Significance
Malaria, caused by intraerythrocytic protozoan parasites of the genus Plasmodium, is by far the deadliest and most prevalent parasitic disease. Most fatalities are attributable to infection by Plasmodium falciparum. The transmission of P. falciparum into Anopheles mosquitoes is absolutely dependent on the ability of the parasite to differentiate into mature gametocytes. Here we show that P. falciparum requires the plant-like phosphatidylcholine synthesis machinery, which is fueled by host serine, and its key enzyme, phosphoethanolamine N-methyltransferase (PfPMT), for gametocyte development, maturation, and transmission. We identified several compounds that inhibit PfPMT activity and gametocyte development. These compounds also inhibited parasite asexual replication. These new chemical entities provide scaffolds for future development of dual-function antimalarials that can block both infection and transmission.
Abstract
Efficient transmission of Plasmodium species between humans and Anopheles mosquitoes is a major contributor to the global burden of malaria. Gametocytogenesis, the process by which parasites switch from asexual replication within human erythrocytes to produce male and female gametocytes, is a critical step in malaria transmission and Plasmodium genetic diversity. Nothing is known about the pathways that regulate gametocytogenesis and only few of the current drugs that inhibit asexual replication are also capable of inhibiting gametocyte development and blocking malaria transmission. Here we provide genetic and pharmacological evidence indicating that the pathway for synthesis of phosphatidylcholine in Plasmodium falciparum membranes from host serine is essential for parasite gametocytogenesis and malaria transmission. Parasites lacking the phosphoethanolamine N-methyltransferase enzyme, which catalyzes the limiting step in this pathway, are severely altered in gametocyte development, are incapable of producing mature-stage gametocytes, and are not transmitted to mosquitoes. Chemical screening identified 11 inhibitors of phosphoethanolamine N-methyltransferase that block parasite intraerythrocytic asexual replication and gametocyte differentiation in the low micromolar range. Kinetic studies in vitro as well as functional complementation assays and lipid metabolic analyses in vivo on the most promising inhibitor NSC-158011 further demonstrated the specificity of inhibition. These studies set the stage for further optimization of NSC-158011 for development of a class of dual activity antimalarials to block both intraerythrocytic asexual replication and gametocytogenesis.
Human malaria parasites exhibit a complex life cycle consisting of asexual phases within human hepatocytes and erythrocytes, with the latter directly responsible for disease manifestations. Within red blood cells, these parasites can also undergo gametocytogenesis, a process during which they interrupt their asexual replication and differentiate to form morphologically and functionally distinct sexual-stage gametocytes (1). These sexual forms serve as precursors for male and female gametes, which develop in the mosquito where they undergo mating, meiosis and several mitotic cycles to produce sporozoites. In Plasmodium falciparum, the causative agent of the most severe form of human malaria, the progression from immature stage I to mature stage V gametocytes takes ∼10 d (2). However, the biological processes that regulate gametocytogenesis remain unknown. Thorough understanding of these processes is crucial to the development of a new generation of dual activity antimalarials that can inhibit both infection and transmission.
Phosphatidylcholine (PC), the predominant phospholipid produced by malaria parasites, plays essential structural and regulatory roles in parasite development and differentiation (reviewed in ref. 3). Lipid metabolic and genetic studies in P. falciparum have demonstrated the presence of two pathways for PC biosynthesis (Fig. S1): the cytidine diphosphate (CDP)-choline pathway, which uses host choline and fatty acids as precursors, and the serine decarboxylase-phosphoethanolamine methyltransferase (SDPM) pathway, which uses host serine and fatty acids as precursors (4). The SDPM pathway involves five parasite-encoded enzymes, of which serine decarboyxlase (PfSD) and phosphoethanolamine methyltransferase (PfPMT) are absent in humans and thus are attractive targets for the development of selective and safe antimalarials (3, 4). In this pathway, serine is converted by PfSD into ethanolamine and then phosphorylated by ethanolamine kinase to form phosphoethanolamine. Phosphoethanolamine is used as a precursor by PfPMT to form phosphocholine via a three-step S-adenosyl methionine (SAM)-dependent methylation reaction (5).
Here, we show that parasites lacking PfPMT are incapable of producing mature gametocytes and are not transmitted to mosquitoes. Using a PfPMT-specific enzyme-coupled assay, we screened a diverse library of small molecules and identified 11 compounds that inhibit PfPMT activity in vitro and block gametocyte development and maturation in cell culture. One compound, NSC-158011, was further validated for its inhibition of PfPMT activity and PC synthesis. NSC-158011 defines a class of chemicals that has never been used in malaria therapy and that can be further optimized to synthesize more specific, selective, and safe dual function antimalarials that inhibit both asexual replication and gametocyte development.
Results
Gametocyte Development and Maturation in P. falciparum Are Modulated by the Phospholipid Precursor Serine.
