information regarding gene expression, two processes appear to have a central role in the response to acetaldehyde, namely the activation of multidrug transporters and the sulfur metabolism pathway.
Gene expression by acetaldehyde is controlled by several transcription factors. Previous results have indicated that Msn2/4p and Hsf1p mediate the response of several stress response genes (HSP26) and ALD genes (4) to this compound. This response is repressed by protein kinase A, as shown by the effects of a mutation in the regulatory subunit Bcy1p which leads to a constitutively high level of protein kinase A (4). The expression of most of the acetaldehyde-induced MET and TPO genes is decreased, to a greater or lesser extent, in mutants with constitutive protein kinase A activity (Fig. 4 and data not shown), which suggests that this pathway somehow regulates the expression of these genes. However, this effect probably does not occur through Msn2/4p transcription factors according to data obtained by Gasch et al. (14). Moreover, in a double msn2 msn4 mutant, there is no reduction in the induction by acetaldehyde for any of the genes considered, as shown for MET16 in Fig. 4A.
Different transcription factors are involved in the activation of different gene families. For instance, the TPO2, TPO3, YPR157W, and YRO2 genes are regulated by Haa1p. Although some connection has been established between this protein and membrane stress proteins (17), the first biological process in which this transcription factor appears to be involved is the response to toxic compounds such as acetaldehyde. We are currently analyzing the mechanisms that control the action of this factor in response to acetaldehyde.
The sulfur metabolism genes tested were regulated by Met4p, and partially by Met31/32p (Fig. 4), the transcription activators that control their expression under nonrepressive conditions for sulfur amino acid biosynthesis. However, the coincidence is not absolute. For MET25, the result obtained is consistent with previous data about its transcriptional activation under nonrepressive conditions for sulfate metabolism (24, 36). In the case of MET3, induction by acetaldehyde appears to be highly dependent on Met31/32p, albeit under nonrepressive conditions, the expression of this gene is strictly dependent on this transcription factor for sulfate metabolism (6). MET16 induction is completely dependent on Met4p and Cbf1p, highly dependent on the Met31/32p transcription factors, and not affected in a met28 mutant. However, the induction of MET16 under nonrepressive conditions of sulfate metabolism was shown to be dependent exclusively on Met4p and Met28p (19).
Interesting results were also obtained regarding the regulation of expression of other acetaldehyde-induced genes by this compound. OYE3 gene expression induction by acetaldehyde is completely dependent on Met31/32p, although there is no known link between this gene and sulfur metabolism. In the case of HSP30, transcriptional regulation still remains nuclear. Acetaldehyde is a very reactive molecule. It can react with sulfite to produce the nontoxic adduct 1-hydroxyethane sulfonate, and metabolic and genetic studies with yeast have suggested that the production of acetaldehyde and the formation of this product help the cell to counteract the toxic effects of sulfite (7). The increase in the expression of genes involved in sulfur metabolism in the presence of acetaldehyde suggests that in our situation, the surplus of this compound could be canceled out by the overproduction of reduced sulfur compounds. Interestingly, the expression of SSU1, the gene encoding the sulfite efflux pump (25), is repressed by acetaldehyde according to our array experiments (Fig. 3). This indicates that yeast cells try to keep reactive sulfur inside the cell. The detoxifying molecule could be sulfite or another sulfur-containing amino acid. Actually, our array experiments point to a homocysteine as a potential candidate (Fig. 3). However, methionine and cysteine help to reduce the toxic effects of acetaldehyde in mammals by reacting with it (22), and according to our results, a mutation in the high-affinity methionine transport gene MUP1 leads to increased acetaldehyde sensitivity (Fig. 5). Glutathione synthesis is not one of the most induced pathways (just 3.5-fold induction of GSH1 gene expression by acetaldehyde and no induction above the threshold for GSH2), but we could not rule out the contribution of this well-known stress-protective molecule. Alternatively, acetaldehyde may simply cause a depletion of the molecule that regulates sulfur metabolism, S-adenosylmethionine (37), leading to a fortuitous activation of the MET network. However, the strong defect of the met4 mutant in dealing with acetaldehyde stress suggests that there is active production of a sulfur compound for detoxifying purposes, not just an artifactual induction of the MET genes.
