Significance
Achieving an AIDS-free generation will require elimination of breast milk transmission of HIV-1, as breastfeeding is a cornerstone of infant survival in developing regions. Antiretroviral prophylaxis considerably reduces postnatal HIV-1 transmission, yet its efficacy is limited by access, adherence, toxicities, and resistance of maternal HIV-1 strains. Alternative, safe strategies of impeding postnatal HIV-1 transmission will be required to eliminate infant HIV-1 infection. In this paper, we identify an innate HIV-neutralizing protein in breast milk, Tenascin-C, which captures and neutralizes HIV-1 virions via binding to the chemokine coreceptor binding site on the HIV-1 Envelope. This protein has the potential to be developed as a prevention strategy for postnatal and other modes of HIV-1 transmission.
Abstract
Achieving an AIDS-free generation will require elimination of postnatal transmission of HIV-1 while maintaining the nutritional and immunologic benefits of breastfeeding for infants in developing regions. Maternal/infant antiretroviral prophylaxis can reduce postnatal HIV-1 transmission, yet toxicities and the development of drug-resistant viral strains may limit the effectiveness of this strategy. Interestingly, in the absence of antiretroviral prophylaxis, greater than 90% of infants exposed to HIV-1 via breastfeeding remain uninfected, despite daily mucosal exposure to the virus for up to 2 y. Moreover, milk of uninfected women inherently neutralizes HIV-1 and prevents virus transmission in animal models, yet the factor(s) responsible for this anti-HIV activity is not well-defined. In this report, we identify a primary HIV-1–neutralizing protein in breast milk, Tenascin-C (TNC). TNC is an extracellular matrix protein important in fetal development and wound healing, yet its antimicrobial properties have not previously been established. Purified TNC captured and neutralized multiclade chronic and transmitted/founder HIV-1 variants, and depletion of TNC abolished the HIV-1–neutralizing activity of milk. TNC bound the HIV-1 Envelope protein at a site that is induced upon engagement of its primary receptor, CD4, and is blocked by V3 loop- (19B and F39F) and chemokine coreceptor binding site-directed (17B) monoclonal antibodies. Our results demonstrate the ability of an innate mucosal host protein found in milk to neutralize HIV-1 via binding to the chemokine coreceptor site, potentially explaining why the majority of HIV-1–exposed breastfed infants are protected against mucosal HIV-1 transmission.
Prevention of HIV-1 transmission via breastfeeding is central to improving infant HIV-free survival in regions of high HIV-1 prevalence. Antiretroviral prophylaxis administered to the infant and/or mother can significantly reduce postnatal HIV-1 transmission (1–3). However, issues of maternal/infant toxicities, adherence, and the development of antiretroviral drug-resistant viruses limit the effectiveness of this prevention strategy (4, 5). Thus, novel strategies to prevent HIV-1 transmission via breastfeeding are vital to eliminating infant HIV-1 infection. Interestingly, despite chronic, daily exposure to the virus for up to 2 y of life, greater than 90% of HIV-1–exposed, breastfed infants will escape infection (6). This low rate of HIV-1 transmission via this mode of transmission suggests that innate or adaptive immune responses in breast milk may protect the majority of infants against virus acquisition. Establishing the mechanism by which the majority of HIV-1–exposed, breastfed infants are naturally protected against HIV-1 acquisition will inform novel strategies to eliminate infant HIV-1 infection.
Breast milk from uninfected individuals is inherently inhibitory of HIV-1 replication (7–9). Moreover, it was recently established that milk of uninfected women abrogates oral HIV-1 transmission in humanized mice (10). Several antiviral glycoproteins contained in milk have been reported to inhibit HIV-1 replication, including lactoferrin (11, 12), mucin-1 (13), and secretory leukocyte protease inhibitor (14, 15). In addition, a recent study reported an association between the prevalence of certain oligosaccharides in milk and the risk of infant HIV-1 acquisition (16), potentially explained by the ability of oligosaccharides to prevent HIV-1 virion interaction with dendritic cells (17, 18). However, a previous report of the HIV-1–inhibitory properties of mucosal fluids noted that the majority of the HIV-neutralizing activity of milk was solely contained in the high molecular mass protein fraction (>500 kDa), which would not be accounted for by previously identified HIV-1–neutralizing factors in breast milk (7). Thus, the primary, high molecular mass HIV-1–neutralizing factor in breast milk remains to be identified, and identification of this factor may inform immunologic strategies to prevent postnatal HIV-1 transmission.
Results
Identification of a High Molecular Mass Innate HIV-1–Neutralizing Protein in Breast Milk.