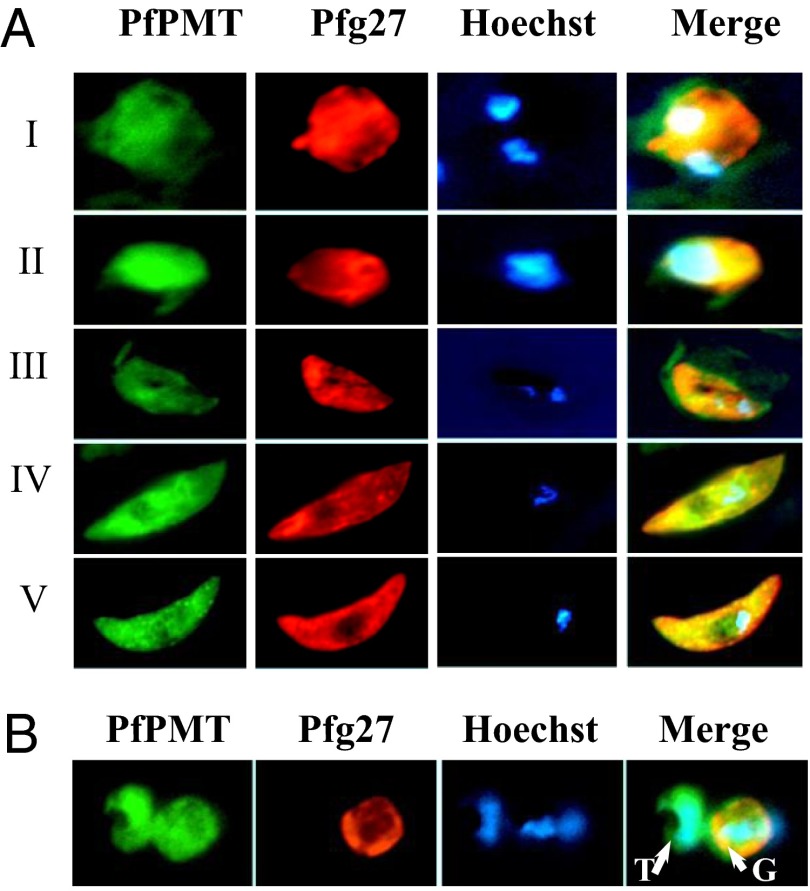
Studies have shown that the PfPMT gene is transcribed in both asexually replicating parasites and gametocytes (6). To validate PfPMT protein expression in gametocytes, a culture of the P. falciparum 3D7 clone at 2% parasitemia and 6% hematocrit in complete medium (PCM culture conditions) was maintained at 37 °C until it reached 10% parasitemia. Parasites were then transferred to culture conditions known to stimulate gametocyte production (GM culture conditions) (7), and stage-I, -II, -III, -IV, and -V gametocytes were collected, fixed, and analyzed by immunofluorescence analysis using anti-PfPMT antibodies. This analysis revealed PfPMT expression in all gametocyte stages (Fig. 1A) as well as in asexually replicating parasites (Fig. 1B). Although PfPMT expression was previously shown to be predominantly associated with membranous structures using a PfPMT-GFP reporter in transgenic parasites (8), both membrane and cytoplasmic localizations of the native enzyme could be detected in wild-type parasites using anti-PfPMT antibodies, suggesting that the protein might exist in both membrane-associated and soluble forms, as was recently shown for the malarial phosphpatidylserine decarboxylase, PkPSD (9). As a control, expression of the gametocyte-specific marker Pfg27 (10) was restricted to gametocytes (Fig. 1 A and B).
Fig. 1.
PfPMT expression during gametocyte development. (A) Wild-type 3D7 parasites were precultured at 2% parasitemia and 6% hematocrit in complete medium and maintained at 37 °C until the cultures reached 10% parasitemia. Parasites were then transferred to GM culture conditions and samples collected over time. Expression of PfPMT and the gametocyte-specific protein Pfg27 in gametocyte stages I, II, III, IV and V was monitored by immunofluoresence analysis using antibodies directed to PfPMT (green) or Pfg27 (red). Areas of overlap between PfPMT and Pfg27 appear in yellow. Nuclear staining was achieved using Hoechst 33258 (blue). (B) Culture sample showing adjacent trophozoite (T)- and gametocyte (G)-infected erythrocytes stained with anti-PfPMT and anti-Pfg27 antibodies.
PfPMT Is Essential for Gametocyte Maturation and Transmission to Mosquitoes.
