Significance
Juvenile hormone (JH) regulates reproductive maturation in insects. In Drosophila melanogaster, JH is necessary for egg maturation. We show that the removal of JH delays both mating and the onset of the production of the major female sex pheromones in D. melanogaster. Importantly, this action of JH was via the Methoprene-tolerant (Met) JH receptor, rather than its paralog Germ-cell expressed, similar to its action in egg maturation. Thus, JH acting via Met coordinates pheromone production and mating with egg maturation. These studies provide insights into the molecular basis of the hormonal regulation of reproductive behavior in insects that may also be applicable to the coordination of the complex behaviors essential for animal reproduction and thus survival.
Abstract
Juvenile hormone (JH) coordinates timing of female reproductive maturation in most insects. In Drosophila melanogaster, JH plays roles in both mating and egg maturation. However, very little is known about the molecular pathways associated with mating. Our behavioral analysis of females genetically lacking the corpora allata, the glands that produce JH, showed that they were courted less by males and mated later than control females. Application of the JH mimic, methoprene, to the allatectomized females just after eclosion rescued both the male courtship and the mating delay. Our studies of the null mutants of the JH receptors, Methoprene tolerant (Met) and germ cell-expressed (gce), showed that lack of Met in Met27 females delayed the onset of mating, whereas lack of Gce had little effect. The Met27 females were shown to be more attractive but less behaviorally receptive to copulation attempts. The behavioral but not the attractiveness phenotype was rescued by the Met genomic transgene. Analysis of the female cuticular hydrocarbon profiles showed that corpora allata ablation caused a delay in production of the major female-specific sex pheromones (the 7,11-C27 and -C29 dienes) and a change in the cuticular hydrocarbon blend. In the Met27 null mutant, by 48 h, the major C27 diene was greatly increased relative to wild type. In contrast, the gce2.5k null mutant females were courted similarly to control females despite changes in certain cuticular hydrocarbons. Our findings indicate that JH acts primarily via Met to modulate the timing of onset of female sex pheromone production and mating.
All aspects of reproductive maturation in animals including gonad development, pheromone production for communication between the sexes, and mating behavior need to be precisely timed and coordinated to ensure reproductive success, and regulatory hormones are the key to this timing and coordination (1, 2). In most female insects, the sesquiterpenoid juvenile hormone (JH) regulates and coordinates reproductive maturation of the ovaries (2, 3) and often sex pheromone synthesis and mating behavior (4, 5). In Drosophila melanogaster, females are unreceptive to male courtship attempts just after eclosion, but by day 2, most become receptive and mate (6, 7). Implantation of JH-secreting corpora allata (CA) (6) just before eclosion or application of methoprene (8), a JH mimic (JHM), caused females to mate precociously. Decreased JH in the apterous mutant (9) or after treatment of females with precocene, a compound cytotoxic for the CA (10), reduced female mating. These findings suggest that JH may play a role in the maturation of female receptivity in D. melanogaster.
The switch from an unreceptive to a receptive state requires coordination of neural activity critical for female mating behaviors with production of sex pheromones that attract the male (11–13). The Drosophila sex pheromones are a subgroup of the cuticular hydrocarbons (CHC) that mediate chemical communication for both sex and species recognition (see refs. 14 and 15 for reviews). In D. melanogaster, two CHCs, (Z,Z)-7,11-C27:2 (C27) and (Z,Z)-7,11-C29:2 (C29) dienes, are female-specific; these dienes are known to play a role in mate choice preference and the onset of male courtship (15–18). Overexpression of the JH esterase-binding protein DmP29 after eclosion, which is thought to reduce the JH titer, caused a reduction in the synthesis of these female-specific dienes (19). The role of JH in the biosynthesis of these CHCs is unknown.
In D. melanogaster, there are two basic helix–loop–helix proteins encoded by the paralogous genes, Methoprene-tolerant (Met) and germ cell-expressed (gce), which act as JH receptors in both metamorphosis and reproductive maturation (20–22). Loss of function of both receptor genes is necessary to mimic the effect of genetic ablation of the CA in larvae, which causes prepupal lethality (23–25). In the adult, loss of Met causes both delayed and reduced fecundity, whereas loss of gce has only a slight effect on ovarian maturation (25). To elucidate the molecular mechanisms underlying JH’s role in the onset of mating in D. melanogaster females, we have genetically ablated the CA in the developing adult and found that the onset of mating behavior was delayed, and this delay could be rescued by JH. This action of JH was dependent only on the Met receptor and was partly due to modulation of sex pheromone production.