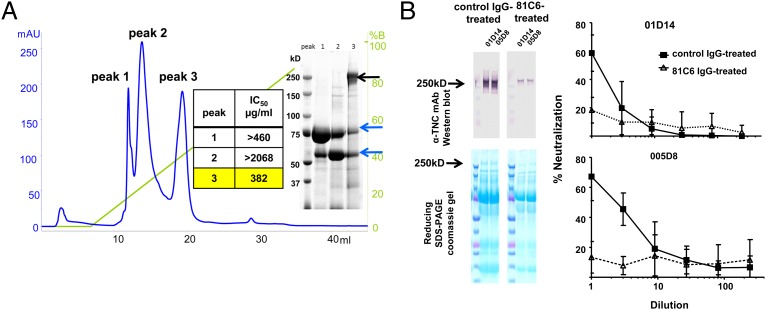
To identify the high molecular mass HIV-1–neutralizing protein in milk, we screened mature milk samples from uninfected women (collected between 2 wk and 7 mo postpartum) for neutralizing activity against a panel of multiclade chronic and transmitted/founder (T/F) pseudoviruses or infectious molecular clones in an HIV-1 neutralization assay in TZM-bl reporter cells (19, 20). The 50% inhibitory dilution (ID50) of milk samples against this panel of HIV-1 strains ranged from 3 to 42 (Fig. S1). We next fractionated a more potently neutralizing milk sample (milk 10; Fig. S1) by size-exclusion chromatography. Consistent with a previous report (7), all of the detectable HIV-1 neutralization activity was contained in the high molecular mass (>500-kDa) fraction [50% inhibitory concentration (IC50), against the chronic, neutralization tier 2 HIV-1 variant C.Du156, 407 µg/mL] (Fig. S2). We further fractionated the active milk fraction by ion-exchange chromatography, narrowing the detectable HIV-1 neutralization activity to a single protein fraction (peak 3, IC50 against C.Du156, 382 μg/mL; Fig. 1A). Reducing SDS/PAGE of this fraction revealed a single unique 250-kDa band that was not visualized in the nonneutralizing fractions (Fig. 1A). Ultra-performance liquid chromatography–tandem mass spectroscopy (LC/MS/MS) of this protein band and comparison of the results with a human protein database revealed 76 unique peptides with >90% likelihood match to the extracellular matrix protein Tenascin-C (TNC). The identity of the protein as TNC was confirmed by Western blot analysis using an anti-TNC mAb (Fig. 1B).
Fig. 1.
TNC is a primary HIV-1–neutralizing protein in breast milk. (A) Anion-exchange chromatography with a linear gradient of 1 M NaCl (green line) of the high molecular mass fraction of milk revealed three distinct protein peaks (blue line). The IC50 of protein peaks against HIV-1 env variant C.Du156 is reported. The unique 250-kDa band on reducing SDS/PAGE in the neutralizing protein fraction (peak 3, black arrow) was identified as TNC by high-resolution LC/MS/MS. Analysis of the smaller protein bands in peak 3 (blue arrows) confirmed matching identity with those contained in inactive fractions (lactoferrin and heavy chain of Ig). (B) Milk of two uninfected women was depleted of TNC by mAb 81C6 and control polyclonal human IgG immunoprecipitation and confirmed by Western blot and SDS/PAGE. Depletion of TNC reduced neutralization activity of the milk samples against HIV-1 C.Du156 Env pseudovirus compared with control IgG-treated milk samples. Line graphs indicate mean percent virus neutralization and SD of two assays performed in duplicate.
HIV-1–Neutralizing Activity of TNC.
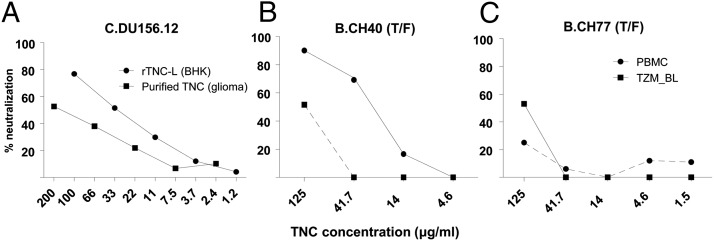
TNC plays a role in fetal brain and mammary gland development, as well as wound healing (21, 22), but no antimicrobial property of this hexameric extracellular matrix protein has previously been described. Depletion of TNC from mature milk samples of two uninfected women using an anti-TNC monoclonal IgG (81C6) (23) resulted in severe reduction of the HIV-1 neutralization activity of the breast milk samples to background levels of the assay (Fig. 1B) (19). Moreover, both TNC purified from a glioma cell line (Millipore) and recombinant TNC (produced in BHK cells) demonstrated dose-dependent HIV-neutralizing activity. Importantly, TNC also neutralized HIV-1 in primary human peripheral blood mononuclear cells (PBMCs) (Fig. 2). To rule out TNC-induced cell toxicity accounting for the neutralizing activity, TNC was incubated with TZM-bl cells in the absence of virus, and luciferase expression was measured after 48 h of incubation. There was no reduction of relative luciferase units (RLUs) at the highest tested concentration of TNC (150 μg/mL) compared with the cell control (mean RLU ± range, 391 ± 10 vs. 362 ± 26, respectively). Moreover, no cell toxicity was observed in the TNC wells by microscopic examination. The HIV-1–neutralizing activity of TNC is directed against the virus and not the target cells, as there was no detectable virus neutralization when TZM-bl target cells were preincubated with TNC before virus inoculation. Purified TNC demonstrated broad-spectrum HIV-1–neutralizing activity against multiclade chronic and T/F HIV-1 Envelope (Env) variants isolated from both adults and postnatally infected infants in the TZM-bl reporter cell assay (IC50 range, 82–158 μg/mL; Fig. S1) in a dose-dependent manner (Fig. 2A). TNC also neutralized the T/F HIV-1 CH40 variant in PBMCs (IC50 of CH40, 27 μg/mL; IC80, 71 μg/mL) more potently than the activity measured in the TZM-bl assay (Fig. 2B). However, the opposite was true with T/F HIV-1 variant CH77 (Fig. 2C), indicating that the neutralizing potency of TNC in PBMCs may be dependent on virus-specific factors. The neutralizing potency of TNC against HIV-1 in TZM-bl reporter cells was higher than that of other breast milk proteins previously shown to neutralize HIV-1, including lactoferrin (11, 24) and mucin-1 (13) (IC50, >300 μg/mL; Fig. S1). TNC neutralized both CCR5 (such as the T/F strains) and CXCR4 (B.MN) coreceptor-tropic HIV-1 variants (Fig. S1). Consistent with the broad activity of innate antimicrobial proteins, TNC also displayed neutralizing activity against the mouse retrovirus murine leukemia virus (MLV; IC50, 109 μg/mL), yet no activity was detected against the simian immunodeficiency virus strain mac251 (>180 μg/mL; Fig. S1). Interestingly, recombinant TNC produced in BHK cells (25), but not CHO or HEK293T cells, recapitulated the HIV-1–neutralizing activity of purified TNC in the TZM-bl neutralization assay, indicating that cell type-specific posttranslational modifications may be important for the neutralizing activity. In fact, TNC produced in various cell types appears to have distinct N-link glycosylation patterns, based on PNGase treatment and Western blot analysis of the resulting deglycosylated forms (Fig. S3). Finally, quantitation of TNC based on relative abundance measured by unbiased mass spectrometry of 10 mature human milk samples revealed a milk TNC concentration range of 2.2–671 µg/mL, spanning the measured IC50 of TNC against HIV-1 variants (Fig. S1).