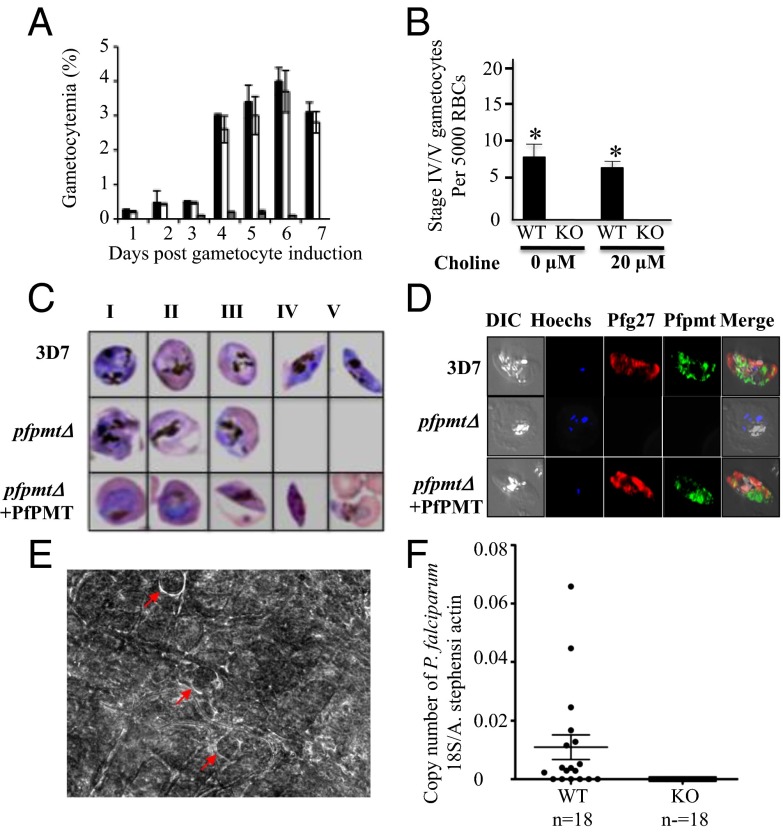
To evaluate the importance of PfPMT and the SDPM pathway in gametocyte development, we compared gametocyte production between wild-type (3D7) and pfpmtΔ parasites lacking PfPMT in the absence or presence of 20 µM choline, the main precursor of the CDP-choline pathway (Fig. 2). Deletion of the PfPMT locus in this strain was obtained following a double cross event leading to replacement of the PfPMT chromosomal gene with the BSD positive selectable marker (8). A complemented strain expressing a wild-type PfPMT on an episome in the pfpmtΔ genetic background was used as a control (8). Because of the slow growth of pfpmtΔ parasites during their intraerythrocytic asexual development, all cultures were grown until they reached 10% parasitemia before they were transferred to GM culture conditions. In wild-type cultures, gametocyte numbers were relatively the same in the absence or presence of choline, with 50% of the gametocytes either at stage IV or V at day 11 following transfer to GM conditions. In contrast, gametocyte production by pfpmtΔ parasites was reduced by 80% compared with the wild-type strain (Fig. 2 A and B). All gametocytes detected in the cultures of knockout parasites were at stages I, II, or III. These stages, however, failed to complete maturation, as no mature gametocytes could be detected even in the presence of choline (Fig. 2 B and C). Episomal expression of the PfPMT gene in the knockout strain restored gametocyte development and maturation (Fig. 2C). To further validate the lack of mature gametocytes in pfpmtΔ cultures, immunofluorescence assays were performed using antibodies against PfPMT and the gametocyte-specific marker Pfg27 on culture samples from 3D7 and pfpmtΔ strains (11, 12) (Fig. 2D). No Pfg27 labeling of stage-IV or -V gametocytes could be detected in the pfpmtΔ strain, whereas stage-IV and -V gametocytes expressing both Pfg27 and PfPMT were detected in wild-type cultures (Fig. 2D). As a control, the pfpmtΔ+PfPMT strain produced all five gametocyte stages at levels similar to those of the parental strain. Together, these results indicate that PfPMT plays a critical role in gametocyte maturation in P. falciparum.
Fig. 2.
Parasites lacking PfPMT are altered in sexual differentiation and transmission. (A) Gametocytemia expressed as percent parasitemia at different days of culture in GM culture conditions for wild-type 3D7, pfpmtΔ+PfPMT and pfpmtΔ mutant strains (black, white, and gray columns, respectively). Data are means ± SDs of triplicate assays. (B) Wild-type (3D7) and pfpmtΔ parasites were precultured at 2% parasitemia and 6% hematocrit in complete medium and maintained at 37 °C until the cultures reached 10% parasitemia in the absence or presence of the CDP-choline precursor choline (20 µM). Parasites were then maintained under GM culture conditions in the absence or presence of 20 µM choline, and the total number of mature gametocytes (IV and V) was determined at day 11. For each condition, a total of 5,000 erythrocytes were counted. (C) Giemsa-stained thin blood smears of gametocyte stages detected in wild-type, pfpmtΔ, and complemented pfpmtΔ+PfPMT parasites. No stage-IV or -V gametocytes could be detected in the knockout strain. (D) Immunofluorescence analysis of wild-type, pfpmtΔ and complemented pfpmtΔ+PfPMT parasites expressing PfPMT under the regulatory control of the P. falciparum CAM1 promoter (9). PfPMT (green), Pfg27 (red), and Hoechst (blue). Error bars indicate SD of light microscopy counts from three independent experiments. *P < 0.05. (E) Phase-contrast images of a wild-type P. falciparum-infected mosquito midgut. Arrows indicate individual P. falciparum 3D7 oocysts. No oocysts could be detected in the midguts of mosquitoes fed on pfpmtΔ-infected red blood cells. Both wild-type and pfpmtΔ parasites were maintained under GM conditions before mosquito feeding. (F) Quantitative PCR results from Anopheles stephensi mosquitoes fed on either wild-type or pfpmt∆ cultures. Each dot indicates individual mosquitoes that were harvested 8 d after artificial blood feeding. Dots on the x axis indicate mosquitoes from which no P. falciparum 18S rRNA could be detected.