Results
Genetic Ablation of the CA in the Developing Females Delays the Onset of Mating.
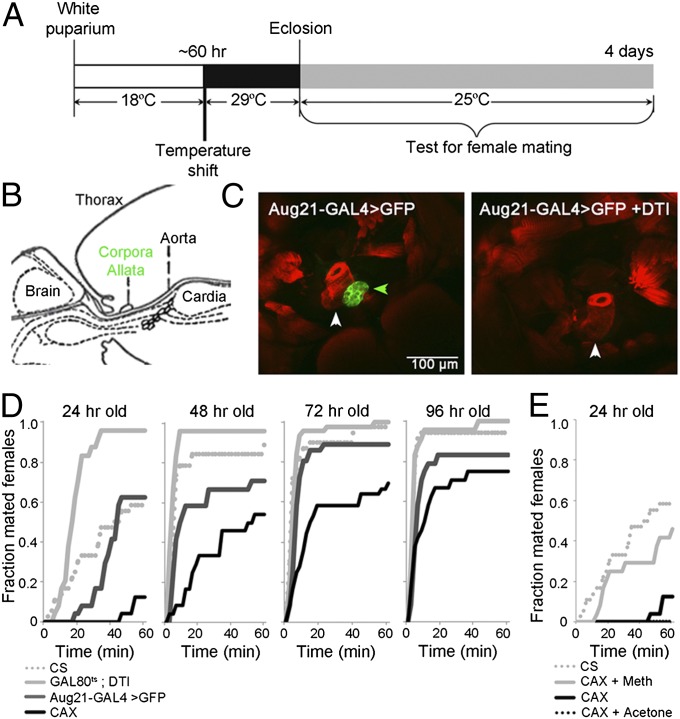
To address the role of JH in female mating behavior, we genetically ablated the CA in developing Drosophila females by targeting the expression of diphtheria toxin (DTI) to the CA via the CA-specific Aug21-galactosidase4 (GAL4) driver (Fig. 1 A–C). Because the removal of the CA is prepupal lethal (23, 24), we used the temperature-sensitive GAL80 (GAL80ts) transgene to bypass this critical period for JH during development. At 60 h after puparium formation (APF), we shifted the pupae from 18 to 29 °C to inactivate the GAL80ts, thus allowing expression of upstream activating sequence (UAS)-DTI (Fig. 1A). At eclosion, the CA were absent as detected by UAS-eGFP driven by Aug21-GAL4 (compare Fig. 1 B and C). The females lacking the CA are designated as allatectomized females (CAX).
Fig. 1.
Genetic ablation of the corpora allata (CA) delays onset of mating in females. (A) Scheme for the genetic ablation of the CA in developing females of genotype w; Aug21-GAL4 > egfp/GAL80ts; UAS-DTI/+. A temperature shift to 29 °C 60 h after puparium formation initiated expression of DTI in the CA . After eclosion, the females were kept in LL at 25 °C. (B) Diagram of the CA position in the adult. (C) Validation of the CA ablation (CAX) by confocal imaging. Normally, Aug21-GAL4 driving UAS-egfp (green) labels the intact CA (Left, green arrow). When UAS-DTI is expressed as in A, the CA is eliminated by the time of eclosion (Right). Genotypes: w; Aug21-GAL4 >egfp/GAL80ts (Left); w; Aug21-GAL4 >egfp/GAL80ts; UAS-DTI/+ (Right). The aorta (white arrow) is seen with phalloidin staining. (Scale bar, 100 µm.) (D) Fraction of CAX and the parental control females mated in the single pair-mating assay at times after eclosion. P < 0.0001 for CAX versus the GAL80ts; DTI control and CAX versus the Aug21-GAL4 > GFP control at all times using the Komogorov Smirnov test. Genotypes: Parental genotype controls are w; GAL80ts /+; UAS-DTI/+ and w; Aug21-GAL4 > GFP/+. CAX is w; GAL80ts/Aug21-GAL4 > egfp; UAS-DTI/+. (E) Fraction of CAX females mated in a single pair-mating assay at 24 h after treatment with methoprene (Meth) or acetone within 2 h after eclosion. No acetone-treated females mated during the 1-h assay period. P < 0.0001 for methoprene-treated CAX females versus acetone-treated control CAX females using the Kolmogorov Smirnov test. For D and E, the number of females tested for each genotype and time point were between 23 and 44.
