Abstract
Cholangiocytes are epithelial cells that line the intra- and extrahepatic ducts of the biliary tree. The main physiologic function of cholangiocytes is modification of hepatocyte-derived bile, an intricate process regulated by hormones, peptides, nucleotides, neurotransmitters, and other molecules through intracellular signaling pathways and cascades. The mechanisms and regulation of bile modification are reviewed herein.
Introduction
Cholangiocytes are a heterogeneous, highly dynamic population of epithelial cells that line a three-dimensional network of bile ducts known as the biliary tree. Their major physiologic function lies in modification of hepatic canalicular (i.e., primary) bile as it is transported along the biliary tree. Bile modification occurs through coordinated transport of various ions, solutes, and water across the cholangiocyte apical and basolateral plasma membranes. It is modulated by hormones, peptides, nucleotides, neurotransmitters, and other molecules, including bile acids, through various intracellular signaling pathways and regulatory cascades. This article reviews the structural and functional diversity of cholangiocytes and the biliary tree and focuses on the physiologic mechanisms and regulation of bile modification. Although brief references may be made, cholangiocyte pathophysiology and immunobiology as well as the digestive properties of bile are beyond the scope of this review.
Overview of Ductal Architecture and Developmental Origin
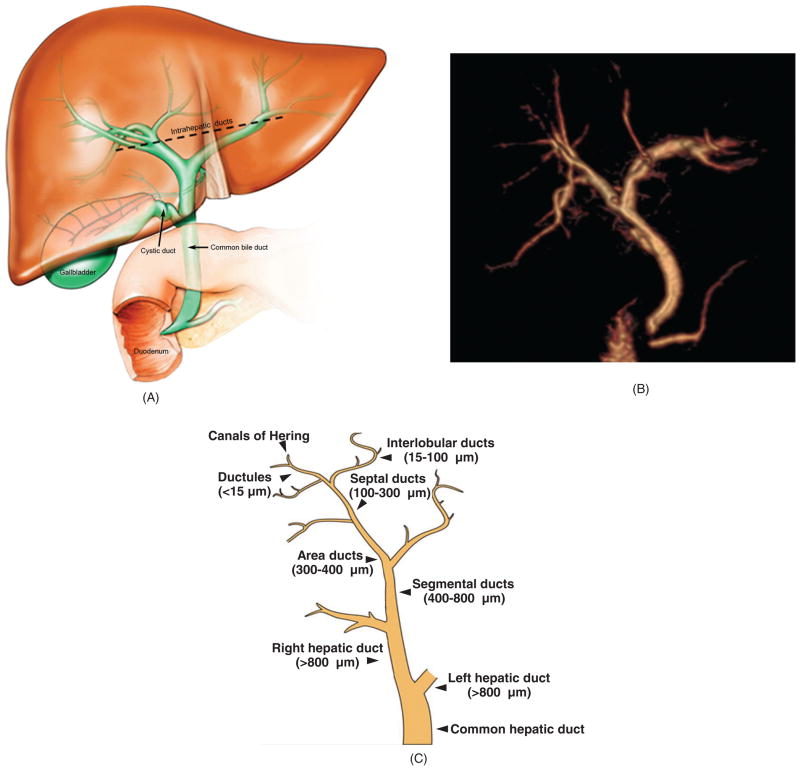
The biliary tree is a complex, three-dimensional network of tubular conduits (collectively referred to as “ducts”) of various sizes and properties. It can be divided grossly into two anatomically and functionally different compartments: intra-hepatic and extrahepatic (162, 221, 247, 256) (Fig. 1A and b). In humans, the nomenclature of the biliary conduits of the intrahepatic compartment is categorized by size and proximity (Fig. 1C). The smallest (< 15 μm in luminal diameter) and most proximal of the conduits are the ductules, which emerge from the canals of Hering, specialized channels lined by both hepatocytes and cholangiocytes which represent the anatomic and physiologic transition from entirely hepatocyte-lined canaliculi to entirely cholangiocyte-lined ductules (24,42,135,167,168,183,195,230,232,234,235,256). These microscopic ductules serially converge to form interlobular ducts, which measure 15 to 100 μm in luminal diameter. Two or more interlobular ducts combine to form septal ducts (lumen diameter 100–300 μm), which then drain into area ducts (luminal diameter 300–400 μm), which are the first of the “large” ducts. Area ducts converge into segmental ducts (luminal diameter 400–800 μm) and then right and left hepatic ducts (lumen diameter >800 μm), which mark the beginning of the extrahepatic biliary tree (162, 167, 221, 247). The extrahepatic biliary tree goes on to include the common hepatic duct, cystic duct, gallbladder (absent in rats), and common bile duct, the distal portion of which drains into the duodenum (162, 221, 247) (Fig. 1).
Figure 1.
Biliary tree architecture. (A) The biliary tree is a three-dimensional network of interconnecting tubular conduits consisting of an intrahepatic (above dashed black line) and an extrahepatic (below dashed black line) portion. (B) Magnetic resonance cholangiogram of human biliary tree (three-dimensional reconstruction). Also seen at the lower portion of the extrahepatic biliary tree are the pancreatic duct and the duodenum, the latter being where biliary and pancreatic contents drain. The gallbladder is surgically absent. (C) The human biliary tree comprises conduits of different sizes extending from the canals of Hering, through which primary or canalicular bile enters the biliary tree, to the terminus of the common bile duct (i.e., where it empties into the duodenum). [B: courtesy of Dr. Naoki Takahashi and Dr. Joel Glockner, Mayo Clinic; C: Reproduced from Masyuk et al. (183), with permission].
Small intrahepatic bile ducts are lined by 4 to 5 cholangiocytes circumferentially (i.e., in cross section), while large intrahepatic bile ducts may be lined by up to 40 cholangiocytes (104, 179, 235). In addition, large intrahepatic ducts are lined peripherally (within their fibromuscular walls) by peribiliary glands, as are the extrahepatic ducts (208).
In rodents, the intrahepatic biliary tree is categorized only into small bile ducts (lumen diameter < 15 μm) and large bile ducts (lumen diameter > 15 μm) (7, 11, 35, 108, 167). As in humans, the lumen of small bile ducts is formed by 4 to 5 cholangiocytes circumferentially, but large bile ducts are encircled by only 8 to 15 cholangiocytes (11, 35, 108, 136).
Current understanding holds that cholangiocytes lining the intrahepatic biliary tree are derived from hepatoblasts, which are also precursor cells to hepatocytes (277). In contrast, cholangiocytes lining the extrahepatic biliary tree derive from endoderm and share a common developmental origin with the pancreas and duodenum (162,221). Thus, cholangiocytes of the intrahepatic biliary tree have a close embryological link to hepatocytes, while those lining the extrahepatic biliary tree and gallbladder have a close embryological link to epithelial cells of the pancreas and duodenum. During morphogenesis, the extrahepatic biliary tree develops before the intrahepatic biliary tree; the mechanisms by which the two anastomose are unknown (162).
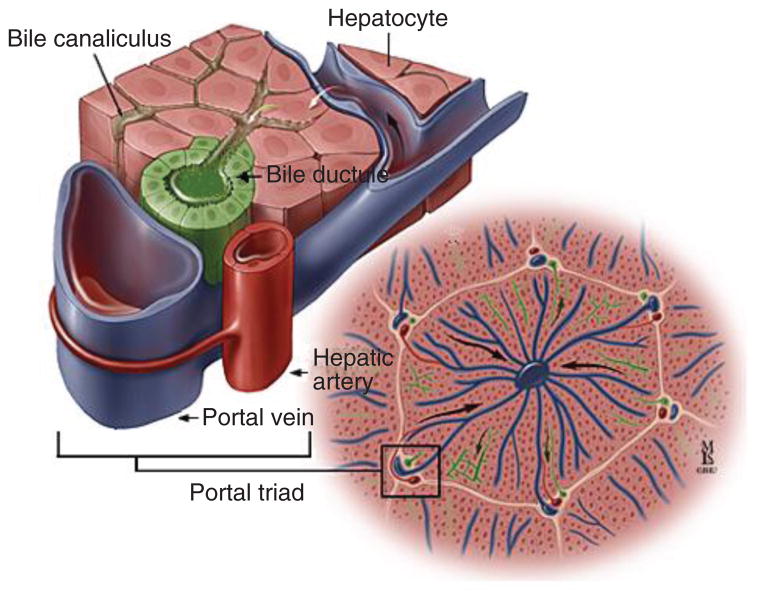
The biliary tree runs along the portal tracts adjacent to a branch of the portal vein and hepatic artery (159); this forms a hallmark anatomical arrangement known as the portal triad (Fig. 2). Portal triads demarcate the liver lobule, which is a basic hepatic architectural unit consisting of plates of hepatocytes lined by sinusoids that radiate toward and are drained by a central vein. Small branches of the hepatic artery form a network of minute vessels around bile ducts, termed the peribiliary vascular plexus, which provides blood supply to the biliary tree. Blood from the peribiliary vascular plexus ultimately drains into a portal venous branch or directly into hepatic sinusoids (96–98, 125, 262, 285). It is now known, despite continued use of the classic name, that portal triads may also include nerves and lymphatic vessels (130).
Figure 2.
Portal triad. Consists of a biliary (represented here as “bile ductule”), hepatic arterial, and portal venous component. [Courtesy of Dr. Anthony Kalloo, Johns Hopkins Gastroenterology & Hepatology (www.hopkins-gi.org)].
Cholangiocyte morphology and ultrastructure
As with bile ducts, cholangiocytes themselves differ in size—from 6 to 15 μm in diameter—as well as in morphology, being flattened or cuboidal in small bile ducts, columnar in large bile ducts (7, 11, 35, 126, 136, 232, 235). Cholangiocyte size generally follows ductal size, that is, large cholangiocytes are found in large ducts (24).
Small and large cholangiocytes differ in their nuclear-to-cytoplasmic ratio; this ratio is greater in small cholangiocytes, suggesting that they are less differentiated and have greater plasticity as compared to large cholangiocytes (35, 179). Indeed in the rat, small cholangiocytes, which are normally mitotically dormant, may proliferate in response to various stimuli (e.g., histamine, secretin, α-naphthylisothiocyanate, and acute carbon tetrachloride) (90,93,105,107,165,196,207) and insults (e.g., partial hepatectomy) (9, 179) and acquire functional features of large cholangiocytes (92,104,171,179). Similar proliferation and plasticity is thought to occur in human and mouse cholangiocytes (4, 207, 242). Therefore, we and others speculate that, in contrast to the more differentiated large cholangiocytes, small cholangiocytes may represent a functional hepatobiliary progenitor cell population (265). However, it remains to be determined experimentally whether this is true.
Cholangiocytes possess an apical (luminal) and a basolateral plasma membrane. Adjacent cholangiocytes are joined by tight junctions (zonula occludens) located near the apical membrane (126, 279) (Fig. 3). These junctions play a central role in establishing and maintaining epithelial cell polarity (246). Cholangiocytes also possess gap junctions, channels that permit direct cytoplasmic communication between adjacent cells (41). Extending from the apical membrane of cholangiocytes are numerous microvilli, which provide a fivefold increase in cell surface area (10, 126, 168, 279) (Fig. 3). Each cholangiocyte also contains a single primary cilium—a long, tubular organelle which extends from the apical plasma membrane and protrudes into the bile duct lumen (31, 123, 126, 185, 189, 190, 193). The primary cilium consists of a membrane-bound axoneme composed of microtubules and a centriole-derived basal body (i.e., kinetosome), a microtubule-organizing center from which the axoneme emerges (155) (Fig. 4). The axoneme of a primary cilium has a 9 + 0 microtubule arrangement, that is, nine peripheral microtubule doublets with no central pair of microtubules, and lacks dynein arms, in contrast to that of a motile cilium which has a 9 + 2 axoneme pattern and requires the motor protein dynein (31, 37, 233).
Figure 3.
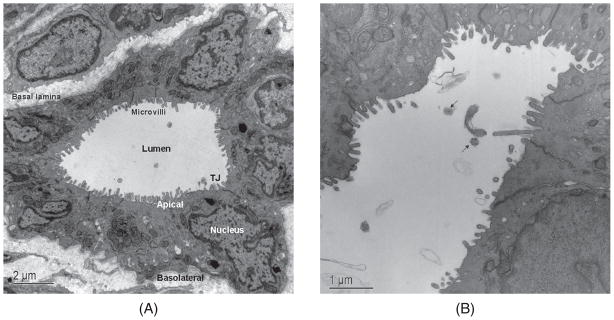
Transmission electron micrographs of rat intrahepatic bile duct cross-sections. (A) Large bile duct lined by 11 cholangiocytes, each with an apical and basolateral plasma membrane and demarcated by tight junctions (TJ) located near the APM. Microvilli on the apical plasma membrane significantly increase the cholangiocyte surface area. (B) Higher power magnification of large bile duct showing partial long view and axial section (black arrows) of primary cilia. [A: reproduced from Masyuk et al. (183), with permission].
Figure 4.
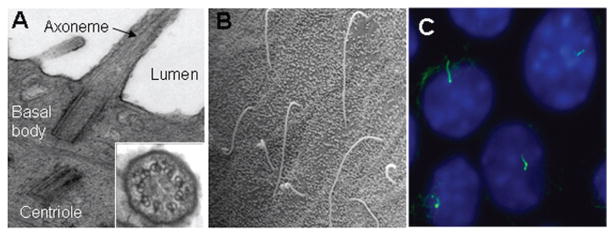
Cholangiocyte primary cilia. (A) Transmission electron microscopy micrograph of primary cilia extending from the cholangiocyte apical plasma membrane and into the ductal lumen with insert showing the 9+0 pattern of the ciliary axoneme. (B) Scanning electron microscopy images of primary cilia in large rat bile duct. (C) Immunofluorescence confocal microscopy image of primary cilia in normal mouse cholangiocyte cell line 10 to 14 days after confluence. In C, cilia were stained with acetylated α-tubulin (green) and nuclei were stained with DAPI (blue). [A and C: reproduced from Masyuk et al. (189), with permission].
In rat liver, primary cilia, which have the same 9+0 ax-oneme pattern, are heterogeneous in length but are generally proportional to the diameter of the duct they are located in (123). In large bile ducts, the cilia measure 6–8 μm in length, approximately twice as long as in small ducts. Basal bodies also vary in size, with ranging from 0.14 to 0.26 μm in diameter and 0.26 to 0.48 μm in length in small and large bile ducts, respectively (123, 189). In cultured normal rat and mouse cholangiocytes, primary cilia appear at day 3 post-confluence and grow progressively up to day 10 to a maximal length of approximately 7 to 10 μm, at which point the majority of cells possess a cilium (123) (Fig. 4). Unlike in rodents, the dimensions of primary cilia along the biliary tree have not been systematically characterized in humans.
Cholangiocytes possess an actin cytoskeleton which is fundamentally involved in structural and functional support of the plasma membrane, establishing and maintaining cell polarity, and regulating vesicle trafficking and membrane protein distribution (68, 69). Coated pits and vesicles have been identified on both the apical and basolateral plasma membrane domains, suggesting that receptor-mediated endocytosis occurs in these regions (127, 128). In addition, lysosomes and multivesicular bodies (MVBs), cellular organelles integrating both endocytic and secretory pathways, are also present near the apical domain of cholangiocytes (183, 186). Some MVBs fuse with lysosomes, resulting in degradation of their contents, while other MVBs fuse with the cholangiocyte apical plasma membrane and exocytically release their contents into the lumen of the bile duct (186). Also released in an exocytic manner from cholangiocytes are exosomes, small (30–100 nm in diameter), membrane-enclosed vesicles (50, 63, 153, 182, 186, 238, 249, 250, 281). Although these vesicles may be released from a variety of cell types and have been implicated in a number of physiological processes, their precise functional significance and processing remains largely obscure (153, 249, 250, 276). One function of exosomes appears to be specific delivery of proteins, lipids, mRNAs and miRNAs to neighboring or distant target cells, thus triggering downstream signaling events therein (225, 238, 249).
Cholangiocytes have a prominent Golgi apparatus located near the apical membrane, an inconspicuous and sparse endoplasmic reticulum, and small perinuclear mitochondria (35, 126, 179, 183). Their nucleus is round to oval, oftentimes notched, and typically located basally (Fig. 3).
Biochemical and functional heterogeneity of cholangiocytes
In addition to being morphologically heterogeneous, cholan-giocytes also demonstrate biochemical and functional heterogeneity. For example, while many proteins are expressed in both small and large cholangiocytes, some proteins are expressed only in small cholangiocytes or vice versa. In humans, both small and large cholangiocytes express pancreatic lipase, pancreatic α-amylase, trypsin (263, 264), and the Cl−-HCO3− exchanger, AE2 (177, 264). In contrast, only small cholangiocytes express blood group antigens (e.g., A and B) (214) and the antiapoptotic protein, Bcl-2 (53,55), while only large cholangiocytes express cytochrome P4502E1 (61, 152). Differential protein expression is also seen in the mouse (94, 202, 254, 273) and rat biliary tree (8, 9, 11, 13, 165, 197, 248).
With respect to functional heterogeneity of cholangiocytes, the working model has it that large but not small cholangiocytes are actively involved in bile modification (94, 104, 136, 179). Recent observations, however, suggest that the functional heterogeneity of large and small cholangiocytes is more complex, and that both large as well as small cholangiocytes may contribute to bile modification (106,283).
Physiology of Bile Formation and Modification
Basic principles
Bile formation and modification require structural and functional integrity of both hepatocytes and cholangiocytes. Initial formation and secretion of primary bile into canaliculi, the rate-limiting step in bile biosynthesis, is driven primarily by active excretion of organic solutes and ions into the canalicular space followed by osmotic entry of water (183). The vectorial transport of bile salts into the canaliculi results in “bile-salt-dependent” canalicular bile secretion, whereas excreted reduced glutathione and bicarbonate (HCO3−) drive “bile-salt-independent” bile secretion (33, 42, 76, 209).