PfPMT Is Essential for P. falciparum Infection into Mosquitoes and Oocysts Formation.
To confirm the role of PfPMT in malaria transmission, mosquito-feeding studies were performed using cultures of wild-type and knockout parasites. Up to 30 oocysts were detected in the midgut of each Anopheles stephensi dissected 8 d after feeding on blood infected with wild-type (3D7) parasites (Fig. 2E). Conversely, no oocysts could be detected in the midgut of mosquitoes that fed on blood infected with pfpmtΔ parasites. To further confirm the lack of oocysts in mosquitoes that fed on pfpmtΔ parasites with more sensitive assays, we performed quantitative RT-PCR analyses to detect the expression of P. falciparum 18S rRNA in the mosquitoes. Consistent with the microscopic data, 18S rRNA were detected in mosquitoes that fed on blood infected with 3D7 parasites, whereas no P. falciparum 18S rRNA could be detected in mosquitoes that fed on blood infected with pfpmtΔ parasites (Fig. 2F). Taken together, these results demonstrate an essential role of PfPMT in gametocyte maturation and malaria transmission.
PfPMT Inhibitors Block Gametocyte Development.
The discovery that PfPMT plays an essential role in gametocyte development and maturation suggests that this enzyme could be an ideal target for the development of transmission-blocking drugs. To identify PfPMT inhibitors with gametocytocidal activity, we screened a library of 3161 diverse compounds from the National Cancer Institute Open Chemical Repository using a previously reported PfPMT-specific enzyme-coupled spectrophotometric methylation assay (13). In this assay, the SAM-dependent activity of a purified recombinant PfPMT is coupled to the activity of S-adenosyl homocysteine nucleosidase (SADN) and adenine deaminase (ADA), resulting in the production of hypoxanthine. A secondary screening to eliminate compounds on the SAHN and ADA coupling enzymes was performed using S-adenosyl homocysteine (SAH) as a substrate (13). Using these two assays, 28 compounds were identified as inhibitors of PfPMT activity (Dataset S1). Of these compounds, 11 inhibited both PfPMT activity and P. falciparum asexual replication, with IC50 values ranging between 0.8 µM (compound NSC-22225) and 5 μM (compound NSC-39225) (Dataset S2). These activities were not affected by exogenous choline, consistent with the nonredundant functions of the CDP-choline and SDPM pathways (3, 4).
The 11 PfPMT inhibitors were then tested for their effects on gametocyte development using the NF54-pfs16-GFP-LUC strain. The compounds were added to infected red blood cell cultures at days 0, 3, and 8 following transfer to conditions that stimulate gametocyte production for 48 h to assess their effects on early-, mid-, and late-stage gametocyte development. Treated parasites were either harvested 48 h (Fig. 3 A and B) following drug application or maintained in culture in the absence of the drugs until harvested at day 16 to determine the gametocytocidal or gametocytostatic activity of the compounds (Fig. 3 C and D). Controls included untreated parasites or the NF54-pfs16-GFP-LUC strain treated with artemisinin, which is known to block both infection and transmission (14). Of the 11 compounds tested, NSC-641296, NSC-668394, NSC-323241, and NSC-158011 showed the strongest gametocytocidal activity (Fig. 3B). NSC-641296 and NSC-668394 inhibited early and late gametocyte stages, and their removal from the culture medium for up to 9 d had little to no effect on gametocyte development (Fig. 3D). NSC-323241 was effective against early-stage gametocytes, and its removal from the culture medium for up to 9 d had no effect on gametocyte development (Fig. 3 B and D). NSC-158011 was also effective against early-stage gametocytes (Fig. 3 B and E) but had modest gametocytocidal activity when added to midstage or late stage gametocytes cultures (Fig. 3 F and G). Removal of NSC-158011 from the culture medium 48 h posttreatment was followed by an increase in gametocytemia (Fig. 3D), suggesting a gametocytostatic effect of the drug.
Fig. 3.
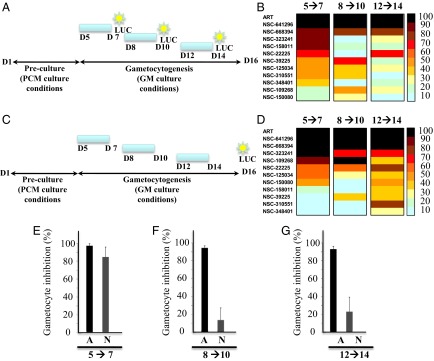
Inhibition of gametocyte development and maturation by PfPMT inhibitors. (A and C) Diagrams of the experimental plan to determine luciferase activity immediately following removal of the compound (A) or on day 16 (C). (B and D) Heat maps representing the effect of PfPMT inhibitors on gametocyte development as measured by luciferase activity in the transgenic line NF54-pfs16-GFP-LUC. Parasites were treated for 48 h (blue bar) with 10 µM of the indicated compound at day 5, 8, or 12 following parasite inoculation. The antimalarial artemisinin (ART) was used as a control at the same concentration. (B) Luciferase activity was measured 48 h posttreatment. (D) Luciferase activity was measured at day 16. (E–G) inhibition of early (E), intermediate (F), and late (G) gametocyte development by artemisinin (A) and NSC-158011 (N). Results are the mean ± SEM of triplicate from three independent assays. Each figure is a representative of one of three independent experiments.