Fig. 2.
TNC neutralizes HIV-1 in a dose-dependent manner in both TZM-bl reporter cells and primary PBMCs. (A) Percent neutralization of HIV-1 pseudovirus Du156 in TZM-bl reporter cells with increasing concentrations of TNC. (B and C) Percent neutralization of clade B T/F HIV-1 variant CH40 (B) and CH77 (C) Renilla luciferase (LucR) reporter HIV-1 infectious molecular clones in TZM-bl cells (squares) and primary PBMCs (circles) with increasing concentration of TNC. Assays were performed in duplicate.
TNC Captures HIV-1 Virions, Blocks Virus–Epithelial Cell Binding, and Binds to the HIV-1 Env Protein in a Charge-Dependent Manner.
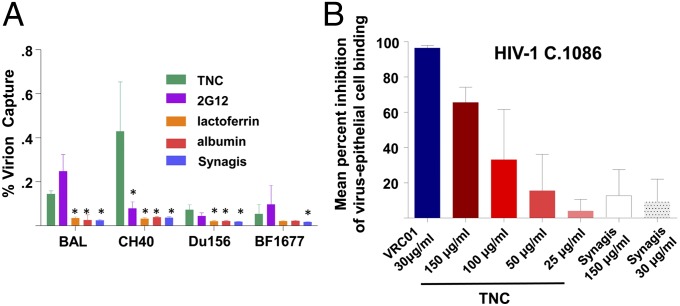
As TNC is a large, multimeric protein, the predicted mechanism of its HIV-1–neutralizing activity is inhibition of virus entry. In fact, purified TNC was able to capture virions with chronic and T/F HIV-1 Env expressed, including those transmitted via breastfeeding, at a significantly higher potency than lactoferrin and albumin, yet at a similar potency to that of a broadly HIV-1–neutralizing monoclonal antibody that is protective against infant HIV-1 acquisition (2G12) (26) (Fig. 3A). As this extracellular matrix protein binds HIV-1 virions and is known to interact with a number of cellular receptors (25), we hypothesized that TNC would be able to block infectious virus from binding to mucosal epithelial cells, representing a potential additional role of TNC in impeding mucosal HIV-1 transmission to infants. Confirming this hypothesis, TNC was able to block up to 66% of infectious virus binding to a monolayer of colonic columnar epithelial cells in a dose-dependent manner (Fig. 3B).
Fig. 3.
TNC captures HIV-1 virions and blocks HIV-1 interaction with colonic epithelial cells. (A) Purified TNC captures HIV-1 virions expressing Env from chronic clade B (B.BAL), chronic clade C (C.Du156), clade B T/F (B.CH40), and clade C infant T/F (C.BF1677) strains with similar potency to anti–HIV-1 Env mAb 2G12 (positive control). Bars indicate the mean percent virus capture, with lines indicating the SD. Asterisks indicate significantly lower (P < 0.05) virion capture compared with TNC, using a two-tailed Mann–Whitney U test (Prism 5; GraphPad Software) to compare the results of two assays performed in triplicate. (B) Purified TNC inhibits infectious T/F C.1086 HIV-1 virus binding to a monolayer of HT-29 colonic epithelial cells in a dose-dependent manner. Results are displayed as mean percent virus inhibition of virion epithelial cell binding compared with virus-only wells of two experiments performed in quadruplicate, with lines indicating the SD.
To define the region of TNC that interacts with the HIV-1 virion, we used surface plasmon resonance (SPR) to detect binding of the HIV-1 Env protein to both the long (TNC-L) and alternatively spliced short (TNC-S) isoforms of TNC (25, 27). The conformation of immobilized TNC was confirmed by intact binding to an anti-TNC mAb (T2H5; Abcam). Both TNC-L and TNC-S, as well as purified TNC, bound to multiclade HIV-1 Env gp120 and gp140 proteins, including chronic HIV-1 variant B.MN gp120 (clade B), ConS gp140 (group M consensus), and the T/F HIV-1 variants Env C.1086 gp120/140 (clade C) and C.CH505 gp140 trimer (28) (Fig. S4). The dissociation constant (Kd) of HIV-1 Env B.MN gp120 binding to TNC-S (Kd 54.8 nM) was equal to that of TNC-L (Kd 58.2 nM) (Fig. S5).
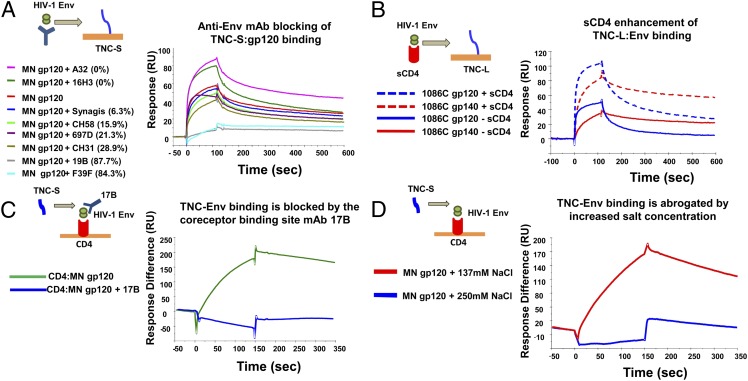
TNC Binds to a CD4-Inducible Epitope on the V3 Loop of the HIV-1 Env Protein in a Region Overlapping the Chemokine Coreceptor Binding Site.