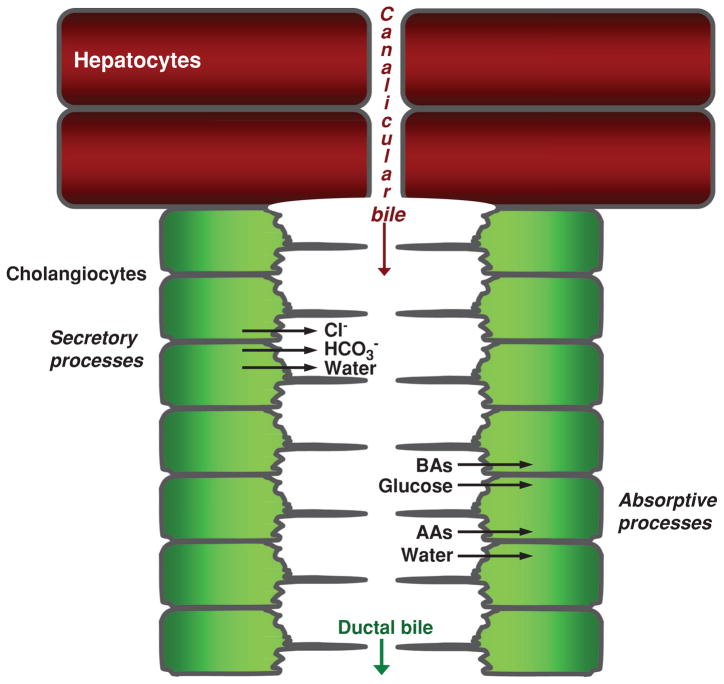
Canalicular bile subsequently enters the lumen of the canals of Hering and then percolates through the biliary tree, where it is extensively modified via a series of reabsorptive and secretory processes executed predominantly by large cholangiocytes and possibly small cholangiocytes (based on data from mice) (Fig. 5) (94, 106). These processes include secretion of Cl−, HCO3−, and water from cholangiocytes and reabsorption of bile acids, amino acids, and glucose into cholangiocytes (Fig. 5) (272). The molecular physiology of bile modification is hereinafter discussed in detail.
Figure 5.
Overview of bile formation and modification. Hepatocytes initiate bile formation by secreting primary bile, composed primarily of water, solutes, and ions, into canaliculi. As canalicular bile flows along the biliary tree, it is subjected to cholangiocyte secretory and absorptive processes, thus resulting in modified ductal bile. Secretion of Cl−, HCO3−, and water and absorption of bile acids (BAs), glucose, amino acids (AAs), and water are the major transport processes determining the chemical composition of ductal bile. [Adapted from Masyuk et al. (183), with permission].
Cholangiocyte secretion
Physiologically, bile secretion is a functional response of the liver to a meal. In the postprandial state, acidic pH and peptides in the duodenal lumen stimulate S cells in the duodenum and jejunum to release secretin into the portal venous circulation. Secretin regulates a number of physiological functions, including secretion of HCO3−-rich bile (42,135,256). HCO3− secreted by cholangiocytes determines the alkalinity, hydration, and pH of bile, minimizes passive cholangiocyte absorption of protonated glycine-conjugated bile acids by forming the biliary HCO3− umbrella, and significantly contributes to the intestinal luminal pool of HCO3− required for digestion (39, 42, 135, 183).
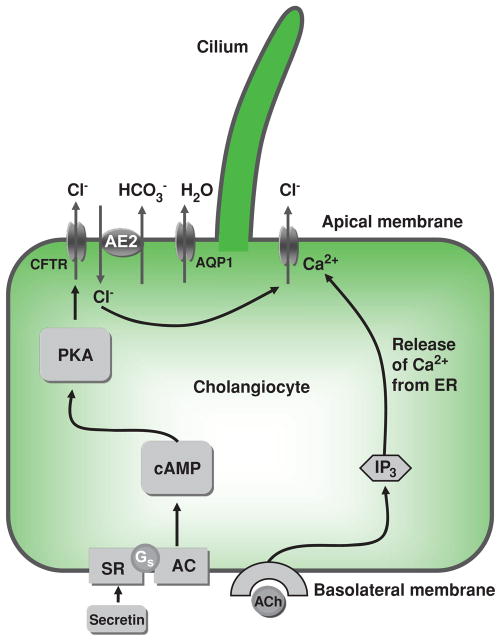
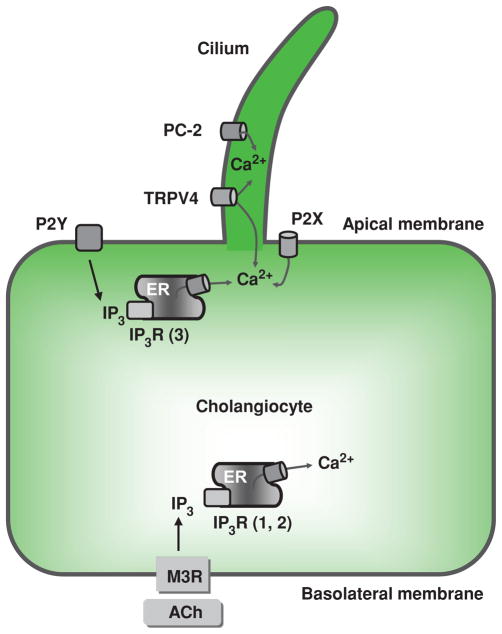
At the molecular level, bile secretion is initiated by opening of various Cl− channels on the cholangiocyte apical plasma membrane, resulting in movement of Cl− out of the cell. An apically located Cl−/HCO3− exchanger is then activated by the established Cl− gradient, and HCO3− is secreted into the bile duct lumen in exchange for Cl−. HCO3− secretion is followed by osmotically driven efflux of water into the ductal lumen (Fig. 6). An alternative mechanism involves stimulation of basolateral M3 muscarinic receptors with acetylcholine (ACh), resulting in a local increase in IP3. This increase in IP3 leads to a release of Ca2+ from basolateral (types I and II) IP3Rs, thus leading to apical Cl−/HCO3− secretion (192, 204).
Figure 6.
Mechanisms of ductal bile modification. According to a widely accepted model, secretin induces HCO3−-rich fluid secretion from cholangiocytes via the cyclic adenosine 3′,5′-monophosphate-protein kinase A (cAMP-PKA) signaling pathway by activation of the apical cystic fibrosis transmembrane conductance regulator (CFTR) Cl−channel resulting in extrusion of Cl− ions, which in turn stimulate the Cl−/HCO3− exchanger AE2 and subsequent secretion of HCO3−. Secreted HCO3− ions drive passive aquaporin 1 (AQP1)-mediated movement of water in response to established osmotic gradients. An alternative mechanism of Cl– efflux is also shown, consisting of acetylcholine (ACh) binding to basolateral M3 muscarinic receptors, which leads to increases in inositol trisphosphate (IP3), which binds to (types I and II) IP3Rs, causing release of Ca2+ and activation of apical Cl− secretion. AC, adenylate cyclase; Gs, Gs protein; PKA, protein kinase A; SR, secretin receptor. [Adapted from Masyuk et al. (183), with permission].
A number of channels, transporters, and exchangers expressed on both the apical and basolateral plasma membranes function in concert to achieve physiologic secretion of bile. These cholangiocyte plasma membrane proteins are depicted in Figure 7, and their characteristics are described individually in the sections below.
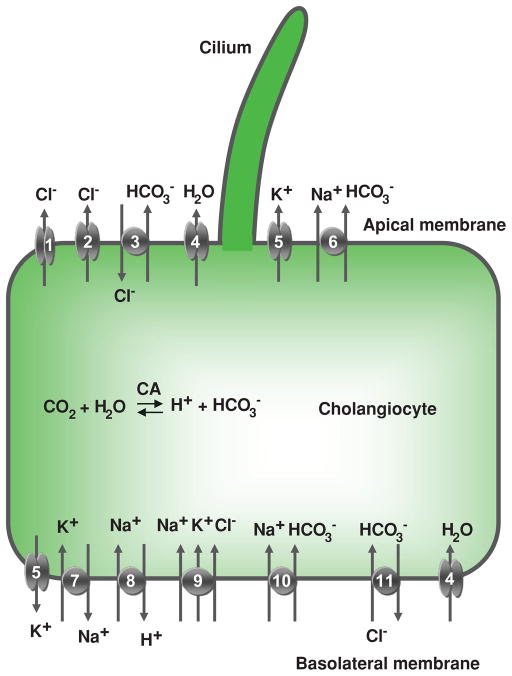
Figure 7.
Cholangiocyte secretion. Located at the cholangiocyte apical plasma membrane are: (1) cyclic adenosine 3′,5′-monophosphate (cAMP)-regulated Cl− channel (cystic fibrosis transmembrane conductance regulator), (2) Ca2+-regulated Cl− channel, (3) Na+-independent Cl−/HCO3− exchanger, (4) water channel, (5) K+ channel (SK2), and (6) Na+-HCO3− cotransporter (in mouse). At the basolateral plasma membrane, cholangiocytes express (5) K+ channels (SK2 and IK-1), (7) Na+/K+-ATPase, (8) Na+/H+ exchanger, (9) Na+- K+-Cl− cotransporter, (10) Na+-HCO3− cotransporter (in rat), (11) Na+-dependent Cl−/HCO3− exchanger (in humans), and (4) water channels (AQP1 and AQP4). Details regarding each of these components of cholangiocyte secretion are found in the text. CA, carbonic anhydrase. [Adapted from Masyuk et al. (183), with permission].
cAMP-regulated Cl− channel, CFTR
Several Cl− conductances with different biophysical characteristics have been demonstrated in cholangiocytes, including ones activated by cyclic adenosine 3′,5′-monophosphate (cAMP), and others by intracellular Ca2+ (86). The cAMP-activated Cl− channel was identified as being the cystic fibrosis transmembrane conductance regulator (CFTR) (88). CFTR (the gene mutated in the human disease cystic fibrosis, which in some cases has a cholestatic phenotype) belongs to a family of adenosine triphosphate (ATP)-binding cassette (ABC) proteins that is expressed in human, rat, and mouse liver, exclusively in large cholangiocytes (7, 62). It contains intracellular N and C termini and has five domains, two of which span the plasma membrane (six times each) (43, 243). These two transmembrane domains are separated by an intracellular regulatory (R) domain containing phosphorylation sites for both protein kinase A (PKA) and protein kinase C (PKC) (54, 84).
Under basal conditions, Cl− transport across the cholangiocyte apical membrane is low; stimulation with cAMP increases it by 20- to 40-fold (88, 199). It was believed that cAMP-dependent PKA phosphorylation of CFTR resulted in CFTR channel opening and efflux of Cl− ions into the bile duct lumen (42, 183) (Fig. 6). However, recent studies challenge the role of CFTR as a primary route of Cl− efflux in bile formation; instead, a regulatory role of CFTR in Cl− secretion by Ca2+-activated Cl− channels has been proposed (84, 204). According to this model, CFTR functions in regulating release of ATP from cholangiocytes into the ductal lumen, the mechanism of which remains uncertain. It is hypothesized that release of ATP occurs through ATP-permeable channels and/or by exocytosis of ATP-containing vesicles (87), and that ATP then activates apically located P2 receptors, resulting in an increase in intracellular Ca2+ and Ca2+-activated Cl− secretion. Thus, CFTR-dependent ATP release followed by Ca2+-activated Cl− secretion is likely a dominant mechanism in the initial steps of bile formation (83, 84, 204).
Ca2+-activated Cl− channels
Ca2+-activated Cl− currents have been recorded in human, rat, and mouse cholangiocytes. Cl− currents occur in response to cholangiocyte swelling, fluid flow, and ATP accumulation, and, can be associated with swelling-activated Cl− channels, fluid flow-stimulated Cl− channels, and ATP-stimulated Cl− channels (73, 86, 88, 89, 282, 283). The molecular identity of the Ca2+-activated Cl− channels in cholangiocytes remains unknown (73, 86, 282, 283).
Ca2+ extrusion systems
The two major Ca2+ extrusion systems of eukaryotic cells are the Na+/Ca2+ exchanger (NCX) and the plasma membrane Ca2+ pump (PMCA). The relative role of these two systems in handling cellular Ca2+ homeostasis varies with the tissue and cell type. The mechanisms and role of these systems in cholangiocyte Ca2+ extrusion are not well understood and are thus only briefly addressed below.
Mammals express three NCX genes coding for NCX 1–3, all of which are expressed at low levels and seem to play a minor role in Ca2+ regulation in the liver (220). Specific information on NCX isoform expression and Ca2+ signaling in cholangiocytes is lacking, but this extrusion system may play a role in preventing Ca2+ overload and hepatocyte injury following oxidative stress (38, 40).
PMCAs belong to the ATP2B family of P-type ATPases and use ATP to pump Ca2+ ions across the plasma membrane against a large concentration gradient (38, 44). They are the sole high-affinity transporter dedicated exclusively to Ca2+ removal from the cell (38). Mammalian PMCAs are encoded by four genes, and alternative splicing generates additional diversity by way of isoforms (259). The major isoform expressed in liver is PMCA1b, but other isoforms, including PMCA4b, are also present (67). In hepatocytes, the PMCA appears to be predominantly in the basolateral (i.e., sinusoidal) membrane, with less of the pump in the apical canalicular membrane. Our unpublished data show that cholangiocytes express PMCA1b and PMCA4b, but their relative levels, cellular localization, and involvement in Ca2+ signaling (e.g., oscillations, secretion, and gene regulation) remain to be determined (203).
Na+-independent Cl−/HCO3− exchanger, SLC4A2/AE2
Cholangiocytes possess a number of specific proteins and mechanisms for intracellular HCO3− homeostasis (252). Among these is the Na+-independent Cl−/HCO3− exchanger, SLC4A2/AE2, which functions in electroneutral and reversible exchange of Cl− and HCO3− across human and rodent cholangiocyte plasma membranes (2, 30, 33, 177, 252, 258). SLC4A2/AE2 (implicated in the pathogenesis of the human disease primary biliary cirrhosis) is a member of the SLC4 family of HCO3− transporter (2, 30, 33, 177, 252, 258); this family consists of eight members, three of which, SLC4A1/AE1, SLC4A2/AE2, and SLC4A3/AE3 (and controversially, SLC4A4/AE4), are Na+ independent (2). SLC4 polypeptides have three structural domains: intracellular N and C terminal domains of 400 to 700 amino acids and 30 to 100 amino acids, respectively, and a C terminal polytopic transmembrane domain of approximately 500 amino acids (2). Apically located SLC4A2/AE2 mediates HCO3− secretion into bile in a Cl− gradient-regulated manner, in accordance with intracellular pH (159), and is also critical to establishing a protective apical cholangiocyte plasma HCO3− umbrella (121). The transcription factor HNF1α (hepatocyte nuclear factor 1α) may upregulate AEb1 and AEb2 expression, suggesting the potential for liver epithelial cells to increase expression of these AE2 isoforms when needed for increased HCO3− secretion (21, 170).
Na+-dependent Cl−/HCO3− exchanger
In humans, the electrogenic cholangiocyte basolateral membrane Na+-dependent Cl−/HCO3− exchanger (NCHE) counterbalances apical HCO3− efflux mediated by AE2, thereby contributing to maintenance of intracellular HCO3−. Carbonic anhydrase II, an enzyme that generates HCO3− and H+ from CO2 and H2O, together with NHE1/SLC9A1 (discussed below), are additional mechanisms that maintain the concentration of intracellular HCO3− (146, 274).
Na+-HCO3− cotransporters
Murine cholangiocytes express an apical plasma membrane Na+-HCO3− cotransport carrier, Slc4a4/Nbc1, that transports one Na+ and three HCO3− into the ductal lumen. Such Na+-HCO3− cotransport was not detected in rat cholangiocytes, suggesting that it is mouse specific (275).
Rat cholangiocytes express a basolateral Na+-HCO3− cotransporter, an analog to human NCHE. This, together with NHE1/SLC9A1 and carbonic anhydrase, functions to maintain a high concentration of intracellular HCO3− (252).
Na+/H+ exchanger
The electroneutral exchange of intracellular H+ for extracellular Na+, which is critical for transepithelial Na+ transport, is performed in different cell types by the NHE/SLC9 family of Na+/H+ exchangers (NHE). NHEs are highly regulated phosphoproteins with two functional domains: an N terminal domain, which constitutes the cation antiport machinery, and a large C terminal cytoplasmic tail domain, which serves a modulatory role by interacting with protein kinases and regulatory factors (52, 218).
The presence of the first of nine Na+/H+ exchanger orthologs (i.e., NHE1/SLC9A1) on the human and rat cholangiocyte basolateral plasma membrane was shown by functional studies (252). NHE1/SLC9A1 has several physiological functions, including regulation of transepithelial HCO3− transport.
K+ channels
Although Cl− channels are believed to provide the main driving force for biliary secretion, complementary actions by membrane K+ channels are also required (72). In secretory epithelia, it is thought that activation of K+ channels leads to membrane hyperpolarization by which the electrical driving force for continued Cl− efflux is maintained (72).
The biliary epithelium possesses two known K+ channels. On the apical plasma membrane, human and rodent cholangiocytes express a Ca2+-activated small conductance K+ channel, SK2 (183). This 63 kDa protein has six transmembrane domains and is activated by small increases in intracellular Ca2+ concentration (81, 226). Activation of apical plasma membrane SK2 results in a small K+ conductance (approximately −4 μA/cm2), which enhances cholangiocyte secretion of Cl− in response to regulatory stimuli (81).