NSC-158011 Is a Competitive Inhibitor of Phosphoethanolamine and Inhibits PfPMT Activity and PC Biosynthesis in Vivo.
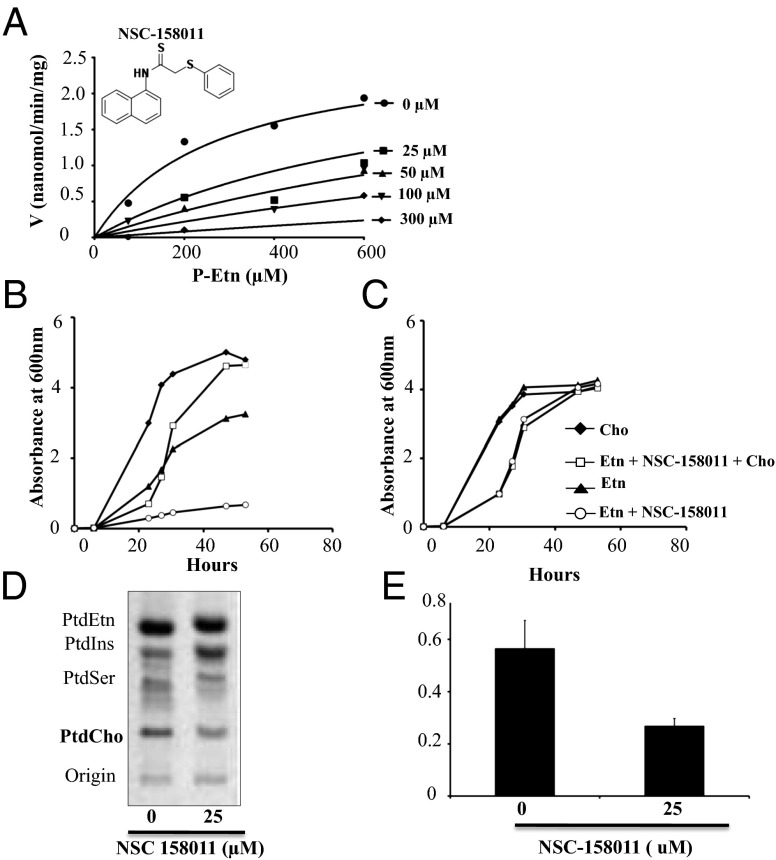
Of the 11 compounds identified in the chemical screen as inhibitors of PfPMT, compound NSC-158011 has the most promising drug-like properties and thus was further investigated for its specificity of inhibition of PfPMT activity. In vitro assays using 14C-SAM as a methyl donor in the absence or presence of increasing concentrations 25, 50, 100, and 300 µM of NSC-158011 showed a dose-dependent inhibition of the enzyme activity (Fig. 4A). Kinetics analyses using a nonlinear fit to the Michaelis–Menten mixed model of inhibition indicated that the compound acts as a competitive inhibitor of phosphoethanolamine (P-Etn) with a Ki of 10.94 ± 4.1 µM. The specificity of inhibition of PfPMT was further demonstrated using a yeast mutant, which relies on PfPMT for survival. Yeast cells do not express a PMT-like activity, but deletion of their two phosphatidylethanolamine methyltransferases, PEM1 and PEM2, in the pem1∆pem2∆ mutant results in choline auxotrophy, a phenotype that can be complemented by expression of PfPMT (5). Unlike wild-type yeast cells, which are only partially inhibited by NSC-158011, the growth of the pem1∆pem2∆ mutant expressing PfPMT was severely affected in the presence of the compound (Fig. 4 B and C). Consistent with a direct inhibition of PfPMT activity and PC biosynthesis, this inhibition was completely reversed in the presence of exogenous choline. Lipid analysis demonstrated a decrease in PC biosynthesis in the drug-treated cells compared with the untreated cells (Fig. 4D). As expected for a decrease in PC biosynthesis, the level of phosphatidylinositol synthesis in these cells increased proportionally. Quantitative analyses showed that the lipid index (PtdCho/PtdIns) in the mutant strain expressing PfPMT under NSC-158011 was decreased by 52% compared with the control. Together, these studies demonstrate a direct inhibition of PfPMT activity in vivo.
Fig. 4.