To map the neutralizing epitope of the HIV-1 Env that is bound by TNC, we first compared the kinetics of TNC binding to HIV-1 Env gp120 and gp140 proteins. TNC-S and TNC-L binding to T/F HIV-1 C.1086 and CH0505 gp140 proteins demonstrated a slower off-rate (binding to TNC-S, Kd 1.55 × 10−3 and 1.56 × 10−3 s−1, respectively) than that of their matched gp120 (binding to TNC-S, Kd 9.1 × 10−3 and 4.5 × 10−3s−1, respectively) (Fig. S4). Thus, the gp120 epitope bound by TNC is partially dependent on the conformation of the gp41–gp120 complex. We then incubated HIV-1 B.MN gp120 with a panel of mAbs directed against defined HIV-1 Env epitopes and determined their ability to block TNC–Env interaction (Fig. 4A), including anti-gp120 conformational C1 (A32, 16H3) (29, 30), anti-V3 loop (19b, F39F) (29), anti-V2 linear (CH58) (31), anti-V2 conformational (697D) (32), anti-CD4 binding site (CH31), and a negative-control anti-RSV mAb (Synagis). TNC binding to HIV-1 Env gp120 was potently blocked by anti–HIV-1 Env mAbs 19B and F39F (84.3% and 87.7%, respectively), both directed against the V3 loop of the Env protein (Fig. 4B). Interestingly, TNC–Env binding was enhanced by Env preincubation with mAb A32 (Fig. 4A), an anti-C1 mAb that induces a conformational change that opens the coreceptor binding site (33) similar to that induced by CD4 receptor engagement. We therefore tested the hypothesis that TNC bound to an epitope of the HIV-1 Env protein whose accessibility is enhanced by CD4 receptor binding. In fact, preincubation of some HIV-1 Env gp120 and gp140 proteins with soluble CD4, including B.MN gp120 and the T/F HIV-1 variant C.1086 gp120 and gp140 proteins, enhanced TNC binding (Fig. 4B), and this increased signal was not due to soluble CD4 binding to the TNC chip (Fig. S6). Moreover, the CD4-inducible (CD4i) mAb 17B, which has a binding site that overlaps that of the chemokine coreceptor (34, 35), blocked TNC binding to the CD4-captured gp120 protein (Fig. 4C). Finally, binding of TNC to CD4-bound gp120 was substantially reduced by increasing the NaCl concentration from 137 to 250 mM (Fig. 4D), indicating that TNC–Env binding is dominated by electrostatic interactions. The strong influence of charge on TNC binding to Env are consistent with the reported electrostatic complementarity of the gp120 V3 loop and chemokine receptors (36–38). Thus, TNC neutralizes HIV-1 via interaction with the chemokine coreceptor binding site on the HIV-1 Env.
Fig. 4.
TNC binds to HIV-1 gp120 at a CD4-inducible epitope that overlaps the chemokine coreceptor binding site via a charge–charge interaction. (A) Interaction of TNC and B.MN gp120 is potently blocked by preincubation of the Env protein with anti-V3 loop mAbs 19B and F393F. Percent blocking is in parentheses. (B) Binding to both the C clade T/F HIV-1 C.1086 Env gp120 and gp140 proteins is enhanced by preincubation of Env proteins with soluble CD4. (C) TNC binding to B.MN gp120 captured by soluble CD4 is abolished by prebinding of mAb 17B, an mAb directed against the chemokine coreceptor binding site. Approximately 1,200 response units (RUs) of 17B mAb bound to B.MN gp120 are captured on the immobilized CD4 surface. (D) Binding of CD4-captured TNC to MN gp120 (100 μg/mL) is abrogated in 250 mM NaCl buffer. All data are representative of at least two experiments.
Finally, we investigated the antiviral function of TNC directed against HIV-1 virions in the pre–CD4-bound conformation compared with the post–CD4-bound open conformation. In fact, the ability of TNC to capture HIV-1 B.MN virions was increased ∼1.5-fold when the virions were preincubated with soluble CD4 (Fig. S7A). However, no increased neutralizing activity was observed when HIV-1 virions were incubated with TZM-bl cells for 10 min on ice before addition of TNC, allowing CD4 interaction but preventing cell–virion fusion, compared with the incubation of TNC and virions before the addition of TZM-bl cells (Fig. S7B). Similarly, we did not detect any increased neutralizing activity in the presence of the nonneutralizing, anti-C1 mAb A32, which induces CD4i conformational change (Fig. S7C). Therefore, although TNC has a higher affinity for CD4-bound gp120 than gp120 alone, it is able to mediate its neutralizing activity against HIV-1 virions without prior gp120 engagement of CD4. As low-level weakly or nonneutralizing HIV Env-specific antibodies are present in breast milk (8) of HIV-infected women, we investigated whether TNC antagonizes the effect of an HIV-neutralizing IgG mAb isolated from colostrum and directed against the same CD4i region of gp120 (39). In fact, TNC did not antagonize the neutralizing effect of CH08 against a tier 1 clade-matched HIV-1 strain (MW965) across a range of mAb concentrations (Fig. S7D), despite being directed against the same region of gp120. Therefore, TNC likely acts in concert with HIV-neutralizing antibodies also present in breast milk.