On the basolateral plasma membrane of human and rat cholangiocytes, two types of Ca2+-activated K+ channels are expressed, SK2 and the intermediate-conductance Ca2+-activated K+ channel 1, IK-1 (72,81,198). These two channels function in a complimentary manner and contribute to cholangiocyte secretory responses by hyperpolarizing the basolateral plasma membrane (72).
Na+/K+ ATPase
Human and rat cholangiocytes possess a Na+/K+-ATPase pump (240, 269, 271), which, as in many other cell types, transports Na+ and K+ against their electrochemical gradients. The Na+/K+-ATPase couples the energy released by hydrolysis of one ATP with transporting 3 Na+ out of and 2 K+ into the cell, creating a strong electrochemical gradient of approximately 60 mV (183). This favors Na+ influx from an extracellular Na+ concentration of approximately 140 mmol/L to an intracellular Na+ concentration of approximately 14 mmol/L, with a rate of transport of 103 to 106 ions/s (86). In cholangiocytes, the inwardly directed Na+ gradient provides energy for transport of organic solutes (e.g., bile acids, amino acids, and glucose).
Na+-K+-Cl− cotransporter
Functional studies suggest that rat cholangiocytes possess an electroneutral Na+-K+-Cl− cotransporter on the basolateral plasma membrane that maintains a high intracellular Cl− concentration (82). In most cell types, Na+-K+-Cl− cotransporters mediate transport of 1Na+:1K+:2Cl− ions into cells in an electrically neutral manner (115). Basolateral Na+-K+-Cl− cotransport and Cl−/HCO3− exchange function as active Cl− uptake mechanisms that maintain the concentration of intracellular Cl− above electrochemical equilibrium. An increase in apical plasma membrane permeability to Cl− also results in activation of basolateral K+ channels (i.e., SK2), through which K+ that is taken up by the Na+-K+-Cl− cotransporter and Na+/K+-ATPase is cycled back out. The lumen-negative transcholangiocyte electrical potential generated by these processes may drive passive paracellular transport of Na+ (and water) from blood to the ductal lumen (183).
Aquaporins
HCO3− secreted into bile duct lumen establishes an osmotic gradient that drives efflux of water from cholangiocytes through water channels known as aquaporins (AQPs) (187, 188). AQPs (AQP0-12) are small (26–34 kDa) integral membrane proteins consisting of 6 transmembrane alpha-helical domains with 5 interhelical loop regions (A–E) and cytoplasmically oriented N and C termini. Connecting loops B (cytoplasmic) and E (extracellular) contain the highly conserved NPA (asparagine-proline-alanine) motif and form the hourglass-shaped structure through which water passes (131). With a diameter of approximately 3 Å, the size of this water pore is sufficient to allow effective passage of water molecules in a single file in either direction (51, 124, 280). AQPs have extremely high water transporting capability, as exemplified by AQP1, which transports approximately 3 × 109 water molecules per channel per second.
In humans, AQP1 is expressed in interlobular and terminal bile ducts (212). In the rat, AQP1 is principally present within intracellular vesicles, and in low amounts, in the basolateral plasma membrane when in the basal state (172, 174); when rat cholangiocytes are stimulated by secretin, AQP1 is subsequently translocated to the apical plasma membrane (128, 129). In the mouse, AQP1 is present in both small and large bile ducts (110, 202); however, although important, AQP1 does not appear to be a rate-limiting factor for ductal bile secretion (202). It is thought that AQP8, expressed particularly abundantly in large murine cholangiocytes, may play the main role in AQP-mediated water transport in murine biliary epithelia (273).
AQP4 is exclusively expressed on the rat cholangiocyte basolateral plasma membrane (173). The high water permeability of AQP4 suggests that AQP4 can effectively balance water permeability between the apical and basolateral membranes (183). In contrast to AQP1, AQP4 expression, and localization in isolated rat cholangiocytes is secretin unresponsive (173). This supports the notion that AQP4 is constitutively present in the basolateral plasma membrane, or alternatively, that its functions are driven by signaling pathways not involving secretin (183).
Studies on enclosed, polarized, microperfused isolated bile duct units (IBDUs) harvested from normal rats and mice have shown AQP-dependent water transport into the lumen of IBDUs in response to inward (i.e., secretory) osmotic gradients (64, 110, 184, 191). Water secretion into the lumen of IBDUs was not inhibited by protamine, a protein that disrupts tight junctions, but was inhibited by mercuric chloride (HgCl2), an AQP-inhibiting compound (64), as well as by small interfering RNAs to AQP1 (253).
Cholangiocyte absorption
Cholangiocytes contribute to ductal bile modification not only by secretion, but also by absorption of ions, bile acids, amino acids, glucose, and other molecules. The cholangiocyte apical and basolateral plasma membrane both express transport proteins that enable these absorptive functions (Fig. 8), and these are individually reviewed in detail below.
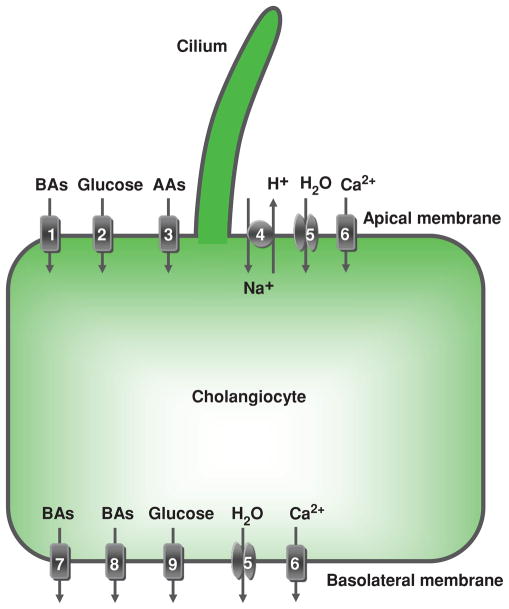
Figure 8.
Cholangiocyte absorption. Located at the cholangiocyte apical plasma membrane are: (1) Na+-dependent bile acid transporter (ASBT), (2) Na+-dependent glucose transporter, (3) unidentified Na+-dependent and Na+-independent amino acid transporters, (4) Na+/H+ exchanger, (5) a water channel (AQP1), and (6) plasma membrane Ca2+ pump (PMCA) (Na+/Ca2+ exchanger not shown due to inadequate data in cholangiocytes). At the basolateral plasma membrane, cholangiocytes express: bile acid transporters (7) t-ASBT and (8) Ostα-Ostβ, (9) glucose transporter, (5) water channels (AQP1 and AQP4), and (6) PMCA (speculated to be present on both the apical and basolateral membrane). These, as well as the secretory transport proteins, function in a coordinated manner to contribute to ductal bile modification by absorbing solutes and water from bile and transporting them out of the cholangiocyte. Details regarding each of these components of cholangiocyte absorption are found in the text. AAs, amino acids; BAs, bile acids. [Adapted from Masyuk et al. (183), with permission].
Na+/H+ exchangers
An NHE ortholog, NHE3/SLC9A3, has been identified on the rat and mouse cholangiocyte apical plasma membrane (201). Functional studies in IBDUs from NHE3/SLC9A3 knockout mice and from normal rats and mice treated with an NHE inhibitor, 5-(N-ethyl-N-isopropyl)-amiloride, have shown that NHE3/SLC9A3 is involved in cholangiocyte fluid absorption and counterbalancing fluid secretion (201). In addition to these functions, NHE3/SLC9A3 may also be a site for regulation of cholangiocyte HCO3− and fluid secretion, presumably through upregulation of CFTR via protein-protein interactions (201). The presence of another ortholog, NHE2/SLC9A2, on the rat cholangiocyte apical plasma membrane has also been reported (176, 252).
Bile acid absorption
A fraction of the bile acids secreted by hepatocytes into bile canaliculi is absorbed by cholangiocytes and returned to hepatocytes instead of being delivered to the intestine. In this pathway, referred to as the cholehepatic shunt (as opposed to the enterohepatic circulation), unconjugated bile acids secreted into bile by hepatocytes in an anionic form become protonated and passively diffuse into cholangiocytes through their apical plasma membrane (120, 183) (Fig. 9). These unconjugated bile acids are then transported into the peribiliary vascular plexus and returned to hepatocytes. They are then resecreted into bile canaliculi, stimulating HCO3− secretion (66). In contrast, conjugated bile acids are transported across the cholangiocyte apical and basolateral membranes by specific transporters (8, 36, 119, 156, 158).
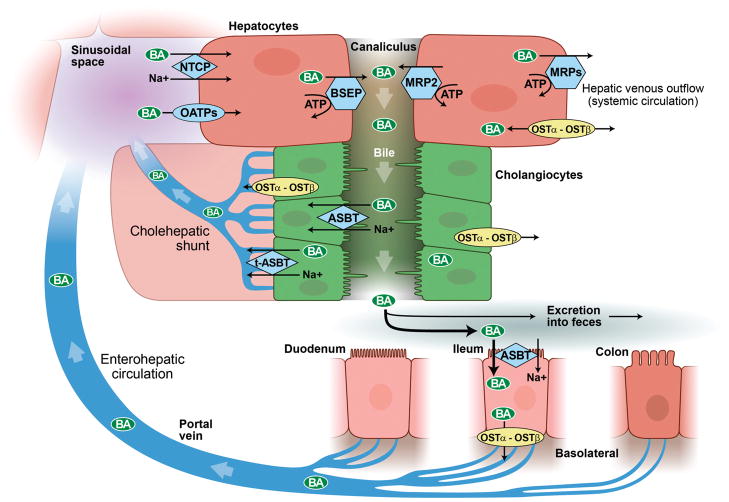
Figure 9.
Enterohepatic circulation and cholehepatic shunt. Bile acids are synthesized by hepatocytes and subsequently secreted into canalicular bile by means of specialized hepatocyte canalicular membrane transporters. Canalicular bile drains into the biliary tree and is modified by the epithelial cells lining it, that is, cholangiocytes. Bile then drains into the proximal small bowel, that is, duodenum, and is metabolized by enteric bacteria. Approximately 95% of bile acids are reabsorbed in the terminal ileum and enter the portal vein to be recycled back to the liver via the enterohepatic circulation. Once in the sinusoids of the liver, bile acids can be taken up by hepatocytes and secreted back into bile. A fraction of (unconjugated) bile acids in the biliary tree are taken up by cholangiocytes at the apical membrane (i.e., prior to reaching the small intestine) and returned to the liver sinusoids via the cholehepatic shunt. (With permission of Mayo Foundation for Medical Education and Research. All Rights Reserved.)
Located on the apical plasma membrane is the Na+-dependent bile acid transporter ASBT/SLC10A2, a 48 kDa integral membrane protein identical to that cloned from rat ileal and renal epithelial cells (8, 156). ASBT/SLC10A2 is a highly efficient transporter of conjugated bile acid that mediates coupled bile acid and Na+ uptake in a 2:1 ratio (65). The low level of ASBT/SLC10A2 expression on the cholangiocyte apical plasma membrane, however, suggests only a modest role of this transporter in conjugated bile acid uptake (66). Alternative splicing of the ASBT gene generates a truncated, 19 kDa form of ASBT/SLC10A2, t-ASBT (158); this truncated form of ASBT is found on the basolateral plasma membrane and has the complementary function of bile acid efflux from the cholangiocyte (158).
Given the cytotoxic effects associated with intracellular accumulation of bile acids, the mechanisms for bile acid efflux from cholangiocytes into the peribiliary vascular plexus are extensive and redundant. These include t-ASBT, multidrug resistance protein 3 (MRP3/ABCB4), MRP4, organic anion transporting polypeptide 3 (Oatp3), and the organic solute transporter Ost alpha-Ost beta (25, 28, 36, 119, 158), the lattermost of which appears to play a central role in this process (25, 28).
Glucose absorption
Glucose initially enters canalicular bile at a concentration equal to plasma (≈ 5.0–7 mmol/L) and is subsequently reabsorbed from bile by cholangiocytes, resulting in a low glucose concentration (< 1.0 mmol/L) in common bile duct bile (114). The vectorial transport of glucose from bile to blood is accounted for by a sodium-dependent glucose cotransporter, SGLT1, expressed on the cholangiocyte apical plasma membrane (as well as in small intestinal epithelial cells, similar to the case of ASBT), and by a facilitative glucose transporter, GLUT1, which is expressed on the basolateral plasma membrane (157).
SGLT1 is one of seven proteins of the SLC5A gene family. It has 14 transmembrane domains and extracellularly oriented N and C termini. Domains 10 to 13 form the sugar binding/translocation domain, and the Na+ binding/translocation domain is located at the N terminus (284). SGLT1 uses the energy of the Na+ electrochemical gradient created by the Na+/K+ ATPase pump to transport glucose across the apical membrane against its gradient (i.e., secondary active transport) (284).
GLUT1, also known as solute carrier family 2, facilitated glucose transporter member 1, has 12 transmembrane domains and cytoplasmically oriented N and C termini. This transporter facilitates energy-independent diffusion of glucose down its gradient across the cholangiocyte basolateral plasma membrane (45).
The transport of biliary glucose by cholangiocytes is functionally important for at least two reasons. First, by absorbing glucose from bile, cholangiocytes actively participate in determining the chemical composition and properties of bile. Second, absorbed glucose provides an osmotic gradient that favors reabsorption of water from bile into cells (114,166,191). It has been shown in humans and rats that as biliary glucose absorption increases following D-glucose infusion into the femoral vein or portal vein, the rate of bile flow decreases (114). Similarly, in isolated rat liver, the rate of bile secretion decreased by approximately one-fifth when it was perfused with a D-glucose-containing solution (166). The converse is also true in that as biliary glucose absorption decreased following administration of phlorizin, a competitive inhibitor of SGLT1, bile flow increased (114, 216). In addition, direct studies on glucose microperfused isolated rat IBDUs showed rapid absorption of glucose via SGLT1 followed by AQP-mediated water absorption (191).
Amino acid absorption
Bile contains the amino acids glutamate, cysteine, and glycine. They are the hydrolysis products of the tripeptide glutathione, the principal driving force for “bile salt-independent” canalicular bile secretion (27, 29, 42). Glutathione, which is secreted by hepatocytes, is degraded in bile by γ-glutamyltranspeptidase, an enzyme found abundantly on the canalicular hepatocyte and ductal cholangiocyte apical plasma membranes (116). Functional studies suggest that cholangiocytes absorb biliary glutamate, cysteine, and glycine by Na+-dependent and independent transport mechanisms that are distinct from those in hepatocytes (26, 42). Absorption of these amino acids by cholangiocytes may conserve them for glutathione resynthesis and thus be involved in “bile salt-independent” canalicular bile secretion (183); in addition, it may generate osmotic gradients of 3 to 12 mOsm that favor water absorption and thereby be directly involved in ductal bile modification (85).
Bidirectional transport of proteins and organic ions
Bile contains a variety of molecules such as proteins and organic anions and cations that are transported bidirectionally by cholangiocytes through their apical and basolateral plasma membranes, that is, both into and from bile, via various channels and pumps. For example, human and rat cholangiocytes express the ATP-dependent conjugate efflux pumps MRP3/Mrp3 and MRP4 (22, 147, 228, 251), which transport a broad range of organic anionic substrates from bile into the systemic circulation (22, 119, 150). Transported in the opposite direction, that is, into bile, are molecules such as epidermal growth factor (EGF) and polymeric IgA. In rat cholangiocytes, EGF is internalized from the basolateral membrane via receptor-mediated endocytosis and then transported into bile by transcytosis (128). In humans, polymeric IgA is secreted into bile by a mechanism involving the IgA receptor (154). Also transported into bile a number of plasma proteins, including ceruloplasmin, asialoceruloplasmin, carcinoembryonic antigen, and asialocarcinoembryonic antigen, into bile (149, 217, 267). The transport of these proteins into bile is believed to occur through anatomical communications between the fenestrated peribiliary capillary endothelium and the basolateral cholangiocyte plasma membrane (255).
Cholangiocytes are continuously exposed to noxious or toxic agents, many of which are organic cations excreted into canalicular bile by hepatocytes (42,206). The transmembrane gradient of such agents may favor their passive entry and accumulation in cholangiocytes with potentially injurious effects (183). Cholangiocytes possess several proteins and mechanisms that can mitigate such effects (Fig. 10). For example, the multidrug resistance P-glycoprotein (also known as ABC subfamily B, a member of which is implicated in the human disease progressive familial intrahepatic cholestasis type 3) gene products, multidrug resistance 1 (MDR1) and Mdr1a, are expressed in human and rat biliary epithelia and localized to the cholangiocyte apical plasma membrane (99, 240, 266). These proteins are ATP-dependent trans-membrane efflux pumps with broad substrate specificity including lipophilic cytotoxic drugs, glucocorticoids, peptide antibiotics, Ca+2 channel blockers, and others (144). The apical localization of MDR1 and Mdr1a in cholangiocytes (99, 240, 266) and Mdr1a-mediated transport of rhodamine 123 (a P-glycoprotein substrate) into the lumen of rat IBDUs suggest that MDR1 and Mdr1a may counteract accumulation of organic cations in cholangiocytes by excreting them back into bile (183). Furthermore, they can excrete into bile exogenous and endogenous lipophilic compounds that have entered cholangiocytes from the peribiliary vascular plexus through the cholangiocyte basolateral plasma membrane (99).