Biochemical and genetic evidence for specific inhibition of PfPMT by NSC-158011. (A) Michaelis–Menten representation of PfPMT activity in the absence or presence of increasing concentrations of NSC-158011 and changing concentrations of the substrate (P-Etn). The lines shown represent the global fit of all data to the nonlinear fit of Michaelis–Menten mixed model of inhibition. The chemical structure of NSC-158011 is represented. (B and C) The pem1∆pem2∆ strains with pYES2.1-PfPMT vector (B) and wild-type strains with empty vector (C) were inoculated into uracil dropout synthetic galactose medium supplemented with 10 μM ethanolamine (Etn) and grown overnight. Cells were harvested and reinoculated at an A600 = 0.005 in uracil dropout synthetic galactose medium supplemented with 100 μM ethanolamine and/or 100 μM choline (Cho), in the absence or presence of 25 μM NSC-158011 as indicated. Cell growth at 30 °C was monitored by A600. (D and E) Labeled phospholipids were extracted from pem1∆pem2∆ strains carrying pYES2.1-PfPMT vector grown for 17 h in synthetic medium containing 8 µCi of [33P] orthophosphoric acid. The lipid classes were resolved by TLC, visualized by PhosphorImager (D), and quantified by liquid scintillation spectrometry (E). Data are the means ± SD for four experiments.
Discussion
The studies described here show that P. falciparum parasites lacking PfPMT are severely altered in gametocyte development and do not produce stage-IV and stage-V gametocytes even in the presence of choline. Reintroduction of PfPMT into pfpmtΔ parasites restored gametocyte differentiation to wild-type levels, indicating that the gametocytogenesis defects seen in pfpmtΔ are solely due to the loss of this enzyme. pfpmtΔ parasites maintained in the presence of high concentrations of choline or monitored for a long period following transfer to GM conditions did not produce stage-IV and -V mature gametocytes, as demonstrated by light and fluorescence microscopy, even though a small number of stage-I, -II, and -III gametocytes (∼20% those found in wild-type parasites) can be detected in these cultures (Fig. 3).
The finding that PfPMT plays an essential role in the production of stage-V gametocytes suggests that PfPMT inhibitors could be used to block malaria transmission into Anopheles mosquitoes. Our screen of >3,000 small molecules identified 28 compounds that inhibit the activity of PfPMT in the low micromolar range including 11 that inhibit asexual replication. The activity of these compounds against asexually replicating parasites was not affected by addition of exogenous choline. This finding is consistent with previous genetic studies that showed that choline supplementation does not complement the developmental defects of pfpmtΔ knockout parasites (8) and confirms that the SDPM and CDP-choline pathways do not play redundant functions in the intraerythrocytic phase of the parasite life cycle (3). Interestingly, these compounds were found to inhibit gametocyte development in a dose-dependent manner. Strong activity was observed particularly with compounds NSC-641296, NSC-668394, NSC-323241, and NSC-158011. The stage specificity of inhibition (early vs. late gametocytes) varied between compounds and, although some compounds showed strong gametocytocidal activity, others were cytostatic. These differential effects could be attributed to differences in specificity of inhibition and killing, stability, or selectivity between these compounds. Because of its chemical structure, which is amenable to further drug development, NSC-158011 was further characterized to determine its specificity of inhibition of PfPMT activity. NSC-158011 was found to be a competitive inhibitor of phosphoethanolamine (Ki ∼ 11 µM). This specificity of inhibition was further demonstrated in vivo using yeast as a surrogate system. NSC-158011 had no effect on wild-type cells, whereas yeast mutants that require PfPMT for survival were highly sensitive to the compound. This selective inhibition was further validated by metabolic labeling of phospholipids with a decrease in PC synthesis in the presence of the compound concomitant with an increase in phosphatidylinositol as was previously shown (13, 15).
With the recent reports of resistance potentially emerging to artemisinin (16), the development of novel compounds that can fulfill a function similar to that of artemisinin to block both infection and transmission will help alleviate the burden on current first-line antimalarial therapies. The compounds described here represent a class of chemicals that will need to be further developed and optimized to design the next generation of specific, selective, and safe dual-function antimalarials that can block both infection and transmission.
Experimental Procedures
Parasite Intraerythrocytic Asexual Development and Gametocyte Differentiation.
P. falciparum 3D7 (wild type), pfpmtΔ, pfpmtΔ+PfPMT, and NF54-pfs16-GFP-LUC (14) parasites expressing a firefly luciferase under the pfs16 promoter were grown under standard conditions (17) using complete media (RPMI 1640 plus 0.5% Albumax I) or complete media lacking choline and serine. Parasite cultures were synchronized twice using 5% d-sorbitol (18). pfpmtΔ knockout parasites were generated following a gene replacement approach as described (8). pfpmtΔ+PfPMT was generated by transfecting pfpmtΔ parasites with a plasmid harboring PfPMT under the control of the CAM promoter. Before transferring parasites into culture conditions known to stimulate gametocyte production (7), parasites were cultured at 0.5% parasitemia and 6% hematocrit in complete media (hereby named PCM) lacking or supplemented with choline or serine, with daily media change. Once parasite cultures reached 10% parasitemia (5 d for wild-type and complemented strains and 6–7 d for pfpmtΔ), the hematocrit in each culture was decreased to 3.6% by adding 10 mL of media (GM conditions) lacking or supplemented with choline or serine (19). Six hours following transfer to GM conditions, 50 mM N-acetylglucosamine (NAG), which kills asexually replicating parasites, was added and cells were maintained in the presence of NAG for 72 h. Culture media were changed every day without addition of erythrocytes. Giemsa-stained blood smears were examined for 12 consecutive days to derive the numbers of asexually replicating parasites and gametocytes. Gametocyte morphology was determined by light microscopy following Giemsa staining. Immunofluorescence, luciferase, and SYBR Green assays were performed as described (8, 14, 20).