Discussion
Despite substantial mucosal virus exposure of nursing infants born to HIV-infected mothers, only a small minority (<10%) acquire HIV-1 via this route. Defining the immune mechanisms responsible for the protection of the overwhelming majority of HIV-1–exposed, breastfed infants may guide strategies to eliminate postnatal and other modes of mucosal HIV-1 transmission. We have identified an innate breast milk protein that neutralizes chronic and T/F HIV-1 variants at its in vivo concentration, TNC. TNC is an extracellular matrix protein known to be important in fetal development and wound healing, but antimicrobial activity has not previously been described for this protein. The presence of this innate antimicrobial protein in milk may contribute to the relatively low rate of HIV-1 transmission via breastfeeding.
TNC appears to mediate its HIV-1–neutralizing activity via capturing HIV-1 virions and binding to the HIV-1 Env proteins of chronic and T/F HIV-1 variants. As a hexameric protein, the multivalency of TNC may contribute to its ability to capture and neutralize the virus. TNC–HIV-1 Env binding mapped to the V3 loop of the gp120 protein, was enhanced in the presence of soluble CD4, and was blocked by the CD4i anti-coreceptor binding site mAb 17B. Thus, this protein is likely exerting its HIV-1–neutralizing activity via blocking chemokine coreceptor contact on the HIV-1 Env protein. Importantly, TNC is able to bind to gp120 and capture virions in the absence of the CD4 molecule (Fig. 4B and Fig. S7A). In an environment where there is limited presence of CD4-expressing cells, such as the breast milk–infant gut interface, TNC interaction with Env may be sufficiently avid to compete with Env binding to the chemokine receptor. Chemokine coreceptor binding to Env requires CD4 triggering, as studies using reconstituted CCR5 showed no detectable binding to Env gp120 without CD4 triggering (40). Therefore, TNC could exert its effect on HIV-1 in the absence of Env–CD4 engagement by capturing the virion via binding the V3 loop and subsequently preventing coreceptor binding.
The V3 loop of the HIV-1 Env is a highly flexible and positively charged domain, characteristics important to coreceptor binding (41) and tropism (42). Indeed, we found that TNC binding to Env is dominated by electrostatic interactions. In fact, a positively charged, heparin-binding domain has been described within the chemokine coreceptor binding site (43), and this region is likely mediating the TNC–Env binding. The charge-dependent interaction of TNC with the HIV-1 Env V3 loop may also explain the nonspecific activity that we detected against the nonhuman retrovirus MLV. The interaction of the V3 loop with the HIV-1 coreceptor CCR5 has been proposed to involve sulfated tyrosines in the N-terminal extracellular domain of CCR5 (44–47). The electrostatic interaction of TNC with gp120 may be similar to that of previously described V3 loop-derived peptides (44), cell-associated heparan sulfate (48), polyanions including dextran sulfate (49), and tyrosine-sulfated antibodies (41). However, whether TNC interacts with the same conserved sulfotyrosine-binding pocket remains to be determined. Because charge plays a dominant role in TNC binding to Env, its inhibitory effect may be expected to be enhanced against viruses harboring gp120 with increased net charge, as in the case of CXCR4-tropic viruses and CCR5-tropic variants with enhanced net charge (50, 51). However, the stronger binding of B.MN gp120 to TNC by SPR compared with other HIV-1 Env variants did not predict the potency of TNC neutralization of this variant, as the CXCR4-tropic MN variant was less potently neutralized than the other HIV-1 variants tested (Fig. S1). Similarly, binding affinity to HIV-1 Env does not always predict the HIV-1 neutralization potency of anti–HIV-1 Env mAbs (28). Because both net charge and V3 loop flexibility can be important for coreceptor binding (50, 52), the flexibility of the V3 loop on monomeric gp120 in the CD4-bound state may not reflect the conformational states of the trimeric spike of the Env (53).
As both the short and long forms of TNC bound to HIV-1 Env, the Env binding site on TNC is likely outside the splice region within the FN-III domain of TNC. Outside this splice region, there are 8 FN-III domains that demonstrate distinct binding to cellular receptors, 14 epidermal-like growth factor domains, and a terminal fibrinogen knob (22, 25), which has been implicated to play a role in regulating the tissue damage response via signaling through TLR-4 (54). It is interesting that human TNC isolated from two different sources, breast milk and a glioma cell line, and recombinant TNC produced in BHK cells neutralized HIV-1, but not all recombinant forms of the protein were able to neutralize the virus. Thus, the ability of TNC to bind to the HIV-1 Env in a charge-dependent manner may be affected by chemical differences introduced posttranslationally, such as the distinct glycosylation we detected in these various TNC products.
In our studies, TNC mediated the majority of the HIV-1–neutralizing activity in milk of an uninfected individual, consistent with a previous report that isolated the HIV-1–neutralizing activity of breast milk to the high molecular mass fraction (7). However, TNC is likely acting in concert with other anti-HIV factors in breast milk, such as lactoferrin, as its neutralizing potency is consistent across distinct HIV-1 variants, unlike that of whole breast milk (Fig. S1). Given its broad-spectrum HIV-1–binding and –neutralizing activity, this innate, mucosal HIV-1 inhibitor could theoretically be developed as an HIV-1 prophylactic agent that could be orally administered to infants before breastfeeding, similar to oral rehydration salts that are routinely administered to infants in developing regions. As an existing component of breast milk, TNC has a unique safety advantage for clinical development as a mucosal prophylaxis agent. Moreover, use of this protein as an oral infant HIV-1 prophylactic agent would not introduce a new antigen to the infant gastrointestinal tract, which has been hypothesized to explain the increased rate of postnatal HIV-1 transmission in the setting of mixed infant feeding (55). Thus, TNC holds promise for development as a safe, HIV-1–neutralizing host protein that can be used for reducing mucosal HIV-1 transmission.
Materials and Methods
Size-Exclusion and Strong Anion-Exchange Chromatography.