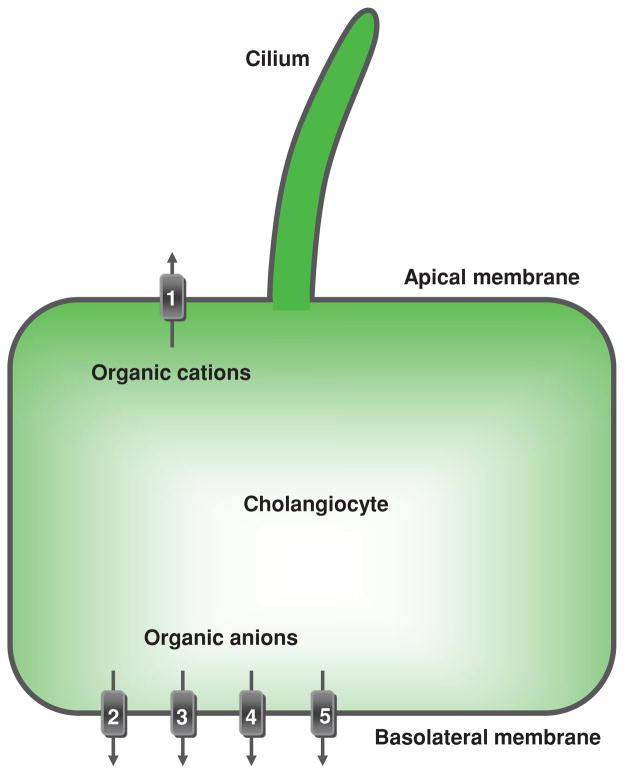
Figure 10.
Cholangiocyte transport of organic anions and cations. Located at the cholangiocyte apical plasma membrane is (1) multidrug resistance 1 protein that excretes organic cations into bile. Located at the basolateral plasma membrane are transporters effectuating efflux of organic anions from cholangiocytes into the peribiliary vascular plexus: multidrug-associated proteins (2) MPR3/Mpr3 and (3) MPR4, (4) organic anion transporting polypeptide 3, and (5) organic solute transporter Ostα-Ostβ. TJ, tight junction. [Adapted from Masyuk et al. (183), with permission].
Intracellular Signaling
To function in a coordinated manner and balance secretion with absorption, cholangiocyte transport proteins are regulated by a variety of extracellular stimuli via intracellular signaling mechanisms. The major cholangiocyte intracellular signaling pathways are associated with cAMP and Ca2+ and are described below.
cAMP signaling
Adenylate cyclase (AC), the enzyme that catalyzes ATP cyclization to generate cAMP and inorganic pyrophosphate, has nine membrane-bound isoforms (AC1-9) that are associated with G-protein-coupled receptors and one soluble G-protein-insensitive AC (sAC, also known as AC10) that have been identified in mammalian cells (132, 260). AC isoforms are tissue specific, have different expression profiles, and are uniquely regulated.
ACs are activated or inhibited depending on the coupling of receptors to heterotrimeric G proteins, which are composed of α, β, and γ subunits. There are several classes of α-subunits, including activating (i.e., αs) and inhibitory (i.e., αi), each of which respectively determines the functional characteristics of a given G protein. The αs-subunit hydrolyzes a molecule of GTP to GDP, and a complex of β-and γ-subunits anchors the G protein to the membrane. Some stimuli induce dissociation of the αs-subunit from Gs protein and binding of it to ACs, resulting in an increase in cAMP production. Other stimuli release αi-, β-, and γ-subunits from the Gi protein complex, which, in turn, inhibit ACs. In addition, different isoforms of ACs are activated (e.g., AC8) or inhibited (e.g., AC5 and AC6) by an increase in intracellular Ca2+ (132, 260).
Rat cholangiocytes express a total of 7 ACs: six membrane-bound ACs (AC4-9) and sAC, suggesting the presence of multiple mechanisms of the cAMP signaling pathway therein (31, 123, 257). Indeed, ACs are heterogeneously expressed along the rat biliary tree and are associated with different secretory stimuli. The expression of Ca2+-insensitive ACs (AC4 and AC7) predominates in small cholangiocytes, whereas large cholangiocytes primarily express Ca2+-sensitive ACs (AC5, AC6, AC8, and AC9) as well as sAC, which is regulated by intracellular HCO3−(31, 123, 257). While AC5, AC6, and AC9 are inhibited by Ca2+, AC8 is Ca2+ activated. Functionally, AC8 and AC9 are linked to cholangiocyte secretion induced by secretin and by β2-adrenergic receptor agonists, respectively, and sAC is linked to cholangiocyte secretion induced by accumulation of intracellular HCO3− (257).
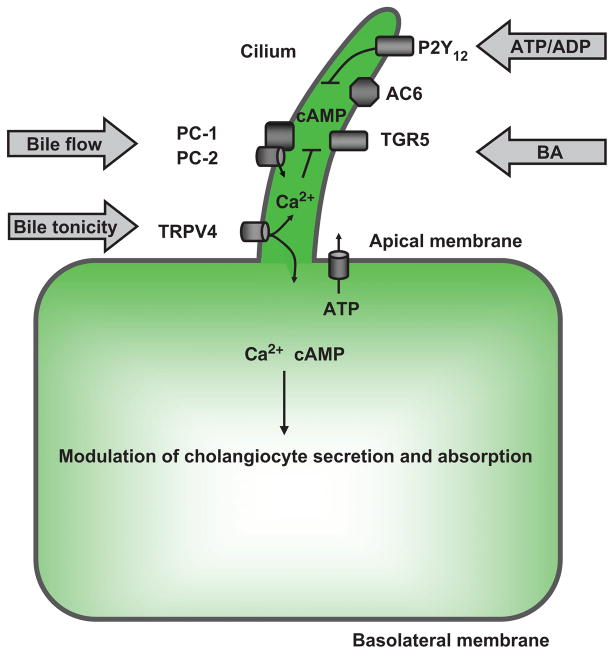
AC4, AC6, and AC8 are also localized to cholangiocyte cilia, where they are involved in cAMP signaling induced mechanically by bile flow or chemically by biliary nucleotides and bile acids (31,123). Bile acid-induced intracellular cAMP signaling is associated with the recently discovered G-protein-coupled bile acid receptor TGR5, which acts as a bile acid sensor coupling biliary bile acid concentrations to bile formation (140–142). TGR5 is expressed in human and rodent cholangiocytes on their apical plasma membrane, on cilia, and intracellularly, and also in other cell types and tissues (141). Intracellular TGR5 is likely the result of its internalization into the cytoplasm in response to bile acids (138), whereas the localization of TGR5 on cholangiocyte cilia may represent the molecular link between bile acids, cilia, and cilia-associated cAMP signaling in cholangiocytes.
In epithelial as well as other cells, cAMP functions as a second messenger to transduce extracellular signals into a cellular response via several cAMP effector proteins, including PKA and exchange proteins activated by cAMP (EPACs) (132, 260). PKA is a heterotetramer with two regulatory and two catalytic subunits that responds to changes in cAMP levels and regulates a wide range of intracellular processes. The regulatory and catalytic subunits of PKA each have four isoforms, RIα, RIβ, RIIα, and RIIβ and Cα, Cβ, Cγ, and PrKX, respectively. The biochemical and functional features of PKA are determined in large part by the structure and properties of the regulatory subunits, which are tissue dependent and variable depend on the cellular state (i.e., basal vs. stimulated) (241). cAMP binds to the PKA regulatory subunits (2 to each regulatory subunit), leading to dissociation and activation of the catalytic subunits that can then effectuate the phosphorylation of different proteins and expression of different genes (241). Rat cholangiocytes express all four regulatory subunits (32); two of them, RIβ and RIIα, are localized to the cholangiocyte cilium (185).
EPAC proteins are a family of cAMP-activated Rap guanine nucleotide exchange factors that regulate cellular processes through PKA-independent mechanisms. There are two different isoforms of EPAC, EPAC1 and EPAC2 (also known as RapGEF3 and RapGEF4, respectively) that are encoded by different genes (229). Both proteins are expressed in rat cholangiocytes (32); one of them, EPAC2, is localized to the cholangiocyte cilium (185).
The expression of multiple isoforms of ACs, regulatory subunits of PKA, and EPACs suggests the diverse effects of cAMP signaling in the physiologic processes of cholangiocytes.
Calcium signaling
Ca2+ signaling in cholangiocytes is initiated by two pathways: (i) activation of G-protein-coupled receptors expressed on both the apical and basolateral plasma membrane and (ii) influx of extracellular Ca2+ into the cell via Ca2+ channels. These are reviewed individually below.
Activation of the G-protein-coupled receptors
Activation of specific G-protein-coupled receptors results in production of inositol 1,4,5-trisphosphate (IP3), which diffuses into the cytoplasm to release Ca2+ from intracellular stores via its interaction with IP3 receptors (IP3R). IP3Rs are tetrameric IP3-gated Ca2+ channels located in the membrane of the endoplasmic reticulum. Upon IP3 binding, the IP3R undergoes large conformational changes resulting in opening of Ca2+ channels and consequent release of Ca2+ from the endoplasmic reticulum and into the cytoplasm (203). Cholangiocytes express all three known isoforms of IP3R (i.e., types I, II, and III); however, IP3R type III is the predominant isoform. This isoform is most abundant near the apical plasma membrane, whereas isoforms I and II are distributed relatively uniformly throughout the cytoplasm (117, 244).
Activation of any of the IP3R isoforms leads to an increase in the intracellular Ca2+ concentration; however, the patterns of intracellular Ca2+ signaling, that is, Ca2+ waves and Ca2+ oscillations, depend on the particular IP3R isoform that is activated. The type III IP3R is involved in initiation of Ca2+ waves, whereas the type I IP3R is likely involved in both the spread of Ca2+ waves and the support of Ca2+ oscillations. The precise role of type II IP3R in intracellular Ca2+ signaling in cholangiocytes is unclear (117, 244). Ca2+ waves induced by the parasympathetic neurotransmitter ACh and ATP are propagated in cholangiocytes from the apical to the basolateral pole. ACh-induced Ca2+ waves cross cholangiocytes at a speed of more than 20 microns/s, whereas ATP-induced Ca2+ waves appear to cross cholangiocytes much slower (117). Both ACh and ATP also induce Ca2+ oscillations (repetitive Ca2+ increases) in cholangiocytes; however, each of these two agonists induces a distinct pattern of Ca2+ oscillations. For example, lower concentrations of ATP predominantly induce sustained Ca2+ oscillations, whereas higher concentrations predominantly induce single, brief Ca2+ increases. In contrast, ACh does not display clear dose dependence, and the spikes it induces tend to be irregular in duration (although typically longer as compared to ATP induced) and frequency (161, 200, 210). This variability may enable different stimuli to elicit nuanced and distinct physiological effects even though they all act through modulation of intracholangiocyte Ca2+ signaling (203).
Ca2+ channels
Cholangiocytes express three types of Ca2+ entry channels that may be involved in intracellular Ca2+ signaling: P2X receptors, polycystin-2 (PC-2), and transient receptor potential channel vanilloid subfamily 4 (TRPV4).
P2X receptors (P2X1-7), which belong to a larger family known as the purinergic receptors, are ligand- (ATP) gated, Ca2+-permeable, nonselective cation channels. They have two transmembrane domains, intracellularly oriented N and C termini, and an extracellular loop containing the ATP-binding site (183). P2X receptor stimulation by extracellular ATP results in opening of the ion-permeable pore, an influx of Ca2+, and depolarization of the cholangiocyte membrane (47). Mz-Cha-1 cells, a human cholangiocarcinoma cell line, express four of seven P2X receptors (P2X2, P2X3, P2X4 and P2X5) (261). Normal rat cholangiocytes express P2X2, P2X3, P2X4 and P2X7 (70, 71), with the P2X4 receptor presumably being the dominant one (70).
PC-2 is a 110 kDa protein, member of the transient receptor potential (TRP) superfamily, that is encoded by the PKD2 gene (mutated in autosomal dominant polycystic kidney disease) and functions as a nonselective cation channel with a high permeability to Ca2+. It contains six transmembrane-spanning domains and intracellular N and C termini. PC-2 localization is concentrated in the endoplasmic reticulum, where it functions as a Ca2+-permeable cation channel (20). In cholangiocytes and other primary cilia-containing epithelial cells, PC-2 colocalizes with ciliary polycystin-1 (PC-1) and other proteins and is a component of a mechanosensory complex (123, 211).
TRPV4 is a Ca2+ entry channel that was first identified as a channel activated by hypotonicity-induced cell swelling (183); it is now clear however, that it might integrate responses to a variety of stimuli (77). It is a member of the OSM9-like transient receptor potential channel subfamily in the TRP superfamily of nonselective cation channels. It has six transmembrane domains, with a pore domain between the fifth and sixth, and intracellular N and C termini. In cholangiocytes, TRPV4 is localized to cilia, where it functions as an osmoreceptor (112, 113, 185). Activation of ciliary TRPV4 induces cholangiocyte HCO3− secretion by a mechanism involving apical ATP release, thus suggesting the importance of ciliary TRPV4 in ductal bile modification (112).
With respect to small versus large cholangiocytes, it is unknown whether these cells have functional differences in Ca2+ signaling. Based on recently published observations (90, 93, 171, 283), we speculate that both small and large cholangiocytes have similar Ca2+-signaling machinery but may respond differentially to extracellular stimuli. Indeed, both small and large cholangiocytes possess the (IP3)/Ca2+-dependent signaling pathway but have different responses to the same agonist. For example, small mouse cholangiocytes proliferate in response to stimulation of the H1 histamine receptor (HRH1) by activation of the IP3/CaMK pathway, whereas large cholangiocytes do not proliferate in response to HRH1 activation (90). It has been also demonstrated that in diseases primarily affecting large cholangiocytes, small cholangiocytes replenish the biliary epithelium by amplification of Ca2+-dependent signaling and acquisition of large cholangiocyte phenotypes (4, 93, 171). Also, both small and large mouse cholangiocytes demonstrate rapid increases in intracellular Ca2+ concentration upon exposure to extracellular nucleotides; however, the magnitude of ATP release is greater in small versus large cholangiocytes, which may reflect differences in ATP-mediated intracellular Ca2+ signaling (283).
Regulation of Bile Modification
Activation or inhibition of the intracellular cAMP and Ca2+ signaling pathways is essential for modulating bile modification via a number of extracellular regulatory factors. These factors may be categorized into four groups: (i) regulatory factors stimulating basal cholangiocyte secretion, (ii) regulatory factors potentiating secretin-stimulated cholangiocyte secretion; (iii) regulatory factors inhibiting basal and secretin-stimulated cholangiocyte secretion or stimulating cholangiocyte absorption, and (iv) bile-associated regulatory factors. Examples of each of these factors are reviewed below and summarized in Table 1.
Table 1.
Factors Regulating Ductal Bile Composition
| Category | Name | Receptors/transporters | Signaling pathways | Targets |
|---|---|---|---|---|
| Stimulate cholangiocyte secretion | Secretin Bombesin VIP Corticosteroids |
SR Unidentified Unidentified Nuclear GcR |
cAMP-PKA Unidentified Unidentified Change in gene expression |
CFTR/AE2/AQP1, NHE3 Cl− channels/AE2, Na+/HCO−3 co-transporter, K+ channels Cl− channels/AE2 AE2, NHE1 |
| Potentiate secretin-stimulated cholangiocyte secretion | Cholinergic neurons Adrenergic neurons |
M1, M3 α1, α2, β1, β2 |
[Ca2+]i-cAMP [Ca2+]i-PKC-cAMP |
CFTR/AE2 CFTR/AE2 |
| Inhibit basal and secretin-stimulated cholangiocyte secretion or stimulate cholangiocyte reabsorption | Somatostatin Gastrin Dopaminergic neurons Endothelin |
sst2 CCK-B/gastrin D2 ETA, ETB |
cGMP, cAMP [Ca2+]i-PKCα, [Ca2+]i-PKCβ [Ca2+]i-PKCγ, cAMP-PKA [Ca2+]i-cAMP |
SR, CFTR/AE2, AQPs CFTR/AE2 CFTR/AE2 CFTR/AE2 |
| Bile-born factors (the first three primarily stimulate cholangiocyte secretion while the last 4 inhibit secretion or stimulate reabsorption) | Nucleotides Bile acids (unconjugated) Bile acids (conjugated) Glucose Amino acids Bile flow Bile osmolarity |
P2X, P2Y Passive transport ASBT SGLT1 Na+-dependent and Na+-independent transport PC-1/PC-2 TRPV4 |
[Ca2+]i [Ca2+]i-PKCα [Ca2+]i –PKCα [Ca2+]i [Ca2+]i |
Cl− channels/AE2 Cl− channels, HCO−3 secretion SR, CFTR/AE2, ASBT, t-ASBT AQPs? AQPs? Cl− channel/AE2 (?) Cl− channel/AE2 (?) |
Abbreviations: SR, secretin receptor; M1, M3, muscarinic acetylcholine receptors; α1, α2, β1, β2, adrenergic receptors; D2, dopaminergic receptor; sst2, somatostatin receptor, subtape 2; CCK-B/gastrin, cholecystokinin-B/gastrin receptor; GcR, glucocorticoid receptor; ETA and ETB, endothelin receptors; P2X and P2Y, nucleotides receptors; PC-1, polycystin 1; PC-2, polycystin 2; ASBT, Na+-dependent bile acid transporter; t-ASBT, truncated form of ASBT; SGLT1, Na+-dependent glucose transporter; TRPV4, osmoreceptor; PKA, protein kinase A; PKC, protein kinase C; CFTR, CFTR Cl− channel; AE2, Cl−/HCO−3 exchanger; AQP1, aquaporin 1; NHE1, Na+/H+ exchanger, isoform 1; NHE3, Na+/H+ exchanger, isoform 3. Adapted from Masyuk et al. 2006 (183).
Regulation of basal cholangiocyte secretion
Secretin
Secretin, a 27-amino acid neuropeptide, has a paramount role in hormonal regulation of ductal bile modification. Secretin receptors are exclusively expressed on the basolateral membrane of cholangiocytes (14, 78, 79), and in rat liver, the secretory response to secretin is greatest in large ducts (7). As mentioned earlier, secretin’s effects are cAMP and PKA-dependent and include secretion of HCO3−-rich fluid into bile.