OYE3 may play a role in acetaldehyde detoxification as well. In H. polymorpha, the overexpression of a gene cluster (HYE) containing three homologues of the Saccharomyces OYE enzyme enhanced resistance to higher concentrations of allyl alcohol (18). The toxic aldehyde acrolein, produced from allyl alcohol by alcohol oxidase activity, can be converted into propionaldehyde by these enzymes, which is less harmful to cells (31). It has been suggested that Oye-like proteins may have a general function in detoxifying dangerous α,β-unsaturated ketones and aldehydes in several organisms (18). Several genes involved in aromatic aldehyde metabolism that belong to the AAD family (9) were up-regulated in the presence of acetaldehyde according to our microarray data (Table 4). However, of these genes, only AAD4 (with an induction of 4.88-fold by acetaldehyde) is likely to be functional in response to oxidative stress (9). The redox activities of these enzymes may be important for eliminating undesired compounds derived from the action of acetaldehyde. It is worth mentioning that other dehydrogenases (e.g., thioredoxin reductase and glutathione reductase) are known to have roles in detoxification, in these cases, of reactive oxygen species.
The strong activation of genes of the major facilitator superfamily of multidrug transporters, such as the TPO2 to -4 genes, may suggest that those membrane proteins are involved in eliminating excess acetaldehyde or the adducts formed when acetaldehyde reacts with various molecules, including sulfur-containing compounds. Tpo proteins may even actively efflux the surplus of acetaldehyde. Aqr1p, a member of the same subfamily of 12 spanner drug-H+ antiporters (DHA12), is involved in conferring resistance to short-chain carboxylic acids, including acetate (34). We cannot rule out the activity of polyamine transporters described for these proteins. Intracellular levels of polyamines are maintained within very narrow limits because a decrease in polyamine levels interferes with cell growth while an excess appears to be toxic (reference 40 and references therein). Control of the polyamine pool can be achieved by synthesis, interconversion with one another to render the necessary amounts of each one, terminal degradation, and transport. Polyamines or the products that may derive from them can be more deleterious for the cell in the presence of acetaldehyde. HSP30 and especially BTN2 (36-fold induction by acetaldehyde) are involved in proton homeostasis (8), and they may be involved in controlling the proton gradient that fuels these antiporters.
We propose a model of acetaldehyde detoxification similar to the system that deals with excessive cadmium (11). In that system, a molecule containing a reactive thiol, reduced glutathione, reacts with cadmium to produce an adduct that is eliminated from the cytoplasm by a transporter called Ycf1p (21). We propose that acetaldehyde reacts spontaneously with sulfite and/or a sulfur-containing amino acid and that the resulting adduct is expelled by the Tpo transporters.
This paper gives new insights into the response of yeast cells to acetaldehyde, a toxic compound that is produced by the metabolic activity of yeast cells and can achieve concentrations similar to those used for our analyses in some natural environments. More analyses are needed to understand particular details of the underlying regulatory mechanisms and to explain the connections between the different processes affected by acetaldehyde.
Acknowledgments
We thank Francisco Estruch for critical reading of the manuscript. We are indebted to F. Estruch, D. Thomas, Y. Surdin-Kerjan, and D. R. Winge for providing us with mutant strains.
This work was supported by grants from the Ministerio de Educación y Ciencia (CICYT ALI99-1224-002-C02 and CICYT AGL2002-01109).