Transmission Studies and Oocyst Formation.
For transmission studies, wild-type (3D7) and pfpmtΔ parasites were maintained in culture conditions known to stimulate gametocyte production for 18 d and monitored daily by light microscopy. Female mosquitoes were infected artificially by membrane feeding (30 min at 37 °C) with wild-type or pfpmtΔ-infected red blood cells. Twenty-four hours after feeding, unfed mosquitoes were discarded, and fully engorged mosquitoes were maintained at 27 °C. Midguts were dissected 8 d after infection, and oocysts stained with 0.05% (wt/vol) mercurochrome in water to monitor infection. For a more sensitive evaluation of parasite presence in mosquito midguts, the presence of parasite 18S rRNA was examined by quantitative PCR (21).
PfPMT Purification and Chemical Screening.
The PfPMT enzyme-coupled assay was performed as described (13). Compounds were obtained from the National Cancer Institute (NCI)/Developmental Therapeutics Program Open Chemical Repository. NCI compounds were diluted to a final concentration of 10 μM. Enzyme activity was measured by monitoring hypoxanthine production at 265 nm every 15 min for 2 h using a UV-visible plate reader using a kinetic mode at 37 °C. Plates included four wells of negative controls (reaction mixture and no PfPMT) and eight wells of positive controls (reaction mixture, PfPMT and no drugs). In addition to these controls, four wells containing amodiaquine were included on each plate. Compounds that inhibited PfPMT by greater than 90% were subsequently tested in a separate plate to determine their possible effect on the coupling enzymes (counter screen). These compounds were added to 96-well reaction plates at a final concentration of 10 μM in a reaction mixture containing 1 mM MnSO4, 0.5 μM BsAda (Bacillus subtilis adenine deaminase), 4.72 μM SAHN, and 100 μM SAH. Plates were read under the same mode as described above. Compounds that were determined to be specific inhibitors of PfPMT were serially diluted twofold across 96-well plates, with the final concentration ranging between 0.3 μM and 10 μM. The change in absorbance over time was calculated, and these values were plotted in Graph Pad Prism software version 5, using log (inhibitor) vs. response to calculate compound IC50 values.
Gametocyte Inhibition Studies.
NF54-pfs16-GFP-LUC strain was used for gametocyte inhibition studies. Protocols for parasite propagation and gametocyte development were followed as described above. Following stimulation of gametocytogenesis, parasites were transferred into 96-well plates. Cultures were treated with 50 mM NAG or a selection of drugs (in 0.1% DMSO) for 48 h at the time of stimulation, as well as 3 d or 6 d poststimulation. After 48 h of treatment, the compounds were removed by replacing the culture medium, and infected red blood cells were either collected or maintained until day 16 with daily media change. Luciferase activity was measured as described earlier.
Yeast Proliferation Assays and Lipid Analyses.
BY4741-pYes2.1 (wild-type) and pem1Δpem2Δ-pYes2.1-PfPMT yeast strains were pregrown overnight in uracil dropout synthetic galactose (4%) (SG-ura) medium supplemented with 10 μM ethanolamine. Cells were harvested by centrifugation, washed twice by resuspension in water, and diluted to an A600 = 0.005 in fresh SG-ura medium supplemented with 100 μM ethanolamine in the absence or presence of 25 μM NSC-158011. Cells were grown at 30 °C, and the growth was monitored by measuring the A600. For phospholipid analyses, pem1∆pem2∆ strains with pYES2.1-PfPMT vector were grown in synthetic uracil dropout medium plus 2 mM ethanolamine and 0.1 mM choline with 4% galactose as a carbon source (SG-Ura). Cells in midlog phase were harvested by centrifugation and washed twice in water. The cells were suspended in SG medium at an A600 of 0.03 in a volume of 2 mL in the absence or presence of 25 µM NSC-158011. Radiolabeling was initiated by adding 8 µCi of [33P]orthophosphoric acid, and growth was continued at 30 °C for 17 h with vigorous shaking. Labeled phospholipids were extracted as described (22), and the lipid classes were resolved by TLC on silica-60 TLC plates in the solvent system of chloroform, methanol, 2-propanol, 0.25% KCl, triethylamine (30:9:25:6:18, vol/vol). The resolved radioactive lipids were visualized by PhosphorImager (GE Typhoon) and quantified by liquid scintillation spectrometry as described (23).
Enzyme Kinetics.
The effect of NSC-158011 on PfPMT was measured using a radiometric assay. Enzymatic assay conditions are: 0.1 M Hepes, KOH (pH8.6), 2 mM Na2EDTA, 10% glycerol, 10% DMSO, 600 µM SAM (100 nCi of [methyl-14C]SAM), and 75–600 µM P-Etn. NSC-158011 was added at concentrations of 25, 50, 100, and 300 µM. Purified enzyme (5 µg) was added, and the reactions were incubated at 37 °C for 30 min and terminated using 1 mL of cold water. The product was then purified by batch purification using AG (H+ resin) using the protocol provided by the manufacturer. The product was eluted from resin using 0.1 M HCl, and total radioactivity was measured using the scintillation mixture.