In cholangiocytes, the secretin signal is inactivated by protein phosphatases 1, 2A, or both (19), which dephosphorylate the regulatory domain of CFTR and promote conformational changes. These changes result in occlusion of the Cl− conductance pathway and restoration of the basal quiescent state. Thus, ductal bile secretion stimulated by secretin is regulated at the level of CFTR by a balance between the activities of kinases (inducing activation) (19) and phosphatases (inducing inactivation) of this channel (16).
An additional mechanism that may contribute to secretin-induced HCO3−-rich choleresis is the Na+/H+ exchanger NHE3, which, as described earlier, is present on the cholangiocyte apical plasma membrane (201). NHE3 stimulates absorption of fluid from the biliary lumen, thus counterbalancing fluid secretion in resting bile duct epithelia (183). PKA, activated by secretin, inhibits NHE3-mediated fluid absorption, and as a result, leads to an increase in net (secretin-induced) fluid secretion (201).
Bombesin
Bombesin, also known as gastrin-releasing peptide, is a 14-amino acid peptide neurotransmitter that directly stimulates cholangiocyte fluid and HCO3− secretion by cAMP, cGMP, intracellular Ca2+, and microtubule-independent mechanisms (57, 59, 60). It is a member of the bombesin ligand family, whose biological activity resides in a common C-terminal sequence. In vivo, bombesin is likely to have both direct and indirect effects on cholangiocyte biliary secretion, the latter by inducing the release of other secretagogues (100, 133, 148, 205). Bombesin-stimulated cholangiocyte secretion is due to increased Cl−/HCO3− exchanger activity, coupled to apical Cl− channels, and is counterbalanced by the electrogenic Na+-HCO3− cotransporter and K+ channels on the basolateral plasma membrane (57, 100).
Vasoactive intestinal peptide
Vasoactive intestinal peptide (VIP), a 28-amino acid neuropeptide thought to be the principal noncholinergic, nonadrenergic neurotransmitter in the gastrointestinal tract, stimulates cholangiocyte HCO3−-rich secretion (58,213,223,268). Human cholangiocytes express one of two known VIP receptors, vasoactive intestinal peptide receptor-1 (VPAC1), which is linked to the cAMP signaling pathway (56, 95). In rats, VIP is a more potent stimulus of biliary secretion than either secretin or bombesin (58). The specific VIP receptors in rodent cholangiocytes have not yet been identified.
Corticosteroids
Activation of glucocorticoid receptors expressed in rat cholangiocytes by dexamethasone or budesonide increases biliary HCO3− secretion in vivo (17). In IBDUs isolated from rats treated with dexamethasone or budesonide, cholangiocyte HCO3− secretion was increased because of upregulation of two exchangers critical for ductal bile modification, namely, the apical Cl−/HCO3− exchanger and basolateral Na+/H+-exchanger (NHE1 isoform) (17).
Potentiation of secretin-stimulated cholangiocyte secretion
ACh and adrenergic agonists do not influence basal cholangiocyte secretion of HCO3− de novo, but they significantly potentiate cholangiocyte HCO3− secretion that has been stimulated by secretin (164, 178). These are described below.
Acetylcholine
The ACh-induced potentiation of secretin-stimulated HCO3− secretion results from activation of muscarinic M1 and M3 receptors that are expressed on the cholangiocyte basolateral plasma membrane followed by an increase of the intracellular Ca2+ concentration (15, 74, 75, 210). In cholangiocytes, ACh does not act via a Ca2+-activated PKC, but presumably by activation of Ca2+-sensitive AC isoforms, whereby it effectuates a twofold increase in cAMP (15). Similarly, in isolated bivascularly perfused rat liver, arterial infusion of ACh accentuates secretin-stimulated HCO3− secretion (118). A decrease of secretin-stimulated bile flow and HCO3− secretion, was observed in cholangiocytes isolated from the liver of bile duct-ligated rats with total vagotomy; this further supports the role of parasympathetic innervation and its neurotransmitter, ACh, in the potentiation of secretin-stimulated HCO3− secretion (165, 178, 180, 181).
Adrenergic agonists
Rat cholangiocytes and Mz-ChA-1 cells express α1-, α2-, β1-, and β2-, and α2A-, α2B-, and α2C-adrenoreceptors, respectively (34, 137, 164). Activation of α1-adrenergic receptors by phenylephrine in bile duct ligated (BDL) rats in vivo resulted in potentiation of secretin-stimulated bile flow and HCO3− secretion. Similarly, in in vitro models, phenylephrine alone did not alter basal cAMP levels in cholangiocytes or expansion of the lumen of enclosed IBDUs (a reflection of cholangiocyte secretion); however, it significantly potentiated secretin-stimulated cAMP levels and IBDU expansion. Phenylephrine’s effects were abolished by benoxanthian hydrochloride, an α1-adrenergic antagonist, again suggesting the potentiating regulatory effects of adrenergic agonists in ductal bile modification. Adrenergic regulation of cholangiocyte function in BDL rats occurs through a Ca2+-dependent, PKC-mediated amplification of the cAMP signaling pathway (164).
Inhibition of basal and secretin-stimulated cholangiocyte secretion
Secretin-stimulated cholangiocyte secretion may be terminated or inhibited by several regulatory factors including somatostatin, gastrin, dopaminergic agonists, alpha2-adrenergic receptor agonists, endothelin (ET), and gamma-aminobutyric acid (GABA) (91, 183).
Somatostatin
Somatostatin is an inhibitory cyclic tetradecapeptide produced by a variety of tissues, including but not limited to neurons in the central and peripheral nervous system and neuroendocrine cells of the gastrointestinal tract (151, 215). In the human, dog, rat, and mouse, somatostatin inhibits both basal and secretin-stimulated ductal fluid secretion (111, 151, 169, 175, 222, 224, 270). Somatostatin exerts a wide array of physiological effects mediated by five somatostatin receptor subtypes (sst1-sst5) (151, 215). Four of them, sst1-sst4, are expressed in rat and mouse cholangiocytes (9, 111, 270). Therein, somatostatin inhibits expression of secretin receptors, secretin-stimulated bile flow, HCO3− secretion, and insertion of exocytic vesicles into the apical plasma membrane through interaction with sst2 receptors, followed by inhibition of the cAMP-signaling pathways (9, 194, 270). In addition, enhanced ductal fluid reabsorption was suggested as one of the major mechanism of somatostatin-induced anticholeresis in mice (111) and dogs (222). Indeed, in IBDUs from normal mice, somatostatin and L-779976, a somatostatin analog specific for sst2, not only inhibited secretin-stimulated ductal fluid secretion, but also directly induced ductal fluid absorption; in sst2-knockout mice, these effects were diminished (111). Thus, somatostatin may regulate ductal bile formation by two mechanisms: (i) inhibiting secretin-stimulated cholangiocyte HCO3− and fluid secretion and (ii) directly stimulating cholangiocyte fluid absorption (111, 222).
Gastrin
Gastrin, a linear peptide produced and secreted by G cells in the duodenum and gastric antrum in response to a variety of stimuli (48), inhibits secretin-stimulated cholangiocyte secretion among other local effects (6,102,103). The inhibitory effect of gastrin on secretin-stimulated cholangiocyte secretion is mediated via activation of the cholecystokinin-B/gastrin receptors, followed by membrane translocation and activation of the Ca2+-dependent PKCα and abolishment of secretin-stimulated cAMP synthesis (103, 109). Gastrin also induces membrane translocation of PKCα in Mz-ChA-1 cells (134). In addition, gastrin is associated with increased expression of PKCα, PKCβ1, and PKCβ2 in BDL rat cholangiocytes (102).
Dopaminergic agonists
Rat cholangiocytes express one of three known dopaminergic receptors, D2, on their basolateral plasma membrane. Through this, quinelorane, a D2 receptor agonist, can induce an increase in intracellular IP3 and Ca2+ levels, resulting in inhibition of secretin-stimulated cholangiocyte secretion. The effect of quinelorane occurs through activation of PKCγ and inhibition of secretin-stimulated increased cAMP levels and PKA activity (101).
Alpha2-adrenergic receptor agonists
In a recent study, it was shown that secretin-stimulated ductal secretion in a BDL animal model could be inhibited by the alpha2-adrenergic receptor agonist UK 14,304 by downregulation of the cAMP system (91). Similarly, degeneration of adrenergic innervation with 6-hydroxidopamine in BDL rats was found to result in a decrease of cholangiocyte cAMP levels and secretin-stimulated choleresis (34). These findings add to a growing body of literature regarding the effects of hepatic nerves and nerve receptor agonists on basal and secretin-stimulated ductal bile secretion, which collectively have demonstrated the presence and importance of neuroregulation of bile secretion and modification (15, 18, 163).
Endothelin
Cholangiocytes express both ET receptors, ETA and ETB. ET-1, a 21-amino-acid polypeptide with multifunctional properties (139), inhibits secretin-stimulated cholangiocyte secretion by interacting with ETA receptors through IP3- and Ca2+-dependent inhibition of AC activity (49).
Gamma-aminobutyric acid
Both small and large rat cholangiocytes express both classes of GABA receptors, GABAA and GABAB. Their activation by GABA, a principle inhibitory neurotransmitter in the nervous system of vertebrates, results in inhibition of secretin-induced increases in cAMP levels and Cl− efflux (171).
Bile-associated regulatory factors
The chemical composition, osmolarity, and flow rates of bile percolating through the biliary tree vary over time and space. These properties significantly affect cholangiocyte secretory and absorptive functions via a number of channels and receptors expressed on the cholangiocyte apical plasma membrane and primary cilia.
ATP and other nucleotides
ATP is recognized as an autocrine/paracrine signaling molecule, acting through activation of specific P2X (reviewed above) and P2Y receptors (71, 82, 83, 87, 200, 227, 231, 236, 237, 287). The P2Y family of purinergic receptors consists of eight members in mammals—P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 (1). Rodent cholangiocytes express six (i.e., P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, and P2Y13) of eight mammalian P2Y receptors (71, 185, 231, 236, 286). Recently, a P2Y receptor functionally similar to cloned human P2Y11 has also been identified in rat cholangiocytes (286).
From a phylogenetic and structural (i.e., amino acid sequence) point of view, and based on selectivity of primary G protein coupling (i.e., signal transduction mechanisms), P2Y receptors can be divided into two subgroups: the first includes P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, which are coupled to Gq protein and associated with activation of phospholipase C and Ca2+ release from IP3-sensitive intracellular stores (1, 46). P2Y11 is also coupled to Gs protein and associated with activation of ACs and an increase in intracellular cAMP (1, 278). The second subgroup of P2Y receptors consists of P2Y12, P2Y13, and P2Y14, which are coupled to Gi protein and associated with inhibition of ACs and decreased cAMP levels (1, 47, 160, 239, 278).
P2X and P2Y receptors are expressed on both the apical and basolateral cholangiocyte plasma membrane (70, 71, 80, 226). P2Y receptors on the apical membrane are equally activated by ATP and other nucleotides (e.g., ADP, UTP, and UDP) (71, 231, 236). In contrast, the basolateral membrane P2Y receptors are preferentially activated by ADP ≥ ATP ≥ UTP (231, 236). Activation of apically located P2Y receptors in rat cholangiocytes causes a rapid and substantial increase in membrane Cl− permeability, favoring efflux of Cl− from the cell into the ductal lumen, which presumably occurs through Ca2+-activated Cl− channels (82, 231, 236). In rat IBDUs, both basolateral and apical membrane P2Y receptor activation results in an increase in intracellular Ca2+ concentration and biliary HCO3− secretion, thus suggesting the importance of this mechanism in ductal bile modification (71, 210). Activation of basolateral P2Y receptors by ATP and other nucleotides is less effective, however, in increasing intracellular Ca2+ concentration compared to apical P2Y receptor activation (71).
One of the P2Y receptors, P2Y12, is also localized to primary cilia in rat cholangiocytes (185). This receptor appears to be critically important in nucleotide-induced cAMP signaling in cholangiocytes. It has been shown that deciliated cholangiocytes (i.e., cholangiocytes that have lost their cilia due to treatment with the deciliating agent, chloral hydrate) still respond to ATP by an increase in intracellular Ca2+ (concentration (123), but ATP-induced changes in cAMP levels were abolished by deciliation. Therefore, biliary ATP and other nucleotides appear to induce an increase in intracellular Ca2+ concentration in cholangiocytes via apically located P2Y receptors through cilia-independent mechanisms (71, 73), whereas their effect on cholangiocyte cAMP signaling is seen only if primary cilia are structurally and functionally intact (185).
The P2Y12 receptor is coupled to the Gi protein and thus is involved in inhibition of the cAMP-signaling pathway. In rat cholangiocytes, activation of the P2Y12 receptor does not affect basal cAMP, but it inhibits cAMP increase after stimulation with forskolin (an activator of AC) via cilia-associated mechanisms involving AC6 (185). This suggests that the cilia-associated P2Y12 receptor is important for downregulation of the cAMP signaling pathway once activated by other signaling molecules (185).
P2Y12 is coexpressed with the ciliary A-kinase anchoring protein (AKAP) signaling complex. The components of this complex include AKAP-150, ACs, PKA, and EPAC. The colocalization of P2Y12, ACs, PKA, and EPAC in cholangiocyte cilia suggests the interconnectivity between EPAC- and PKA-mediated signaling in these organelles (185).
Bile acids
As mentioned earlier, unconjugated bile acids absorbed by cholangiocytes indirectly stimulate a HCO3−-rich choleresis when chole hepatically shunted. They also increase intra-cellular Ca2+ concentration and Cl− efflux through Cl− channels expressed on the cholangiocyte apical plasma membrane, thus stimulating HCO3− secretion via Ca2+-activated Cl− channels either directly (245) or more likely through CFTR-dependent ATP secretion (84).
In a BDL rat model, wherein the number of cholangiocytes dramatically increases as a result of ductular proliferation, taurocholic and taurolithocholic acid interact with cholangiocytes, and an associated increase in secretin receptor gene expression, cAMP levels, and Cl−/HCO3− exchanger activity is seen; these suggest the role of bile acids in stimulating cholangiocyte ductal secretion (3, 6, 12, 24, 181). Tauroursodeoxycholic acid (TUDCA) and taurohyodeoxycholic (THDCA) acid also have choleretic effects; treatment with TUDCA was associated with increased HCO3− secretion, and THDCA treatment led to an increase in biliary secretion of phospholipids (23). TUDCA effects on cholangiocyte secretion occur through activation of the Ca2+-dependent PKC-α (3). In addition, in a bile acid-fed rat model, different conjugated bile acids upregulated or downregulated the expression of the bile acids transporters ASBT and t-ASBT, further suggesting their functional importance in regulation of ductal bile modification (3, 5, 145).
Bile flow
Bile flowing through the ductal lumen exerts mechanical forces on the cholangiocyte apical plasma membrane is. The cholangiocyte senses bile flow via the primary cilium on its apical plasma membrane as well as by yet unknown mechanisms associated with ATP release in response to mechanical forces applied to the apical plasma membrane (123,185,282).
Cholangiocyte primary cilia express PC-1 and PC-2 that act as a mechanoreceptor and a Ca2+ channel, respectively. The flow rate in the lumen of rat intrahepatic bile ducts causes a cilia-mediated increase in intracholangiocyte Ca2+ concentration and inhibition of cAMP signaling, suggesting that luminal mechanical stimuli affect both the intracellular Ca2+ and cAMP signaling pathways (190). Bile flow also induces a cilia-mediated increase in intracellular pH, a reflection of Cl− and HCO3− transport, thus suggesting that mechanical stimuli transmitted to cholangiocytes via cilia activate the intracellular Ca2+ second messenger pathway, followed by Ca2+-dependent regulation of Cl− transport (190).
Recent findings also suggest that the flow-induced increase in intracellular Ca2+ concentration in various epithelia is caused or augmented by a flow- or stretch-induced ATP release that acts as a paracrine signal activating epithelial P2 receptors (73, 129, 219). The involvement of cilia in this process is essential (122).
Bile osmolality
Cholangiocytes express a permeable Ca2+ ion channel on their apical plasma membrane and cilia, TRPV4, that is exquisitely sensitive to minor changes in osmolality of the extracellular milieu and involved in functional responses of cholangiocytes to changes in bile osmolality (112). TRPV4 is activated by extracellular hypotonicity and inhibited by extracellular hypertonicity. Its activation leads to an increase in intracellular Ca2+ via influx of Ca2+ into the cholangiocyte (77). Exposure of cholangiocytes to hypotonicity induces an increase in intracellular Ca2+, cilia-dependent ATP release and HCO3− secretion, suggesting an important role of cilia-associated TRPV4 in ductal bile modification (112). Indeed, a retrograde infusion of 4αPDD, an agonist to TRPV4, into rat intrahepatic bile ducts in vivo induced ductal bile secretion; this effect was abolished by Gd3+, a TRP channel inhibitor (112).
Cholangiocyte primary cilia
Primary cilia act as cellular antennae that can detect and transmit signals that influence cholangiocyte function (155). The sensory, including mechano-, osmo-, and chemosensation, and signaling functions of cholangiocyte cilia are associated with specific receptors and channels (Fig. 11). For instance, a mechanoreceptor, PC-1, and a Ca2+ channel, PC-2 appear to be key components for mechanosensory function of cholangiocyte cilia (190). A Ca2+-permeable ion channel, TRPV4, is involved in the mechanism of ciliary osmosensory function (112). A G-protein-coupled nucleotide receptor, P2Y12, and the bile acid receptor, TGR5, are among the key elements of ciliary chemosensation (143,185). Importantly, chemosignals are delivered to cholangiocyte cilia not only as single small or large signaling molecules but also by sophisticated vesicles such as exosomes (186).
Figure 11.