Milk samples from HIV-1–uninfected women between 2 wk and 7 mo after delivery were delipidized by centrifugation at 21,000 × g, filtered, and concentrated 10× before loading on a protein liquid chromatography size-exclusion column (Superdex 200 10/300GL; GE Healthcare). Proteins were further fractionated using a 1 M NaCl elution gradient (Source 15Q 4.6/100 PE; GE Healthcare).
Mass Spectrometry.
Protein bands were processed (www.genome.duke.edu/cores/proteomics/sample-preparation) and analyzed using data-dependent acquisition on a nanoscale capillary LC/MS/MS system (nanoAcquity LC and SYNAPT G2 HDMS; Waters). Data were processed with the Mascot pipeline (Demon, Distiller, and Server 2.2; Matrix Sciences) and searched against a forward/reverse UniProt_human database (56). For quantitation of TNC concentration in milk, an internal standard of yeast alcohol dehydrogenase (Waters) was added as a surrogate standard. Samples were analyzed using ion mobility-assisted data-independent acquisition on a nanoscale capillary LC/MS/MS system. Approximate mole amount was calculated using the Top 3 method (57).
TNC Depletion and HIV-1 Neutralization.
Ten milligrams of anti-TNC IgG1 mAb 81C6 (58) was coupled to 600 mg cyanogen bromide-activated Sepharose beads (GE Healthcare), incubated with delipidized/filtered milk overnight, and then centrifuged to remove the protein-bound beads. For virus neutralization assays, purified protein and milk samples were incubated with 293T cell-produced HIV-1 infectious molecular clones (CH40, CH77, CH58, CM235), or HIV-1 Env pseudoviruses (all other variants) were tested for neutralization potency in TZM-bl target cells (19, 20). Additionally, an HIV-1 infectious molecular clone (NL-LucR.T2A-CH040.ecto) (59) produced in human PBMCs was incubated with purified TNC and tested for neutralization in activated human PBMCs (60, 61). For determining the interaction between TNC and breast milk HIV-neutralizing antibodies, HIV MW965 virions were incubated for 1 h with either the colostrum HIV-neutralizing mAb CH08 (39), TNC (final concentration 175 μg/mL), or CH08 and TNC, and then TZM-bl cells were added. For determining whether Env–CD4 engagement enhanced the neutralizing activity of TNC, TZM-bl cells were incubated with HIV virions (HIV DU156.12) on ice for 10 min, and then TNC was added (final concentration 350 μg/mL). Neutralization was measured after 48 h as a reduction in RLUs compared with the virus-only control. To rule out the possibility of endotoxin-mediated neutralization in the PBMC assay, endotoxin was measured in the purified TNC lots and the amount of endotoxin detected (≤0.3 ng/mL; Limulus Amebocyte Lysate Pyrogent Plus Kit; Lonza) was found to be 10-fold lower than that which mediates 80% HIV-1 neutralization in the PBMC neutralization assay (62).
HIV-1 Virion Capture and Inhibition of Epithelial Cell Binding.
To assess the ability of TNC to capture HIV-1 virions, 0.3 µg of purified breast milk proteins TNC (Millipore), lactorferrin (Sigma), mucin-1 (Abcam), or 2G12 mAb (National Institutes of Health AIDS Reagent Program) were coated on a 96-well plate and blocked with 50g/100 ml BSA (Sigma). HIV-1 Env pseudovirions (10–20 ng of Gag p24) were incubated in the well for 2 h at 37 °C, and unbound virions were removed by washing. The bound virions were quantitated by p24 ELISA (PerkinElmer). Percent virus capture was calculated as follows: (p24 amount of bound virions)/(p24 amount added to the well).
To determine the ability of TNC to impede infectious virus binding to colonic epithelial cells, a modified previously reported protocol was used (63). Colonic HT-29 cells (ATCC) were grown to confluence on a 96-well flat-bottom plate in modified McCoy’s 5a medium supplemented with 10% FBS and antibiotics. The HT-29 cells were washed once with serum-free media and treated with 100 μL of 50 µg/mL mitomycin C for 1 h to prevent further division, followed by two washes. Then, 3.6 × 106 50% tissue culture infectious dose of HIV-1 C.1086 Env-IMC-LucR infectious molecular clone diluted in serum-free media was incubated with increasing concentrations of purified TNC for 1 h at 37 °C, and then added in quadruplicate to the colonic epithelial cell monolayer and plates were incubated at 37 °C for 4 h. Monolayers were washed twice with PBS to remove free virus, and 1 × 104 TZM-bl reporter cells were added to each well of the virus-bound monolayer. After 48 h, luciferase reagent (Bright-Glo; Promega) was added to the well and the RLUs were measured. Percent inhibition was calculated by dividing the RLUs of each well by the median RLUs of epithelial-bound virus that was not preincubated with TNC. The anti-RSV mAb Synagis was used as a negative control, whereas the broadly neutralizing anti–HIV-1 CD4 binding site mAb VRC01 (64) was used as a positive control.
Recombinant TNC Expression.
Recombinant TNC was expressed in BHK cells with the pNUT vector and purified with a combination of ammonium sulfate precipitation, gel filtration, and anion-exchange chromatography (25). TNC was also expressed in CHO cells from the pEE14 expression vector or in HEK cells by transient expression in serum-free media adapted from recombinant fibronectin expression and purified with ammonium sulfate precipitation and gel-filtration chromatography.
HIV-1 Env Binding.