REFERENCES
- 1.Albertsen, M., I. Bellahn, R. Krämer, and S. Waffenschmidt. 2003. Localization and function of the yeast multidrug transporter Tpo1p. J. Biol. Chem. 278:12820-12825. [DOI] [PubMed] [Google Scholar]
- 2.Alexandre, H., V. Ansanay-Galeote, S. Dequin, and B. Blondin. 2001. Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae. FEBS Lett. 498:98-103. [DOI] [PubMed] [Google Scholar]
- 3.Aranda, A., A. Querol, and M. del Olmo. 2002. Correlation between acetaldehyde and ethanol resistance and expression of HSP genes in yeast strains isolated during the biological aging of sherry wines. Arch. Microbiol. 177:304-312. [DOI] [PubMed] [Google Scholar]
- 4.Aranda, A., and M. del Olmo. 2003. Response to acetaldehyde stress in the yeast Saccharomyces cerevisiae involves a strain-dependent regulation of several ALD genes and is mediated by the general stress response pathway. Yeast 20:749-759. [DOI] [PubMed] [Google Scholar]
- 5.Backhus, L. E., J. DeRisi, P. O. Brown, and L. F. Bisson. 2001. Functional genomic analysis of a commercial wine strain of Saccharomyces cerevisiae under differing nitrogen conditions. FEMS Yeast Res. 1413:1-15. [DOI] [PubMed] [Google Scholar]
- 6.Blaisseau, P.-L., A.-D. Isnard, Y. Surdin-Kerjan, and D. Thomas. 1997. Met31p and Met32p, two related zinc finger proteins, are involved in transcriptional regulation of yeast sulfur amino acid metabolism. Mol. Cell. Biol. 17:3640-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casalone, E., C. M. Colella, S. Daly, E. Gallori, L. Moriani, and M. Polsinelli. 1992. Mechanism of resistance to sulfite in Saccharomyces cerevisiae. Curr. Genet. 22:435-440. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay, S., N. E. Muzaffar, F. Sherman, and D. A. Pearce. 2000. The yeast model for batten disease: mutations in btn1, btn2, and hsp30 alter pH homeostasis. J. Bacteriol. 182:6418-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delneri, D., D. C. Gardner, and S. G. Oliver. 1999. Analysis of the seven-member AAD gene set demonstrates that genetic redundancy in yeast may be more apparent than real. Genetics 153:1591-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 11.Dormer, U. H., J. Westwater, N. F. McLaren, N. A. Kent, J. Mellor, and D. J. Damieson. 2000. Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J. Biol. Chem. 275:32611-32616. [DOI] [PubMed] [Google Scholar]
- 12.Estruch, F., and M. Carlson. 1993. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 13:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazzio, T. G., C. Kooperberg, J. P. Goldmark, C. Neal, R. Basom, J. Delrow, and T. Tsukiyama. 2001. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol. Cell. Biol. 21:6450-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isnard, A. D., D. Thomas, and Y. Surdin-Kerjan. 1996. The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J. Mol. Biol. 262:473-484. [DOI] [PubMed] [Google Scholar]
- 16.Jones, R. P. 1990. Roles for replicative deactivation in yeast-ethanol fermentation. CRC Crit. Rev. Biotechnol. 10:205-222. [DOI] [PubMed] [Google Scholar]
- 17.Keller, G., E. Ray, P. O. Brown, and D. R. Winge. 2001. Haa1, a protein homologous to the copper-regulated transcription factor AceI, is a novel transcriptional activator. J. Biol. Chem. 276:38697-38702. [DOI] [PubMed] [Google Scholar]
- 18.Komduur, J. A., A. Leao, I. Monastyrska, M. Veenhuis, and J. A. K. Kiel. 2002. Old yellow enzyme confers resistance of Hansenula polymorpha towards allyl alcohol. Curr. Genet. 41:401-406. [DOI] [PubMed] [Google Scholar]
- 19.Kuras, L., H. Cherest, Y. Surdin-Kerjan, and D. Thomas. 1996. A heteromeric complex containing the centromere binding factor 1 and two basic leucine zipper factors, Met4 and Met28, mediates the transcription activation of yeast sulfur metabolism. EMBO J. 15:2519-2529. [PMC free article] [PubMed] [Google Scholar]