Ca2+ signaling in cholangiocytes. At the cholangiocyte apical plasma membrane, Ca2+ signaling is initiated by activation of the G-protein-coupled receptors (e.g., P2Y), resulting in production of inositol 1,4,5-trisphosphate (IP3). IP3, via the IP3 receptor (IP3R)-isoform 3, induces Ca2+ release from the endoplasmic reticulum (ER), increasing the intracellular Ca2+ concentration. Intracellular Ca2+ concentration is also increased as a result of entry of extracellular Ca2+ via apically and ciliary located PC-2, P2X, and TRPV4 that function as Ca2+ channels. Activation of basolaterally located muscarinic receptors (M3R) by acetylcholine (ACh) also results in production of IP3 that in turn releases Ca2+ from ER via the IP3R isoforms 1 and 2. TJ, tight junction. [Adapted from Masyuk et al. (183), with permission].
Regulation of cholangiocyte secretion and absorption by bile-associated factors are in many cases cilia dependent. These notions have been summarized in a working model as shown in Figure 12. According to the model, mechanical and/or osmotic stimuli (i.e., bile flow and bile tonicity) affect intracellular Ca2+ and cAMP signaling pathways through cilia-associated proteins PC-1, PC-2, and TRPV4 (73, 112, 190), simultaneously inducing a release of ATP into the ductal lumen. ATP and ADP, in turn, induce an increase in intracellular Ca2+ via apically located P2Y1, P2Y2, and P2Y4 purinergic receptors and inhibit cAMP signaling. The mechanisms providing mechano-, osmo-, and chemosensory functions of cholangiocyte cilia overlap, and, in fact, one stimulus (e.g., bile flow or bile osmolarity) may generate another stimulus (e.g., ATP release), thereby affecting ductal bile modification in a coordinated manner.
Figure 12.
Cholangiocyte cilia in bile modification. A working model shows the potential involvement of mechano-, osmo-, and chemosensory functions of cholangiocyte cilia in intracellular signaling associated with ductal bile modification. In response to mechanical stimuli (e.g., bile flow), cilia-associated PC-1 and PC-2 form a functional complex allowing entry of extracellular Ca2+, which in turn suppresses cyclic adenosine 3′,5′-monophosphate (cAMP) concentration in cholangio-cytes via cilia-associated AC6. In response to osmostimuli (i.e., changes in bile tonicity), cilia-associated TRPV4 is activated when bile tonicity decreases or inhibited when bile tonicity increases, causing changes in intracellular Ca2+ concentration, which in turn affect cholangiocyte ATP release via unknown mechanisms. As an example of chemostimuli, biliary ATP, released by hepatocytes and/or cholangiocytes, as well as ATP degradation product ADP, inhibit ciliary cAMP signaling via cilia-associated P2Y12, whereas bile acids induce an increase in cAMP levels via cilia-associated bile acid receptor, TGR5. Intracellular cAMP and Ca2+ signaling induced by extracellular stimuli via cilia-associated mechanisms may affect apically and basolaterally located transport mechanisms depicted in Figures 7 and 9 resulting in increased or decreased cholangiocyte secretion or absorption. [Adapted from Masyuk et al. (183), with permission].
The physiological significance of cholangiocyte cilia in ductal bile modification is not fully understood. Bile flow is thought to normally be pulsatile and, thus, the momentary variations in bile flow may alter the mechanical forces acting on cholangiocyte cilia; this may, as a result, affect cholangiocyte secretory and absorptive functions. Changes in bile osmolality may also have regulatory significance; bile is considered isotonic, but given that cholangiocytes participate in both secretion and absorption, it is plausible that their secretory and absorptive functions can generate transient changes in bile tonicity that could inhibit or activate ciliary osmosensory proteins, that is, TRPV4. The activation or inhibition of ciliary TRPV4, in turn, could inhibit or induce ion and water transport by cholangiocytes, restoring the isotonicity of bile. Further, changes in bile flow and tonicity may induce ATP release, which in turn would activate ciliary P2Y12 and coordinate ductal bile modification through intracellular Ca2+ and cAMP signaling pathways (Fig. 11) (112, 123).
Conclusion
This review has summarized the current knowledge of the physiology of cholangiocytes with an emphasis on bile modification. Salient mechanistic discoveries at the molecular and cellular level continue to be made and have included: (i) identification of a wide variety of transporters and receptors expressed on the cholangiocyte apical and basolateral plasma membranes, (ii) demonstration of the central role of ATP in regulation of ductal bile modification, (iii) clarification of the functional role of different ACs and downstream effectors of cAMP in cholangiocyte physiology, and (iv) perception of the important role of cholangiocyte primary cilia in mechano-, osmo-, and chemosensation. The continued application of novel techniques of molecular and cell biology to cholangiocyte physiology will enable better understanding of the diversity and complexity of cholangiocyte transport systems, their coordinated function, and their regulation.
Acknowledgments
The authors would like to thank Dr. Emanuel Strehler and Dr. Sergio Gradilone for their scientific input and personal communications regarding cholangiocyte Ca2+ extrusion and entry systems.
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153–161. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041–1052. doi: 10.1053/jhep.2002.32712. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Franchitto A, Demorrow S, Onori P, Gaudio E, Wise C, Francis H, Venter J, Kopriva S, Mancinelli R, Carpino G, Stagnitti F, Ueno Y, Han Y, Meng F, Glaser S. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL, Mauldin J, Lesage G. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226–1237. doi: 10.1053/gast.2002.36055. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers RE, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol. 1997;273:G518–G529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- 7.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 8.Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734–1740. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- 9.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 10.Alpini G, Lenzi R, Zhai WR, Slott PA, Liu MH, Sarkozi L, Tavoloni N. Bile secretory function of intrahepatic biliary epithelium in the rat. Am J Physiol. 1989;257:G124–G133. doi: 10.1152/ajpgi.1989.257.1.G124. [DOI] [PubMed] [Google Scholar]
- 11.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 12.Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 13.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage GD, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol. 1997;272:G289–297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 14.Alpini G, Ulrich CD, II, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 15.Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser SS, Benedetti A. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349–1362. doi: 10.1172/JCI119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvaro D, Benedetti A, Marucci L, Delle Monache M, Monterubbianesi R, Di Cosimo E, Perego L, Macarri G, Glaser S, Le Sage G, Alpini G. The function of alkaline phosphatase in the liver: Regulation of intrahepatic biliary epithelium secretory activities in the rat. Hepatology. 2000;32:174–184. doi: 10.1053/jhep.2000.9078. [DOI] [PubMed] [Google Scholar]
- 17.Alvaro D, Gigliozzi A, Marucci L, Alpini G, Barbaro B, Monterubbianesi R, Minetola L, Mancino MG, Medina JF, Attili AF, Benedetti A. Corticosteroids modulate the secretory processes of the rat intrahepatic biliary epithelium. Gastroenterology. 2002;122:1058–1069. doi: 10.1053/gast.2002.32374. [DOI] [PubMed] [Google Scholar]
- 18.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: A neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Alvaro D, Mennone A, Boyer JL. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3- exchange in cholangiocytes. Am J Physiol. 1997;273:G303–G313. doi: 10.1152/ajpgi.1997.273.2.G303. [DOI] [PubMed] [Google Scholar]
- 20.Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun. 2004;322:1364–1373. doi: 10.1016/j.bbrc.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 21.Aranda V, Martinez I, Melero S, Lecanda J, Banales JM, Prieto J, Medina JF. Shared apical sorting of anion exchanger isoforms AE2a, AE2b1, and AE2b2 in primary hepatocytes. Biochem Biophys Res Commun. 2004;319:1040–1046. doi: 10.1016/j.bbrc.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 22.Arrese M, Trauner M. Molecular aspects of bile formation and cholestasis. Trends Mol Med. 2003;9:558–564. doi: 10.1016/j.molmed.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Baiocchi L, Alpini G, Glaser S, Angelico M, Alvaro D, Francis H, Marzioni M, Phinizy JL, Barbaro B, LeSage G. Taurohyodeoxycholate-and tauroursodeoxycholate-induced hypercholeresis is augmented in bile duct ligated rats. J Hepatol. 2003;38:136–147. doi: 10.1016/s0168-8278(02)00358-6. [DOI] [PubMed] [Google Scholar]
- 24.Baiocchi L, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bile secretion. J Hepatol. 1999;31:179–191. doi: 10.1016/s0168-8278(99)80180-9. [DOI] [PubMed] [Google Scholar]
- 25.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 26.Ballatori N, Jacob R, Barrett C, Boyer JL. Biliary catabolism of glutathione and differential reabsorption of its amino acid constituents. Am J Physiol. 1988;254:G1–G7. doi: 10.1152/ajpgi.1988.254.1.G1. [DOI] [PubMed] [Google Scholar]
- 27.Ballatori N, Jacob R, Boyer JL. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986;261:7860–7865. [PubMed] [Google Scholar]
- 28.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: A key membrane transporter of bile acids and conjugated steroids. Front Biosci. 2009;14:2829–2844. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992;263:G617–G624. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- 30.Banales JM, Arenas F, Rodriguez-Ortigosa CM, Saez E, Uriarte I, Doctor RB, Prieto J, Medina JF. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 31.Banales JM, Masyuk TV, Bogert PS, Huang BQ, Gradilone SA, Lee SO, Stroope AJ, Masyuk AI, Medina JF, LaRusso NF. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. Am J Pathol. 2008;173:1637–1646. doi: 10.2353/ajpath.2008.080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banales JM, Prieto J, Medina JF. Cholangiocyte anion exchange and biliary bicarbonate excretion. World J Gastroenterol. 2006;12:3496–3511. doi: 10.3748/wjg.v12.i22.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbaro B, Glaser S, Francis H, Taffetani S, Marzioni M, LeSage G, Alpini G. Nerve regulation of cholangiocyte functions. In: Alpini G, Alvaro D, Marzioni M, LeSage G, LaRusso N, editors. The Pathophysiology of the Biliary Epithelia. Georgetown: Lamdes Bioscience, Eurekah; 2004. pp. 200–210. [Google Scholar]
- 35.Benedetti A, Bassotti C, Rapino K, Marucci L, Jezequel AM. A morphometric study of the epithelium lining the rat intrahepatic biliary tree. J Hepatol. 1996;24:335–342. doi: 10.1016/s0168-8278(96)80014-6. [DOI] [PubMed] [Google Scholar]
- 36.Benedetti A, Di Sario A, Marucci L, Svegliati-Baroni G, Schteingart CD, Ton-Nu HT, Hofmann AF. Carrier-mediated transport of conjugated bile acids across the basolateral membrane of biliary epithelial cells. Am J Physiol. 1997;272:G1416–G1424. doi: 10.1152/ajpgi.1997.272.6.G1416. [DOI] [PubMed] [Google Scholar]
- 37.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- 39.Beuers U, Hohenester S, de Buy Wenniger LJ, Kremer AE, Jansen PL, Elferink RP. The biliary HCO(3) (-) umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 40.Blaustein MP, Lederer WJ. Sodium/calcium exchange: Its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 41.Bode HP, Wang L, Cassio D, Leite MF, St-Pierre MV, Hirata K, Okazaki K, Sears ML, Meda P, Nathanson MH, Dufour JF. Expression and regulation of gap junctions in rat cholangiocytes. Hepatology. 2002;36:631–640. doi: 10.1053/jhep.2002.35274. [DOI] [PubMed] [Google Scholar]
- 42.Boyer JL. Bile formation and cholestasis. In: Schiff ERSM, Maddrey WC, editors. Schiff’s Diseases of the Liver. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 135–165. [Google Scholar]
- 43.Bradbury NA. Intracellular CFTR: Localization and function. Physiol Rev. 1999;79:S175–S191. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- 44.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 45.Brown GK. Glucose transporters: Structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237–246. doi: 10.1023/a:1005632012591. [DOI] [PubMed] [Google Scholar]
- 46.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol (Oxf) 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 48.Calatayud S, Alvarez A, Victor VM. Gastrin: An acid-releasing, proliferative and immunomodulatory peptide? Mini Rev Med Chem. 2010;10:8–19. doi: 10.2174/138955710791112532. [DOI] [PubMed] [Google Scholar]
- 49.Caligiuri A, Glaser S, Rodgers RE, Phinizy JL, Robertson W, Papa E, Pinzani M, Alpini G. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol. 1998;275:G835–G846. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- 50.Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi K, Parolini I, Malavasi F, Peschle C, Sargiacomo M, Testa U. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006;119:4486–4498. doi: 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 51.Carbrey JM, Agre P. Discovery of the aquaporins and development of the field. Handb Exp Pharmacol. 2009:3–28. doi: 10.1007/978-3-540-79885-9_1. [DOI] [PubMed] [Google Scholar]
- 52.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 53.Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol. 1998;275:G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- 54.Chappe V, Hinkson DA, Howell LD, Evagelidis A, Liao J, Chang XB, Riordan JR, Hanrahan JW. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 2004;101:390–395. doi: 10.1073/pnas.0303411101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charlotte F, L’Hermine A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994;144:460–465. [PMC free article] [PubMed] [Google Scholar]
- 56.Chignard N, Mergey M, Barbu V, Finzi L, Tiret E, Paul A, Housset C. VPAC1 expression is regulated by FXR agonists in the human gallbladder epithelium. Hepatology. 2005;42:549–557. doi: 10.1002/hep.20806. [DOI] [PubMed] [Google Scholar]
- 57.Cho WK, Boyer JL. Characterization of ion transport mechanisms involved in bombesin-stimulated biliary secretion in rat cholangiocytes. J Hepatol. 1999;30:1045–1051. doi: 10.1016/s0168-8278(99)80258-x. [DOI] [PubMed] [Google Scholar]
- 58.Cho WK, Boyer JL. Vasoactive intestinal polypeptide is a potent regulator of bile secretion from rat cholangiocytes. Gastroenterology. 1999;117:420–428. doi: 10.1053/gast.1999.0029900420. [DOI] [PubMed] [Google Scholar]
- 59.Cho WK, Mennone A, Boyer JL. Intracellular pH regulation in bombesin-stimulated secretion in isolated bile duct units from rat liver. Am J Physiol. 1998;275:G1028–G1036. doi: 10.1152/ajpgi.1998.275.5.G1028. [DOI] [PubMed] [Google Scholar]
- 60.Cho WK, Mennone A, Rydberg SA, Boyer JL. Bombesin stimulates bicarbonate secretion from rat cholangiocytes: Implications for neural regulation of bile secretion. Gastroenterology. 1997;113:311–321. doi: 10.1016/s0016-5085(97)70109-4. [DOI] [PubMed] [Google Scholar]
- 61.Clawson GA. Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res. 1989;8:104–112. doi: 10.1159/000157141. [DOI] [PubMed] [Google Scholar]
- 62.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 63.Conde-Vancells J, Rodriguez-Suarez E, Embade N, Gil D, Matthiesen R, Valle M, Elortza F, Lu SC, Mato JM, Falcon-Perez JM. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cova E, Gong A, Marinelli RA, LaRusso NF. Water movement across rat bile duct units is transcellular and channel-mediated. Hepatology. 2001;34:456–463. doi: 10.1053/jhep.2001.27092. [DOI] [PubMed] [Google Scholar]
- 65.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson PA, Shneider B, Hofmann A. Bile formation and the enterohepatic circulation. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. San Diego, CA: Elsevier Academic Press; 2006. pp. 1437–1462. [Google Scholar]
- 67.Delgado-Coello B, Trejo R, Mas-Oliva J. Is there a specific role for the plasma membrane Ca2 +-ATPase in the hepatocyte? Mol Cell Biochem. 2006;285:1–15. doi: 10.1007/s11010-005-9060-z. [DOI] [PubMed] [Google Scholar]
- 68.Doctor RB, Dahl R, Fouassier L, Kilic G, Fitz JG. Cholangiocytes exhibit dynamic, actin-dependent apical membrane turnover. Am J Physiol Cell Physiol. 2002;282:C1042–C1052. doi: 10.1152/ajpcell.00367.2001. [DOI] [PubMed] [Google Scholar]
- 69.Doctor RB, Fouassier L. Emerging roles of the actin cytoskeleton in cholangiocyte function and disease. Semin Liver Dis. 2002;22:263–276. doi: 10.1055/s-2002-34504. [DOI] [PubMed] [Google Scholar]
- 70.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: Identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G779–G786. doi: 10.1152/ajpgi.00325.2004. [DOI] [PubMed] [Google Scholar]
- 71.Dranoff JA, Masyuk AI, Kruglov EA, LaRusso NF, Nathanson MH. Polarized expression and function of P2Y ATP receptors in rat bile duct epithelia. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1059–G1067. doi: 10.1152/ajpgi.2001.281.4.G1059. [DOI] [PubMed] [Google Scholar]
- 72.Dutta AK, Khimji AK, Sathe M, Kresge C, Parameswara V, Esser V, Rockey DC, Feranchak AP. Identification and functional characterization of the intermediate-conductance Ca(2+)-activated K(+) channel (IK-1) in biliary epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1009–G1018. doi: 10.1152/ajpgi.00223.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dutta AK, Woo K, Doctor RB, Fitz JG, Feranchak AP. Extracellular nucleotides stimulate Cl- currents in biliary epithelia through receptor-mediated IP3 and Ca2+ release. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1004–G1015. doi: 10.1152/ajpgi.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elsing C, Hubner C, Fitscher BA, Kassner A, Stremmel W. Muscarinic acetylcholine receptor stimulation of biliary epithelial cells and its effect on bile secretion in the isolated perfused liver [corrected] Hepatology. 1997;25:804–813. doi: 10.1002/hep.510250404. [DOI] [PubMed] [Google Scholar]