Recombinant and purified TNC proteins (25) were immobilized by amine coupling to SPR sensor chip CM5 (65) and washed overnight in pH 7.4 buffer. HIV-1 Env proteins (66) were injected at 100 μg/mL over immobilized surfaces and binding was measured with a BIAcore 3000 (GE Healthcare). Nonspecific binding was subtracted over a surface immobilized with anti-RSV IgG mAb (Synagis). Binding activity of immobilized TNC was confirmed by injecting anti-TNC mAb (clone T2H5; Abcam). MN gp120 was incubated with saturating concentrations of anti-gp120 mAbs for 1 h and injected over a TNC-immobilized surface. Percent blocking was calculated as follows: (response with gp120 in buffer – response with gp120 bound to mAb)/(response with gp120 in buffer). MAbs included: a negative-control anti-RSV (Synagis), anti-gp120 conformational C1 (A32, 16H3), anti-V3 loop (19b, F39F, 17B), anti-V2 linear (CH58), anti-V2 conformational (697D), and anti-CD4 binding site (CH31). For CD4 induction of HIV-1 Env binding, gp120 or gp140 protein was preincubated with soluble CD4 at a 1:1 molar ratio and binding to TNC was measured as above. The effect of salt on TNC binding to CD4-captured Env gp120 was assessed by increasing the salt concentration in the gp120 sample buffer to 250 mM NaCl and using PBS (pH 7.4) with 250 mM NaCl as a running buffer.
Supplementary Material
Acknowledgments
We are grateful to Dr. Darell Bigner, Charles Pegram, Dr. Christina Ochsenbauer, Dr. John Kappes, Dr. Barton Haynes, Dr. Tony Moody, Dr. Thomas Denny, Linda Walker, Dr. James Robinson, Dr. John Mascola, Dr. Susan Zolla-Pazner, and Dr. David Montefiori for provision of reagents and discussions. Funding for this work included The Doris Duke Charitable Foundation Clinical Scientist Development Award (to S.R.P.), Duke University School of Medicine (S.M.A. and S.R.P.), Center for HIV/AIDS Vaccine Immunology (U19 AI067854), and National Institutes of Health Grants K08AI087992 (to S.R.P.) and CA047056 (to H.P.E.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307336110/-/DCSupplemental.
References
- 1.Chasela CS, et al. BAN Study Group Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362(24):2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro RL, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumwenda NI, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 4.Fogel J, et al. Analysis of nevirapine resistance in HIV-infected infants who received extended nevirapine or nevirapine/zidovudine prophylaxis. AIDS. 2011;25(7):911–917. doi: 10.1097/QAD.0b013e328344fedc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae WH, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22(13):1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutsoudis A, et al. Breastfeeding and HIV International Transmission Study Group Late postnatal transmission of HIV-1 in breast-fed children: An individual patient data meta-analysis. J Infect Dis. 2004;189(12):2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 7.Kazmi SH, et al. Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin Vaccine Immunol. 2006;13(10):1111–1118. doi: 10.1128/CDLI.00426-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouda GG, et al. Center for HIV/AIDS Vaccine Immunology HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J Virol. 2011;85(18):9555–9567. doi: 10.1128/JVI.05174-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newburg DS, Viscidi RP, Ruff A, Yolken RH. A human milk factor inhibits binding of human immunodeficiency virus to the CD4 receptor. Pediatr Res. 1992;31(1):22–28. doi: 10.1203/00006450-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wahl A, et al. Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog. 2012;8(6):e1002732. doi: 10.1371/journal.ppat.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmsen MC, et al. Antiviral effects of plasma and milk proteins: Lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis. 1995;172(2):380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 12.Moriuchi M, Moriuchi H. A milk protein lactoferrin enhances human T cell leukemia virus type I and suppresses HIV-1 infection. J Immunol. 2001;166(6):4231–4236. doi: 10.4049/jimmunol.166.6.4231. [DOI] [PubMed] [Google Scholar]
- 13.Habte HH, et al. Inhibition of human immunodeficiency virus type 1 activity by purified human breast milk mucin (MUC1) in an inhibition assay. Neonatology. 2008;93(3):162–170. doi: 10.1159/000108414. [DOI] [PubMed] [Google Scholar]
- 14.Hocini H, et al. Secretory leukocyte protease inhibitor inhibits infection of monocytes and lymphocytes with human immunodeficiency virus type 1 but does not interfere with transcytosis of cell-associated virus across tight epithelial barriers. Clin Diagn Lab Immunol. 2000;7(3):515–518. doi: 10.1128/cdli.7.3.515-518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeely TB, et al. Secretory leukocyte protease inhibitor: A human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96(1):456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bode L, et al. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. 2012;96(4):831–839. doi: 10.3945/ajcn.112.039503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naarding MA, et al. Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes. J Clin Invest. 2005;115(11):3256–3264. doi: 10.1172/JCI25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) Br J Nutr. 2009;101(4):482–486. doi: 10.1017/s0007114508025804. [DOI] [PubMed] [Google Scholar]
- 19.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei X, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson HP, Bourdon MA. Tenascin: An extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- 22.Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cell Mol Life Sci. 2011;68(19):3175–3199. doi: 10.1007/s00018-011-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy-Ullrich JE, et al. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991;115(4):1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkhout B, et al. Characterization of the anti-HIV effects of native lactoferrin and other milk proteins and protein-derived peptides. Antiviral Res. 2002;55(2):341–355. doi: 10.1016/s0166-3542(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 25.Aukhil I, Joshi P, Yan Y, Erickson HP. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993;268(4):2542–2553. [PubMed] [Google Scholar]
- 26.Hofmann-Lehmann R, et al. Passive immunization against oral AIDS virus transmission: An approach to prevent mother-to-infant HIV-1 transmission? J Med Primatol. 2001;30(4):190–196. doi: 10.1034/j.1600-0684.2001.d01-52.x. [DOI] [PubMed] [Google Scholar]