- 20.Kuras, L., R. Barbey, and D. Thomas. 1997. Assembly of a bZip/bHLH transcription activation complex: formation of the yeast Cbf1/Met4/Met28 complex is regulated through Met28 stimulation of Cbf1 DNA binding. EMBO J. 16:2447-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, Z. S., Y. P. Lu, R. G. Zhen, M. Szczypka, D. J. Thiele, and P. A. Rea. 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionat)cadmium. Proc. Natl. Acad. Sci. USA 94:42-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber, C. S. 2002. S-Adenosyl-l-methionine: its role in the treatment of liver disorders. Am. J. Clin. Nutr. 76:1183S-1187S. [DOI] [PubMed]
- 23.Martínez, P., L. Pérez-Rodríguez, and T. Benítez. 1997. Evolution of flor yeast population during the biological aging of fine sherry wine. Am. J. Enol. Vitic. 48:160-168. [Google Scholar]
- 24.Mountain, H. A., A. S. Bystrom, and C. Korch. 1993. The general amino acid control regulates MET4, which encodes a methionine-pathway-specific transcriptional activator of Saccharomyces cerevisiae. Mol. Microbiol. 7:215-228. [DOI] [PubMed] [Google Scholar]
- 25.Park, H., and A. T. Bakalinsky. 2000. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881-888. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen, I. T., M. K. Sliwinski, and M. R. Saier, Jr. 1998. Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 430:116-125. [DOI] [PubMed] [Google Scholar]
- 27.Sá-Correia, I., and S. Tenreiro. 2002. The multidrug resistance transporters of the major facilitator superfamily, 6 years after disclosure of Saccharomyces cerevisiae genome sequence. J. Biotechnol. 98:215-226. [DOI] [PubMed] [Google Scholar]
- 28.Saito, K., D. J. Thiele, M. Davio, O. Lockridge, and V. Massey. 1991. The cloning and expression of a gene encoding old yellow enzyme from Saccharomyces carlbergensis. J. Biol. Chem. 266:20720-20724. [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell (ed.). 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Shankar, C. S., A. Aneez, M. S. Ramakrishnan, and S. Umesh-Kumar. 1996. Mitochondrial NADH dehydrogenase activity and ability to tolerate acetaldehyde determine faster ethanol production in Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. 40:145-150. [DOI] [PubMed] [Google Scholar]
- 31.Slott, V. L., and B. F. Hales. 1985. Teratogenicity and embryolethality of acrolein and structurally related compounds in rats. Teratology 32:65-72. [DOI] [PubMed] [Google Scholar]
- 32.Stanley, G. A., and N. B. Pamment. 1992. Transport and intracellular accumulation of acetaldehyde in Saccharomyces cerevisiae. Biotechnol. Bioeng. 42:24-29. [DOI] [PubMed] [Google Scholar]
- 33.Stanley, G. A., N. G. Douglas, E. J. Every, T. Tzanatos, and N. B. Pamment. 1993. Inhibition and stimulation of yeast growth by acetaldehyde. Biotechnol. Lett. 15:1199-1204. [Google Scholar]
- 34.Tenreiro, S., P. A. Nunes, C. A. Viegas, M. S. Neves, M. C. Teixeira, M. G. Cabral, and I. Sá-Correia. 2002. AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter on the major facilitator superfamily that confer resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 292:741-748. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, D., and Y. Surdin-Kerjan. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61:503-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas, D., I. Jacquemin, and Y. Surdin-Kerjan. 1992. MET4, a leucine zipper protein, and centromere binding factor I are both required for transcriptional activation of sulfur metabolism in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, D., H. Cherest, and Y. Surdin-Kerjan. 1989. Elements involved in S-adenosylmethionine-mediated regulation of the Saccharomyces cerevisiae MET25 gene. Mol. Cell. Biol. 9:3292-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomitori, H., K. Kashiwagi, K. Sakata, Y. Kakinuma, and K. Igarashi. 1999. Identification of a gene for a polyamine transport protein in yeast. J. Biol. Chem. 274:3265-3267. [DOI] [PubMed]
- 39.Tomitori, H., K. Kashiwagi, T. Asakawa, Y. Kakinuma, A. J. Michael, and K. Igarashi. 2001. Multiple polyamine transport systems on the vacuolar membrane in yeast. Biochem. J. 353:681-688. [DOI] [PMC free article] [PubMed]
- 40.Urdiales, J. L., M. A. Medina, and F. Sánchez-Jiménez. 2001. Polyamine metabolism revisited. Eur. J. Gastroenterol. Hepatol. 13:1015-1019. [DOI] [PubMed] [Google Scholar]