- 75.Elsing C, Kassner A, Hubner C, Buhli H, Stremmel W. Absorptive and secretory mechanisms in biliary epithelial cells. J Hepatol. 1996;24(Suppl 1):121–127. [PubMed] [Google Scholar]
- 76.Esteller A. Physiology of bile secretion. World J Gastroenterol. 2008;14:5641–5649. doi: 10.3748/wjg.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog Biophys Mol Biol. 2010;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 78.Farouk M, Vigna SR, Haebig JE, Gettys TW, McVey DC, Chari R, Pruthi RS, Meyers WC. Secretin receptors in a new preparation of plasma membranes from intrahepatic biliary epithelium. J Surg Res. 1993;54:1–6. doi: 10.1006/jsre.1993.1001. [DOI] [PubMed] [Google Scholar]
- 79.Farouk M, Vigna SR, McVey DC, Meyers WC. Localization and characterization of secretin binding sites expressed by rat bile duct epithelium. Gastroenterology. 1992;102:963–968. doi: 10.1016/0016-5085(92)90183-y. [DOI] [PubMed] [Google Scholar]
- 80.Feranchak AP. Ion channels in digestive health and disease. J Pediatr Gastroenterol Nutr. 2003;37:230–241. doi: 10.1097/00005176-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: The role of apamin-sensitive SK channels. Gastroenterology. 2004;127:903–913. doi: 10.1053/j.gastro.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 82.Feranchak AP, Fitz JG. Adenosine triphosphate release and purinergic regulation of cholangiocyte transport. Semin Liver Dis. 2002;22:251–262. doi: 10.1055/s-2002-34503. [DOI] [PubMed] [Google Scholar]
- 83.Feranchak AP, Fitz JG. Thinking outside the cell: The role of extracellular adenosine triphosphate in bile formation. Gastroenterology. 2007;133:1726–1728. doi: 10.1053/j.gastro.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 84.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 85.Fitz JG. Cellular mechanism of bile secretion. In: Alpini G, Alvaro D, Marzioni M, LeSage G, LaRusso N, editors. Hepatology. A Textbook of Liver Disease. Philadelphia: WB Saunders; 1996. pp. 362–375. [Google Scholar]
- 86.Fitz JG. Cholangiocyte ion channels: Target for drug development. In: Alpini G, Alvaro D, Marzioni M, LeSage G, LaRusso N, editors. The Pathophysiology of the Biliary Epithelia. Georgetown: Lamdes Bioscience, Eurekah; 2004. pp. 72–80. [Google Scholar]
- 87.Fitz JG. Regulation of cellular ATP release. Trans Am Clin Climatol Assoc. 2007;118:199–208. [PMC free article] [PubMed] [Google Scholar]
- 88.Fitz JG, Basavappa S, McGill J, Melhus O, Cohn JA. Regulation of membrane chloride currents in rat bile duct epithelial cells. J Clin Invest. 1993;91:319–328. doi: 10.1172/JCI116188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, Fitz JG, Doctor RB. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33:166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 90.Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, Glaser S, Mancino MG, Marucci L, Benedetti A, Alpini G. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–C1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 92.Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP(3)/Ca(2+) and cAMP-dependent signaling mechanisms. Lab Invest. 2011;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2 +and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukushima K, Ueno Y. Bioinformatic approach for understanding the heterogeneity of cholangiocytes. World J Gastroenterol. 2006;12:3481–3486. doi: 10.3748/wjg.v12.i22.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardner DF, Kilberg MS, Wolfe MM, McGuigan JE, Misbin RI. Preferential binding of vasoactive intestinal peptide to hepatic nonparenchymal cells. Am J Physiol. 1985;248:G663–G669. doi: 10.1152/ajpgi.1985.248.6.G663. [DOI] [PubMed] [Google Scholar]
- 96.Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, Glaser S, Alvaro D, Onori P. Cholangiocytes and blood supply. World J Gastroenterol. 2006;12:3546–3552. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: A morphological study. Gastroenterology. 1996;111:1118–1124. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 98.Gaudio E, Pannarale L, Carpino F, Marinozzi G. Microcorrosion casting in normal and pathological biliary tree morphology. Scanning Microsc. 1988;2:471–475. [PubMed] [Google Scholar]
- 99.Gigliozzi A, Fraioli F, Sundaram P, Lee J, Mennone A, Alvaro D, Boyer JL. Molecular identification and functional characterization of Mdr1a in rat cholangiocytes. Gastroenterology. 2000;119:1113–1122. doi: 10.1053/gast.2000.18156. [DOI] [PubMed] [Google Scholar]
- 100.Glad H, Svendsen P, Ainsworth MA, Olsen O, Rehfeld JF, Schaffalitzky de Muckadell OB. The effect of gastrin-releasing peptide on porcine pancreaticobiliary bicarbonate secretion is mediated by secretin. Scand J Gastroenterol. 1994;29:195–202. doi: 10.3109/00365529409090463. [DOI] [PubMed] [Google Scholar]
- 101.Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol Gastrointest Liver Physiol. 2003;284:G683–G694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- 102.Glaser S, Alvaro D, Ueno Y, Francis H, Marzioni M, Phinizy JL, Baumann B, Mancino MG, Venter J, LeSage G, Alpini G. Gastrin reverses established cholangiocyte proliferation and enhanced secretin-stimulated ductal secretion of BDL rats by activation of apoptosis through increased expression of Ca2+-dependent PKC isoforms. Liver Int. 2003;23:78–88. doi: 10.1034/j.1600-0676.2003.00814.x. [DOI] [PubMed] [Google Scholar]
- 103.Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 104.Glaser S, Francis H, Demorrow S, Lesage G, Fava G, Marzioni M, Venter J, Alpini G. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol. 2006;12:3523–3536. doi: 10.3748/wjg.v12.i22.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glaser S, Wang M, Ueno Y, Venter J, Wang K, Chen H, Alpini G, Holterman A. Differential transcriptional characteristics of small and large biliary epithelial cells derived from small and large bile ducts. Am J Physiol Gastrointest Liver Physiol. 2010;299:G769–G777. doi: 10.1152/ajpgi.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva SE, Savage JM, Alpini GD. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol. 1997;273:G1061–G1070. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- 110.Gong AY, Masyuk AI, Splinter PL, Huebert RC, Tietz PS, LaRusso NF. Channel-mediated water movement across enclosed or perfused mouse intrahepatic bile duct units. Am J Physiol Cell Physiol. 2002;283:C338–C346. doi: 10.1152/ajpcell.00162.2001. [DOI] [PubMed] [Google Scholar]
- 111.Gong AY, Tietz PS, Muff MA, Splinter PL, Huebert RC, Strowski MZ, Chen XM, LaRusso NF. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol. 2003;284:C1205–C1214. doi: 10.1152/ajpcell.00313.2002. [DOI] [PubMed] [Google Scholar]
- 112.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gradilone SA, Masyuk TV, Huang BQ, Banales JM, Lehmann GL, Radtke BN, Stroope A, Masyuk AI, Splinter PL, LaRusso NF. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology. 2010;139:304–314. e302. doi: 10.1053/j.gastro.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guzelian P, Boyer JL. Glucose reabsorption from bile. Evidence for a biliohepatic circulation. J Clin Invest. 1974;53:526–535. doi: 10.1172/JCI107586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haas M, Forbush B., III The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 116.Hammond CL, Lee TK, Ballatori N. Novel roles for glutathione in gene expression, cell death, and membrane transport of organic solutes. J Hepatol. 2001;34:946–954. doi: 10.1016/s0168-8278(01)00037-x. [DOI] [PubMed] [Google Scholar]
- 117.Hirata K, Dufour JF, Shibao K, Knickelbein R, O’Neill AF, Bode HP, Cassio D, St-Pierre MV, Larusso NF, Leite MF, Nathanson MH. Regulation of Ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirata K, Nathanson MH. Bile duct epithelia regulate biliary bicarbonate excretion in normal rat liver. Gastroenterology. 2001;121:396–406. doi: 10.1053/gast.2001.26280. [DOI] [PubMed] [Google Scholar]
- 119.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3) J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 120.Hofmann AF. The enterohepatic circulation of bile acids in mammals: Form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 121.Hohenester S, de Buy Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Oude Elferink RP, Beuers U. A biliary HCO(3) (-) umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2011;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 122.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 2008;4:155–170. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–G509. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 124.Ishibashi K, Hara S, Kondo S. Aquaporin water channels in mammals. Clin Exp Nephrol. 2009;13:107–117. doi: 10.1007/s10157-008-0118-6. [DOI] [PubMed] [Google Scholar]
- 125.Ishida F, Terada T, Nakanuma Y. Histologic and scanning electron microscopic observations of intrahepatic peribiliary glands in normal human livers. Lab Invest. 1989;60:260–265. [PubMed] [Google Scholar]
- 126.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 127.Ishii M, Vroman B, LaRusso NF. Fluid-phase endocytosis by intrahepatic bile duct epithelial cells isolated from normal rat liver. J Histochem Cytochem. 1990;38:515–524. doi: 10.1177/38.4.2319122. [DOI] [PubMed] [Google Scholar]
- 128.Ishii M, Vroman B, LaRusso NF. Morphologic demonstration of receptor-mediated endocytosis of epidermal growth factor by isolated bile duct epithelial cells. Gastroenterology. 1990;98:1284–1291. doi: 10.1016/0016-5085(90)90346-3. [DOI] [PubMed] [Google Scholar]
- 129.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J. Flow-induced [Ca2+]i increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol. 2007;18:2062–2070. doi: 10.1681/ASN.2006070700. [DOI] [PubMed] [Google Scholar]
- 130.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, Fleming WH. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One. 2008;3:e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- 132.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: A tale of two systems. J Mol Biol. 2006;362:623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaminski DL, Deshpande YG. Effect of somatostatin and bombesin on secretin-stimulated ductular bile flow in dogs. Gastroenterology. 1983;85:1239–1247. [PubMed] [Google Scholar]
- 134.Kanno N, Glaser S, Chowdhury U, Phinizy JL, Baiocchi L, Francis H, LeSage G, Alpini G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 135.Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 136.Kanno N, LeSage G, Glaser S, Alvaro D, Alpini G. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 137.Kanno N, Lesage G, Phinizy JL, Glaser S, Francis H, Alpini G. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329–1340. doi: 10.1053/jhep.2002.33330. [DOI] [PubMed] [Google Scholar]
- 138.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 139.Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68:195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keitel V, Donner M, Winandy S, Kubitz R, Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 141.Keitel V, Haussinger D. TGR5 in the biliary tree. Dig Dis. 2011;29:45–47. doi: 10.1159/000324127. [DOI] [PubMed] [Google Scholar]
- 142.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Gorg B, Selbach O, Haussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 143.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–789. doi: 10.1515/BC.2010.077. [DOI] [PubMed] [Google Scholar]
- 144.Kimura Y, Matsuo M, Takahashi K, Saeki T, Kioka N, Amachi T, Ueda K. ATP hydrolysis-dependent multidrug efflux transporter: MDR1/P-glycoprotein. Curr Drug Metab. 2004;5:1–10. doi: 10.2174/1389200043489090. [DOI] [PubMed] [Google Scholar]
- 145.Kip NS, Lazaridis KN, Masyuk AI, Splinter PL, Huebert RC, LaRusso NF. Differential expression of cholangiocyte and ileal bile acid transporters following bile acid supplementation and depletion. World J Gastroenterol. 2004;10:1440–1446. doi: 10.3748/wjg.v10.i10.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kivela AJ, Kivela J, Saarnio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol. 2005;11:155–163. doi: 10.3748/wjg.v11.i2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Elferink RP, Baas F, Borst P. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kortz WJ, Nashold JR, Delong E, Meyers WC. Effects of bombesin on fasting bile formation. Ann Surg. 1986;203:1–7. doi: 10.1097/00000658-198601000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kressner MS, Stockert RJ, Morell AG, Sternlieb I. Origins of biliary copper. Hepatology. 1984;4:867–870. doi: 10.1002/hep.1840040512. [DOI] [PubMed] [Google Scholar]
- 150.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 151.Kumar U, Grant M. Somatostatin and somatostatin receptors. Results Probl Cell Differ. 50:137–184. doi: 10.1007/400_2009_29. [DOI] [PubMed] [Google Scholar]
- 152.Lakehal F, Wendum D, Barbu V, Becquemont L, Poupon R, Balladur P, Hannoun L, Ballet F, Beaune PH, Housset C. Phase I and phase II drug-metabolizing enzymes are expressed and heterogeneously distributed in the biliary epithelium. Hepatology. 1999;30:1498–1506. doi: 10.1002/hep.510300619. [DOI] [PubMed] [Google Scholar]
- 153.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: Emerging roles in cell and tissue polarity. Trends Cell Biol. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.LaRusso NF. Proteins in bile: How they get there and what they do. Am J Physiol. 1984;247:G199–G205. doi: 10.1152/ajpgi.1984.247.3.G199. [DOI] [PubMed] [Google Scholar]
- 155.Larusso NF, Masyuk TV. The role of cilia in the regulation of bile flow. Dig Dis. 2011;29:6–12. doi: 10.1159/000324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714–2721. doi: 10.1172/JCI119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lazaridis KN, Pham L, Vroman B, de Groen PC, LaRusso NF. Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am J Physiol. 1997;272:G1168–G1174. doi: 10.1152/ajpgi.1997.272.5.G1168. [DOI] [PubMed] [Google Scholar]
- 158.Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, LaRusso NF. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci U S A. 2000;97:11092–11097. doi: 10.1073/pnas.200325297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lecchi S, Spirli C, Cadamuro M, Fiorotto R, Strazzabosco M. Cholangiocyte biology as relevant to cystic liver diseases. In: Murray KF, editor. Clinical Gastroenterology: Fibrocystic Diseases of the Liver. New York: Humana Press; 2010. pp. 23–43. [Google Scholar]
- 160.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 161.Leite MF, Nathanson M. Ca2+ signaling in the liver. In: Arias I, Boyer J, Chisari F, Fausto N, Schachter D, Shafritz D, editors. The Liver: Biology and Pathobiology. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 537–554. [Google Scholar]
- 162.Lemaigre FP. Mechanisms of liver development: Concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 163.LeSag EG, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 164.LeSage GD, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S, Barbaro B, Fava G, Ueno Y, Alpini G. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 165.LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, Phinizy JL, Baiocchi L, Francis H, Lasater J, Ugili L, Alpini G. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–319. doi: 10.1002/hep.510290242. [DOI] [PubMed] [Google Scholar]
- 166.Lira M, Schteingart CD, Steinbach JH, Lambert K, McRoberts JA, Hofmann AF. Sugar absorption by the biliary ductular epithelium of the rat: Evidence for two transport systems. Gastroenterology. 1992;102:563–571. doi: 10.1016/0016-5085(92)90104-7. [DOI] [PubMed] [Google Scholar]
- 167.Ludwig J. New concepts in biliary cirrhosis. Semin Liver Dis. 1987;7:293–301. doi: 10.1055/s-2008-1040584. [DOI] [PubMed] [Google Scholar]
- 168.Ludwig J, Ritman EL, LaRusso NF, Sheedy PF, Zumpe G. Anatomy of the human biliary system studied by quantitative computer-aided three-dimensional imaging techniques. Hepatology. 1998;27:893–899. doi: 10.1002/hep.510270401. [DOI] [PubMed] [Google Scholar]
- 169.Magnusson I. Anticholeretic effects of substance P and somatostatin. Acta Chir Scand Suppl. 1984;521:1–57. [PubMed] [Google Scholar]
- 170.Malumbres R, Lecanda J, Melero S, Ciesielczyk P, Prieto J, Medina JF. HNF1alpha upregulates the human AE2 anion exchanger gene (SLC4A2) from an alternate promoter. Biochem Biophys Res Commun. 2003;311:233–240. doi: 10.1016/j.bbrc.2003.09.200. [DOI] [PubMed] [Google Scholar]
- 171.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, Demorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpini G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marinelli RA, Pham L, Agre P, LaRusso NF. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane. Evidence for a secretin-induced vesicular translocation of aquaporin-1. J Biol Chem. 1997;272:12984–12988. doi: 10.1074/jbc.272.20.12984. [DOI] [PubMed] [Google Scholar]
- 173.Marinelli RA, Pham LD, Tietz PS, LaRusso NF. Expression of aquaporin-4 water channels in rat cholangiocytes. Hepatology. 2000;31:1313–1317. doi: 10.1053/jhep.2000.7986. [DOI] [PubMed] [Google Scholar]
- 174.Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol. 1999;276:G280–G286. doi: 10.1152/ajpgi.1999.276.1.G280. [DOI] [PubMed] [Google Scholar]
- 175.Marteau P, Chretien Y, Calmus Y, Parc R, Poupon R. Pharmacological effect of somatostatin on bile secretion in man. Digestion. 1989;42:16–21. doi: 10.1159/000199820. [DOI] [PubMed] [Google Scholar]
- 176.Marti U, Elsing C, Renner EL, Liechti-Gallati S, Reichen J. Differential expression of Na+,H(+)-antiporter mRNA in biliary epithelial cells and in hepatocytes. J Hepatol. 1996;24:498–502. doi: 10.1016/s0168-8278(96)80172-3. [DOI] [PubMed] [Google Scholar]