We then examined mating of CAX females using two different assays. Initially we used a group assay in which groups of 12 3- to 4-d-old don juan males carrying green fluorescent protein (GFP)-labeled sperm and 12 females were placed in a food vial; the number mated after 1 h was assessed by the presence of GFP-labeled sperm in the female spermatheca. In this assay (Fig. S1A), significantly fewer CAX females at 24 and 30 h after eclosion mated during the 1-h period compared with the parental controls. By 48 h, there was no significant difference between the CAX and control females. When the number of pairs in the vial was reduced to either three or one pairs per vial, the number of CAX females mating at 48 h was significantly lower than the parental controls; at 72 h the difference between CAX and control females was only seen for the single pair (Fig. S1B).
In contrast, in the single-pair assay in a courtship chamber, CAX females mated significantly less with the Canton S (CS) males than the controls at all times up to 96 h (Fig. 1D). Of those that mated, the time to copulation decreased with the increasing age of the females across all genotypes (Fig. S1C). However, the time to copulation for CAX females was significantly longer than that of the control females through 72 h. Exposure to the JHM methoprene just after eclosion reduced the time for mating of CAX females at 24 h to the control level (Fig. S1C). The high percentage mating during the 1-h period for the CS and parental control lines at 24 h in this single-pair assay is at least partly due to the temperature shifts necessary for the allatectomy. When CS pupae and adults were subjected to a similar temperature shift regimen, they showed increased mating at 24 h compared with those reared continuously at 25 °C (Fig. S1D). The reason for the very high percentage mating of the transgenic GAL80ts; DTI parental line (Fig. 1D) is unknown.
Together, these findings suggest that in the absence of JH, there is a significant delay in the onset of female mating. Apparently, this delay can be partially overcome in the presence of other flies because similar percentages of grouped CAX and control females mated at 48 h, indicating that social cues may be important as well as individual maturation.
Application of the JH Mimic, Methoprene, Rescues the Delay in the Onset of Mating of the CAX Females.
In the group-mating assay, treatment of CAX females within 2 h of eclosion with increasing doses of JHM led to an increased number mating at 24 h compared with that of acetone-treated females (Fig. S2A). The 50% effective dose (ED50) was found to be ∼0.72 pmoles methoprene. Moreover, application of 6.4 pmoles or higher doses of methoprene to the CAX females increased the percent mating above that of parental controls to about 54–60%. When we assayed mating in the single-pair assay at 24 h, the application of 6.4 pmoles methoprene caused 50% of the treated CAX females to mate within the 1-h assay, whereas none of the control acetone-treated CAX females mated (Fig. 1E). Together, these findings indicate that the lack of JH caused the observed delay in the onset of mating of the CAX females.
JH Acts Primarily Via Methoprene tolerant (Met) to Modulate Mating in Females.
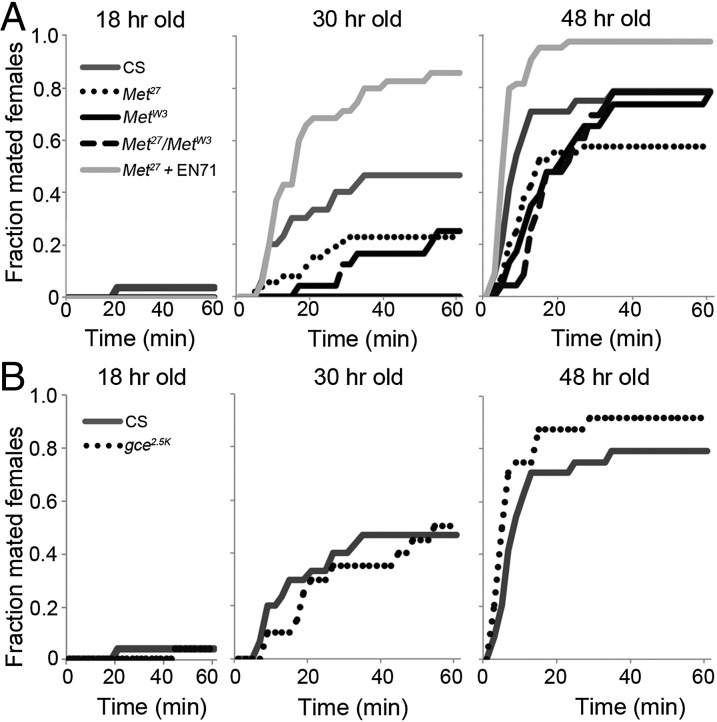
The above analysis of the CAX females indicated that JH was necessary for the normal timing of the onset of female mating. To determine whether JH acts through the same receptors to control the onset of female mating as it does for controlling metamorphosis (21), we first examined females homozygous for the Met27 null allele, a small deletion in the 5′ regulatory region that prevents Met transcription (26), and another Met mutant allele, MetW3, a missense mutation in the ORF (27). In the single-pair assay, the onset of mating of homozygous Met27, homozygous MetW3, and transheterozygous Met27/MetW3 females was significantly delayed compared with wild-type CS females at both 30 and 48 h after eclosion (Fig. 2A). Expression of the Met genomic rescue transgene EN71 (26) in the homozygous null Met27 background prevented the delay in mating at both times (Fig. 2A). The reason for the higher level of mating seen in the rescued mutant compared with the CS wild type is likely due to differences in attractiveness (see below). Consistent with these findings in the single-pair assay, these two Met mutants also showed delayed onset of mating in the group assay (Fig. S2B).