- 27.Siri A, et al. Human tenascin: Primary structure, pre-mRNA splicing patterns and localization of the epitopes recognized by two monoclonal antibodies. Nucleic Acids Res. 1991;19(3):525–531. doi: 10.1093/nar/19.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao HX, et al. NISC Comparative Sequencing Program Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496(7446):469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70(3):1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao F, et al. Cross-reactive monoclonal antibodies to multiple HIV-1 subtype and SIVcpz envelope glycoproteins. Virology. 2009;394(1):91–98. doi: 10.1016/j.virol.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HX, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38(1):176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorny MK, et al. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68(12):8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao HX, et al. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoproteins. J Virol. 2004;78(10):5270–5278. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattentau QJ, Moore JP. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182(1):185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thali M, et al. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67(7):3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CC, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310(5750):1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, et al. Coreceptor tropism in human immunodeficiency virus type 1 subtype D: High prevalence of CXCR4 tropism and heterogeneous composition of viral populations. J Virol. 2007;81(15):7885–7893. doi: 10.1128/JVI.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López de Victoria A, Kieslich CA, Rizos AK, Krambovitis E, Morikis D. Clustering of HIV-1 subtypes based on gp120 V3 loop electrostatic properties. BMC Biophys. 2012;5:3. doi: 10.1186/2046-1682-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman J, et al. Isolation of HIV-1-neutralizing mucosal monoclonal antibodies from human colostrum. PLoS One. 2012;7(5):e37648. doi: 10.1371/journal.pone.0037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navratilova I, Sodroski J, Myszka DG. Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal Biochem. 2005;339(2):271–281. doi: 10.1016/j.ab.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Huang CC, et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317(5846):1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edo-Matas D, van Dort KA, Setiawan LC, Schuitemaker H, Kootstra NA. Comparison of in vivo and in vitro evolution of CCR5 to CXCR4 coreceptor use of primary human immunodeficiency virus type 1 variants. Virology. 2011;412(2):269–277. doi: 10.1016/j.virol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Crublet E, Andrieu JP, Vivès RR, Lortat-Jacob H. The HIV-1 envelope glycoprotein gp120 features four heparan sulfate binding domains, including the co-receptor binding site. J Biol Chem. 2008;283(22):15193–15200. doi: 10.1074/jbc.M800066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morikis D, Rizos AK, Spandidos DA, Krambovitis E. Electrostatic modeling of peptides derived from the V3-loop of HIV-1 gp120: Implications of the interaction with chemokine receptor CCR5. Int J Mol Med. 2007;19(3):343–351. [PubMed] [Google Scholar]
- 45.Baritaki S, et al. Ionic interaction of the HIV-1 V3 domain with CCR5 and deregulation of T lymphocyte function. Biochem Biophys Res Commun. 2002;298(4):574–580. doi: 10.1016/s0006-291x(02)02511-1. [DOI] [PubMed] [Google Scholar]
- 46.Farzan M, et al. A tyrosine-sulfated peptide based on the N terminus of CCR5 interacts with a CD4-enhanced epitope of the HIV-1 gp120 envelope glycoprotein and inhibits HIV-1 entry. J Biol Chem. 2000;275(43):33516–33521. doi: 10.1074/jbc.M007228200. [DOI] [PubMed] [Google Scholar]
- 47.Cormier EG, et al. Specific interaction of CCR5 amino-terminal domain peptides containing sulfotyrosines with HIV-1 envelope glycoprotein gp120. Proc Natl Acad Sci USA. 2000;97(11):5762–5767. doi: 10.1073/pnas.97.11.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rider CC, et al. Anti-HIV-1 activity of chemically modified heparins: Correlation between binding to the V3 loop of gp120 and inhibition of cellular HIV-1 infection in vitro. Biochemistry. 1994;33(22):6974–6980. doi: 10.1021/bi00188a029. [DOI] [PubMed] [Google Scholar]
- 49.Callahan LN, Phelan M, Mallinson M, Norcross MA. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J Virol. 1991;65(3):1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman TL, Doms RW. HIV-1 envelope determinants for cell tropism and chemokine receptor use. Mol Membr Biol. 1999;16(1):57–65. doi: 10.1080/096876899294760. [DOI] [PubMed] [Google Scholar]
- 51.Repits J, et al. Primary HIV-1 R5 isolates from end-stage disease display enhanced viral fitness in parallel with increased gp120 net charge. Virology. 2008;379(1):125–134. doi: 10.1016/j.virol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Chandramouli B, et al. Structural dynamics of V3 loop with different electrostatics: Implications on co-receptor recognition: A molecular dynamics study of HIV gp120. J Biomol Struct Dyn. 2013;31(4):403–413. doi: 10.1080/07391102.2012.703068. [DOI] [PubMed] [Google Scholar]
- 53.Yuan W, Bazick J, Sodroski J. Characterization of the multiple conformational states of free monomeric and trimeric human immunodeficiency virus envelope glycoproteins after fixation by cross-linker. J Virol. 2006;80(14):6725–6737. doi: 10.1128/JVI.00118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 55.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. South African Vitamin A Study Group Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: A prospective cohort study. Lancet. 1999;354(9177):471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 56.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 57.Silva MF, Tóth IV, Rangel AO. Determination of mercury in fish by cold vapor atomic absorption spectrophotometry using a multicommuted flow injection analysis system. Anal Sci. 2006;22(6):861–864. doi: 10.2116/analsci.22.861. [DOI] [PubMed] [Google Scholar]
- 58.Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res. 1983;43(6):2796–2805. [PubMed] [Google Scholar]
- 59.Ochsenbauer C, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86(5):2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edmonds TG, et al. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408(1):1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown BK, et al. The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS One. 2012;7(4):e29454. doi: 10.1371/journal.pone.0029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moody MA, et al. Anti-phospholipid human monoclonal antibodies inhibit CCR5-tropic HIV-1 and induce beta-chemokines. J Exp Med. 2010;207(4):763–776. doi: 10.1084/jem.20091281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fouda GG, et al. Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology. 2013;10:3. doi: 10.1186/1742-4690-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alam SM, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178(7):4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao HX, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353(2):268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.