- 177.Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immuno-histochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 178.Marzioni M, Fava G, Alvaro D, Alpini G, Benedetti A. Control of cholangiocyte adaptive responses by visceral hormones and neuropeptides. Clin Rev Allergy Immunol. 2009;36:13–22. doi: 10.1007/s12016-008-8090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 180.Marzioni M, LeSage GD, Glaser S, Patel T, Marienfeld C, Ueno Y, Francis H, Alvaro D, Tadlock L, Benedetti A, Marucci L, Baiocchi L, Phinizy JL, Alpini G. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct-ligated rats. Am J Physiol Gastrointest Liver Physiol. 2003;284:G837–G852. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- 181.Marzioni M, Saccomanno S, Candelaresi C, Rychlicki C, Agostinelli L, Trozzi L, De Minicis S, Benedetti A. Clinical implications of novel aspects of biliary pathophysiology. Dig Liver Dis. 2010;42:238–244. doi: 10.1016/j.dld.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 182.Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colom-batto P, Brunetto M, Yen TS, Houghton M, Pileri P, Abrignani S. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 183.Masyuk A, Masyuk T, LaRusso N. Physiology of Cholangiocytes. New York: Academic Pess; 2006. [Google Scholar]
- 184.Masyuk AI, Gong AY, Kip S, Burke MJ, LaRusso NF. Perfused rat intrahepatic bile ducts secrete and absorb water, solute, and ions. Gastroenterology. 2000;119:1672–1680. doi: 10.1053/gast.2000.20248. [DOI] [PubMed] [Google Scholar]
- 185.Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–G734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, Larusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–G999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Masyuk AI, LaRusso NF. Aquaporins in the hepatobiliary system. Hepatology. 2006;43:S75–S81. doi: 10.1002/hep.20996. [DOI] [PubMed] [Google Scholar]
- 188.Masyuk AI, Marinelli RA, LaRusso NF. Water transport by epithelia of the digestive tract. Gastroenterology. 2002;122:545–562. doi: 10.1053/gast.2002.31035. [DOI] [PubMed] [Google Scholar]
- 189.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2 +and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Masyuk AI, Masyuk TV, Tietz PS, Splinter PL, LaRusso NF. Intrahepatic bile ducts transport water in response to absorbed glucose. Am J Physiol Cell Physiol. 2002;283:C785–C791. doi: 10.1152/ajpcell.00118.2002. [DOI] [PubMed] [Google Scholar]
- 192.Masyuk AI, LaRusso NF. Physiology of cholangiocytes. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 5. London, UK: Academic Press; 2012. pp. 1531–1457. [Google Scholar]
- 193.Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 194.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 195.Masyuk TV, Ritman EL, LaRusso NF. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. Am J Pathol. 2001;158:2079–2088. doi: 10.1016/S0002-9440(10)64679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Masyuk TV, Ritman EL, LaRusso NF. Hepatic artery and portal vein remodeling in rat liver: Vascular response to selective cholangiocyte proliferation. Am J Pathol. 2003;162:1175–1182. doi: 10.1016/S0002-9440(10)63913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mathis GA, Walls SA, D’Amico P, Gengo TF, Sirica AE. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989;9:477–485. doi: 10.1002/hep.1840090323. [DOI] [PubMed] [Google Scholar]
- 198.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McGill JM, Basavappa S, Gettys TW, Fitz JG. Secretin activates Cl-channels in bile duct epithelial cells through a cAMP-dependent mechanism. Am J Physiol. 1994;266:G731–G736. doi: 10.1152/ajpgi.1994.266.4.G731. [DOI] [PubMed] [Google Scholar]
- 200.McGill JM, Basavappa S, Mangel AW, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology. 1994;107:236–243. doi: 10.1016/0016-5085(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 201.Mennone A, Biemesderfer D, Negoianu D, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Aronson PS, Boyer JL. Role of sodium/hydrogen exchanger isoform NHE3 in fluid secretion and absorption in mouse and rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2001;280:G247–G254. doi: 10.1152/ajpgi.2001.280.2.G247. [DOI] [PubMed] [Google Scholar]
- 202.Mennone A, Verkman AS, Boyer JL. Unimpaired osmotic water permeability and fluid secretion in bile duct epithelia of AQP1 null mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G739–G746. doi: 10.1152/ajpgi.00540.2001. [DOI] [PubMed] [Google Scholar]
- 203.Minagawa N, Ehrlich BE, Nathanson MH. Calcium signaling in cholangiocytes. World J Gastroenterol. 2006;12:3466–3470. doi: 10.3748/wjg.v12.i22.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, Larusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miyata M, Rayford PL, Thompson JC. Hormonal (gastrin, secretin, cholecystokinin) and secretory effects of bombesin and duodenal acidification in dogs. Surgery. 1980;87:209–215. [PubMed] [Google Scholar]
- 206.Moseley R. Bile secretion. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 380–403. [Google Scholar]
- 207.Munshi MK, Priester S, Gaudio E, Yang F, Alpini G, Mancinelli R, Wise C, Meng F, Franchitto A, Onori P, Glaser SS. Regulation of biliary proliferation by neuroendocrine factors: Implications for the pathogenesis of cholestatic liver diseases. Am J Pathol. 2011;178:472–484. doi: 10.1016/j.ajpath.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nakanuma Y. Tutorial review for understanding of cholangiopathy. Int J Hepatol. 2012;2012:547840. doi: 10.1155/2012/547840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 210.Nathanson MH, Burgstahler AD, Mennone A, Boyer JL. Characterization of cytosolic Ca2 +signaling in rat bile duct epithelia. Am J Physiol. 1996;271:G86–G96. doi: 10.1152/ajpgi.1996.271.1.G86. [DOI] [PubMed] [Google Scholar]
- 211.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 212.Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nyberg B, Einarsson K, Sonnenfeld T. Evidence that vasoactive intestinal peptide induces ductular secretion of bile in humans. Gastroenterology. 1989;96:920–924. [PubMed] [Google Scholar]
- 214.Okada Y, Jinno K, Moriwaki S, Shimoe T, Tsuji T, Murakami M, Thurin J, Koprowski H. Blood group antigens in the intrahepatic biliary tree. I. Distribution in the normal liver. J Hepatol. 1988;6:63–70. doi: 10.1016/s0168-8278(88)80463-x. [DOI] [PubMed] [Google Scholar]
- 215.Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 216.Olson JR, Fujimoto JM. Demonstration of a D-glucose transport system in the biliary tree of the rat by use of the segmented retrograde intrabiliary injection technique. Biochem Pharmacol. 1980;29:213–219. doi: 10.1016/0006-2952(80)90331-7. [DOI] [PubMed] [Google Scholar]
- 217.Orlans E, Peppard JV, Payne AW, Fitzharris BM, Mullock BM, Hinton RH, Hall JG. Comparative aspects of the hepatobiliary transport of IgA. Ann N Y Acad Sci. 1983;409:411–427. doi: 10.1111/j.1749-6632.1983.tb26886.x. [DOI] [PubMed] [Google Scholar]
- 218.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 219.Praetorius HA, Leipziger J. Fluid flow sensing and triggered nucleotide release in epithelia. J Physiol. 2008;586:2669. doi: 10.1113/jphysiol.2008.155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- 221.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2009;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 222.Rene E, Danzinger RG, Hofmann AF, Nakagaki M. Pharmacologic effect of somatostatin on bile formation in the dog. Enhanced ductular reabsorption as the major mechanism of anticholeresis. Gastroenterology. 1983;84:120–129. [PubMed] [Google Scholar]
- 223.Ricci GL, Fevery J. The action of VIP on bile secretion and bile acid output in the non-anaesthetized rat. Biochem Pharmacol. 1985;34:3765–3767. doi: 10.1016/0006-2952(85)90243-6. [DOI] [PubMed] [Google Scholar]
- 224.Ricci GL, Fevery J. Somatostatin inhibits the effect of secretin on bile flow and on hepatic bilirubin transport in the rat. Gut. 1989;30:1266–1269. doi: 10.1136/gut.30.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 226.Roman R, Feranchak AP, Troetsch M, Dunkelberg JC, Kilic G, Schlenker T, Schaack J, Fitz JG. Molecular characterization of volume-sensitive SK(Ca) channels in human liver cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G116–G122. doi: 10.1152/ajpgi.2002.282.1.G116. [DOI] [PubMed] [Google Scholar]
- 227.Roman RM, Fitz JG. Emerging roles of purinergic signaling in gastrointestinal epithelial secretion and hepatobiliary function. Gastroenterology. 1999;116:964–979. doi: 10.1016/s0016-5085(99)70081-8. [DOI] [PubMed] [Google Scholar]
- 228.Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J Pathol. 2003;200:553–560. doi: 10.1002/path.1379. [DOI] [PubMed] [Google Scholar]
- 229.Roscioni SS, Elzinga CR, Schmidt M. Epac: Effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- 230.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: Canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 231.Salter KD, Fitz JG, Roman RM. Domain-specific purinergic signaling in polarized rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2000;278:G492–G500. doi: 10.1152/ajpgi.2000.278.3.G492. [DOI] [PubMed] [Google Scholar]
- 232.Sasaki H, Schaffner F, Popper H. Bile ductules in cholestasis: Morphologic evidence for secretion and absorption in man. Lab Invest. 1967;16:84–95. [PubMed] [Google Scholar]
- 233.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saxena R, Theise N. Canals of Hering: Recent insights and current knowledge. Semin Liver Dis. 2004;24:43–48. doi: 10.1055/s-2004-823100. [DOI] [PubMed] [Google Scholar]
- 235.Schaffner F, Popper H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- 236.Schlenker T, Romac JM, Sharara AI, Roman RM, Kim SJ, LaRusso N, Liddle RA, Fitz JG. Regulation of biliary secretion through apical purinergic receptors in cultured rat cholangiocytes. Am J Physiol. 1997;273:G1108–G1117. doi: 10.1152/ajpgi.1997.273.5.G1108. [DOI] [PubMed] [Google Scholar]
- 237.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci U S A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schorey JS, Bhatnagar S. Exosome function: From tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 240.Scoazec JY, Bringuier AF, Medina JF, Martinez-Anso E, Veissiere D, Feldmann G, Housset C. The plasma membrane polarity of human biliary epithelial cells: In situ immunohistochemical analysis and functional implications. J Hepatol. 1997;26:543–553. doi: 10.1016/s0168-8278(97)80419-9. [DOI] [PubMed] [Google Scholar]
- 241.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 242.Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology. 1998;27:317–331. doi: 10.1002/hep.510270202. [DOI] [PubMed] [Google Scholar]
- 243.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 244.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shimokura GH, McGill JM, Schlenker T, Fitz JG. Ursodeoxycholate increases cytosolic calcium concentration and activates Cl- currents in a biliary cell line. Gastroenterology. 1995;109:965–972. doi: 10.1016/0016-5085(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 246.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 247.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 248.Silva FG, Sherrill K, Spurgeon S, Sudhof TC, Stone DK. High-level expression of the 32.5-kilodalton calelectrin in ductal epithelia as revealed by immunocytochemistry. Differentiation. 1986;33:175–183. doi: 10.1111/j.1432-0436.1986.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 249.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 250.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: Current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 251.Soroka CJ, Lee JM, Azzaroli F, Boyer JL. Cellular localization and upregulation of multidrug resistance-associated protein 3 in hepatocytes and cholangiocytes during obstructive cholestasis in rat liver. Hepatology. 2001;33:783–791. doi: 10.1053/jhep.2001.23501. [DOI] [PubMed] [Google Scholar]
- 252.Spirli C, Granato A, Zsembery K, Anglani F, Okolicsanyi L, LaRusso NF, Crepaldi G, Strazzabosco M. Functional polarity of Na+/H+ and Cl-/HCO3- exchangers in a rat cholangiocyte cell line. Am J Physiol. 1998;275:G1236–G1245. doi: 10.1152/ajpgi.1998.275.6.G1236. [DOI] [PubMed] [Google Scholar]
- 253.Splinter PL, Masyuk AI, LaRusso NF. Specific inhibition of AQP1 water channels in isolated rat intrahepatic bile duct units by small interfering RNAs. J Biol Chem. 2003;278:6268–6274. doi: 10.1074/jbc.M212079200. [DOI] [PubMed] [Google Scholar]
- 254.Splinter PL, Masyuk AI, Marinelli RA, LaRusso NF. AQP4 transfected into mouse cholangiocytes promotes water transport in biliary epithelia. Hepatology. 2004;39:109–116. doi: 10.1002/hep.20033. [DOI] [PubMed] [Google Scholar]
- 255.Sternlieb I. Special article: Functional implications of human portal and bile ductular ultrastructure. Gastroenterology. 1972;63:321–327. [PubMed] [Google Scholar]
- 256.Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec (Hoboken) 2008;291:653–660. doi: 10.1002/ar.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, Alpini G. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Strazzabosco M, Joplin R, Zsembery A, Wallace L, Spirli C, Fabris L, Granato A, Rossanese A, Poci C, Neuberger JM, Okolicsanyi L, Crepaldi G. Na(+)-dependent and -independent Cl-/HCO-3 exchange mediate cellular HCO3- transport in cultured human intrahepatic bile duct cells. Hepatology. 1997;25:976–985. doi: 10.1002/hep.510250431. [DOI] [PubMed] [Google Scholar]
- 259.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 260.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: Multiplicities of signaling. Mol Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 261.Taylor AL, Schwiebert LM, Smith JJ, King C, Jones JR, Sorscher EJ, Schwiebert EM. Epithelial P2X purinergic receptor channel expression and function. J Clin Invest. 1999;104:875–884. doi: 10.1172/JCI7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8:139–149. doi: 10.1016/0168-8278(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 263.Terada T, Kono N, Nakanuma Y. Immunohistochemical and immunoelectron microscopic analyses of alpha-amylase isozymes in human intrahepatic biliary epithelium and hepatocytes. J Histochem Cytochem. 1992;40:1627–1635. doi: 10.1177/40.11.1431051. [DOI] [PubMed] [Google Scholar]
- 264.Terada T, Morita T, Hoso M, Nakanuma Y. Pancreatic enzymes in the epithelium of intrahepatic large bile ducts and in hepatic bile in patients with extrahepatic bile duct obstruction. J Clin Pathol. 1994;47:924–927. doi: 10.1136/jcp.47.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 266.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willing-ham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Thomas P, Zamcheck N. Role of the liver in clearance and excretion of circulating carcinoembryonic antigen (CEA) Dig Dis Sci. 1983;28:216–224. doi: 10.1007/BF01295116. [DOI] [PubMed] [Google Scholar]
- 268.Thulin L, Hellgren M. Choleretic effect of vasoactive intestinal peptide. Acta Chir Scand. 1976;142:235–237. [PubMed] [Google Scholar]
- 269.Tietz P, Levine S, Holman R, Fretham C, LaRusso NF. Characterization of apical and basolateral plasma membrane domains derived from cultured rat cholangiocytes. Anal Biochem. 1997;254:192–199. doi: 10.1006/abio.1997.2431. [DOI] [PubMed] [Google Scholar]
- 270.Tietz PS, Alpini G, Pham LD, Larusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol. 1995;269:G110–G118. doi: 10.1152/ajpgi.1995.269.1.G110. [DOI] [PubMed] [Google Scholar]
- 271.Tietz PS, Holman RT, Miller LJ, LaRusso NF. Isolation and characterization of rat cholangiocyte vesicles enriched in apical or basolateral plasma membrane domains. Biochemistry. 1995;34:15436–15443. doi: 10.1021/bi00047a007. [DOI] [PubMed] [Google Scholar]
- 272.Trauner M, Boyer JL. Bile salt transporters: Molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 273.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, LeSage G, Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 274.Ueno Y, Ishii M, Takahashi S, Igarashi T, Toyota T, LaRusso NF. Different susceptibility of mice to immune-mediated cholangitis induced by immunization with carbonic anhydrase II. Lab Invest. 1998;78:629–637. [PubMed] [Google Scholar]
- 275.Uriarte I, Banales JM, Saez E, Arenas F, Oude Elferink RP, Pri-eto J, Medina JF. Bicarbonate secretion of mouse cholangiocytes involves Na(+)-HCO(3)(-) cotransport in addition to Na(+)-independent Cl(-)/HCO(3)(-) exchange. Hepatology. 2010;51:891–902. doi: 10.1002/hep.23403. [DOI] [PubMed] [Google Scholar]
- 276.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: A common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 277.Vestentoft PS, Jelnes P, Hopkinson BM, Vainer B, Mollgard K, Quistorff B, Bisgaard HC. Three-dimensional reconstructions of intrahepatic bile duct tubulogenesis in human liver. BMC Deve Biol. 2011;11:56. doi: 10.1186/1471-213X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 279.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303–313. [PubMed] [Google Scholar]
- 280.Walz T, Fujiyoshi Y, Engel A. The AQP structure and functional implications. Handb Exp Pharmacol. 2009;(190):31–56. doi: 10.1007/978-3-540-79885-9_2. [DOI] [PubMed] [Google Scholar]
- 281.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl- transport in biliary epithelial cells through a PKCzeta-dependent pathway. J Physiol. 2008;586:2779–2798. doi: 10.1113/jphysiol.2008.153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Woo K, Sathe M, Kresge C, Esser V, Ueno Y, Venter J, Glaser SS, Alpini G, Feranchak AP. Adenosine triphosphate release and purinergic (P2) receptor-mediated secretion in small and large mouse cholangiocytes. Hepatology. 2010;52:1819–1828. doi: 10.1002/hep.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 285.Yamamoto K, Sherman I, Phillips MJ, Fisher MM. Three-dimensional observations of the hepatic arterial terminations in rat, hamster and human liver by scanning electron microscopy of microvascular casts. Hepatology. 1985;5:452–456. doi: 10.1002/hep.1840050318. [DOI] [PubMed] [Google Scholar]
- 286.Yu J, Sheung N, Soliman EM, Spirli C, Dranoff JA. Transcriptional regulation of IL-6 in bile duct epithelia by extracellular ATP. Am J Physiol Gastrointest Liver Physiol. 2009;296:G563–G571. doi: 10.1152/ajpgi.90502.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zsembery A, Spirli C, Granato A, LaRusso NF, Okolicsanyi L, Crepaldi G, Strazzabosco M. Purinergic regulation of acid/base transport in human and rat biliary epithelial cell lines. Hepatology. 1998;28:914–920. doi: 10.1002/hep.510280403. [DOI] [PubMed] [Google Scholar]