Statistical Analyses.
Statistical analyses were performed using an unpaired Student t test. Differences were considered statistically significant when P < 0.05. Graphs were plotted and analyzed using GraphPad.
Supplementary Material
Acknowledgments
Pfg27 antibodies were kindly provided by Dr. Nirbhay Kumar (Tulane University). The compounds used in the chemical screening were provided by the National Cancer Institute. This research was supported by National Institutes of Health (NIH) and Department of Defense Grants AI51507, AI007603, and PR033005, and BWF Award 1006267 (to C.B.M.). C.B.M. is a recipient of the Investigators of Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. D.A.F. acknowledges support from the NIH (Grant R01 AI079709).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313965110/-/DCSupplemental.
References
- 1.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alano P. Plasmodium falciparum gametocytes: Still many secrets of a hidden life. Mol Microbiol. 2007;66(2):291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 3.Bobenchik AM, Augagneur Y, Hao B, Hoch JC, Ben Mamoun C. Phosphoethanolamine methyltransferases in phosphocholine biosynthesis: Functions and potential for antiparasite therapy. FEMS Microbiol Rev. 2011;35(4):609–619. doi: 10.1111/j.1574-6976.2011.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci USA. 2004;101(16):6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pessi G, Choi JY, Reynolds JM, Voelker DR, Mamoun CB. In vivo evidence for the specificity of Plasmodium falciparum phosphoethanolamine methyltransferase and its coupling to the Kennedy pathway. J Biol Chem. 2005;280(13):12461–12466. doi: 10.1074/jbc.M414626200. [DOI] [PubMed] [Google Scholar]
- 6.Young JA, et al. The Plasmodium falciparum sexual development transcriptome: A microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143(1):67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Bruce MC, Alano P, Duthie S, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100(Pt 2):191–200. doi: 10.1017/s0031182000061199. [DOI] [PubMed] [Google Scholar]
- 8.Witola WH, et al. Disruption of the Plasmodium falciparum PfPMT gene results in a complete loss of phosphatidylcholine biosynthesis via the serine-decarboxylase-phosphoethanolamine-methyltransferase pathway and severe growth and survival defects. J Biol Chem. 2008;283(41):27636–27643. doi: 10.1074/jbc.M804360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JY, Augagneur Y, Ben Mamoun C, Voelker DR. Identification of gene encoding Plasmodium knowlesi phosphatidylserine decarboxylase by genetic complementation in yeast and characterization of in vitro maturation of encoded enzyme. J Biol Chem. 2012;287(1):222–232. doi: 10.1074/jbc.M111.313676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Sharma I, Kogkasuriyachai D, Kumar N. Structure of a gametocyte protein essential for sexual development in Plasmodium falciparum. Nat Struct Biol. 2003;10(3):197–203. doi: 10.1038/nsb899. [DOI] [PubMed] [Google Scholar]
- 11.Wizel B, Kumar N. Identification of a continuous and cross-reacting epitope for Plasmodium falciparum transmission-blocking immunity. Proc Natl Acad Sci USA. 1991;88(21):9533–9537. doi: 10.1073/pnas.88.21.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witola WH, Pessi G, El Bissati K, Reynolds JM, Mamoun CB. Localization of the phosphoethanolamine methyltransferase of the human malaria parasite Plasmodium falciparum to the Golgi apparatus. J Biol Chem. 2006;281(30):21305–21311. doi: 10.1074/jbc.M603260200. [DOI] [PubMed] [Google Scholar]
- 13.Bobenchik AM, et al. Identification of inhibitors of Plasmodium falciparum phosphoethanolamine methyltransferase using an enzyme-coupled transmethylation assay. BMC Biochem. 2010;11:4. doi: 10.1186/1471-2091-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adjalley SH, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA. 2011;108(47):E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pessi G, Ben Mamoun C. Pathways for phosphatidylcholine biosynthesis: Targets and strategies for antimalarial drugs. Future Med Future Lipidol. 2006;1(2):173–180. [Google Scholar]
- 16.Dondorp AM, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011;365(12):1073–1075. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 18.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65(3):418–420. [PubMed] [Google Scholar]
- 19.Carter R, Ranford-Cartwright L, Alano P. The culture and preparation of gametocytes of Plasmodium falciparum for immunochemical, molecular, and mosquito infectivity studies. Methods Mol Biol. 1993;21:67–88. doi: 10.1385/0-89603-239-6:67. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JD, et al. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51(6):1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rougemont M, et al. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42(12):5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trotter PJ, Pedretti J, Voelker DR. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J Biol Chem. 1993;268(28):21416–21424. [PubMed] [Google Scholar]
- 23.Trotter PJ, Pedretti J, Yates R, Voelker DR. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiáe. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J Biol Chem. 1995;270(11):6071–6080. doi: 10.1074/jbc.270.11.6071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.