Fig. 2.
Role of the JH receptors, Met and Gce, in onset of mating in females. (A) Mating of the wild-type CS and the Met allele females with and without the Met genomic rescue transgene EN71 with CS males in the single pair-mating assay at times after eclosion. At 30 h, P < 0.0001 for Met27, MetW3, MetW3/Met27, and Met27 EN71 versus CS and for Met27 versus Met27 EN71; at 48 h, P < 0.0001 for Met27, MetW3, and Met27 EN71 versus CS; P = 0.002 for MetW3/Met27 versus CS, using the Kolmogorov Smirnov test. Genotypes: CS, wvMetW3/wvMetW3 (MetW3), wvMet27/wvMet27 (Met27), wvMetW3/wvMet27 (MetW3/Met27), and wvMet27/ wvMet27; EN71/+ (Met27 + EN71). (B) Mating of homozygous gce null allele (gce2.5k) females and CS control females in the single-pair assay. P = 0.013, 0.06, and P < 0.0001for gce2.5k versus CS at 18, 30, and 48 h, respectively, using the Kolmogorov Smirnov test. Genotypes: CS and w gce2.5k/w gce2.5k. Numbers of females tested for each genotype and time point were between 23 and 59 for A and between 20 and 30 for B.
If JH acts only via the Met receptor, then the application of the JHM methoprene should not affect mating behavior of the Met27 null females. The application of either 6.4 or 64 pmoles methoprene to these females soon after eclosion significantly increased the fraction of females mating at both 24 and 48 h (Fig. S2C), suggesting that in the absence of Met, the paralogous JH receptor, Gce, may play a role in mating.
In Drosophila, Gce is partially redundant to Met during development such that simultaneous loss of function of both JH receptor genes, Met and gce, is necessary to recapitulate the effects of allatectomy during larval life (23–25). Therefore, to determine whether gce had any effect on the maturation of mating behavior, we used a null allele of gce (gce2.5k), which is a 2.5 kb deletion in the gce locus that causes loss of gce transcripts (25). The complete loss of function of gce had no significant effect on the percent mating at 30 h after eclosion, but at 48 h more gce mutant females than CS control females mated (Fig. 2B). This difference may be due to the different genetic backgrounds of these two strains. Lack of Gce had no significant effect on the time to onset of copulation at either 30 or 48 h (Fig. S2D). These findings suggest that Met is the primary receptor that mediates the modulatory effect of JH on the timing of mating in females.
JH Signaling Modulates the Attractiveness of D. melanogaster Females.
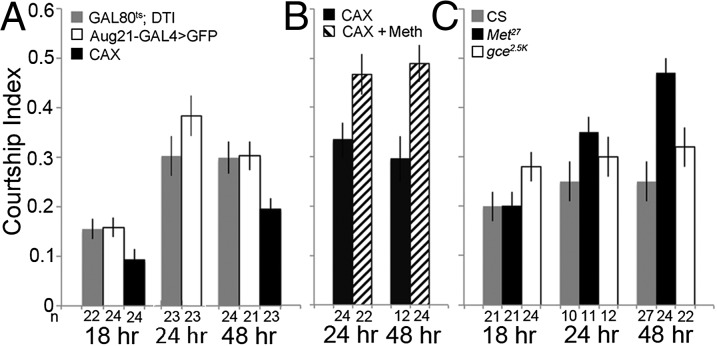
In the presence of wild-type adult males, onset of mating depends on both the females’ acceptance or rejection behaviors and their attractiveness to the males (28). To assess the attractiveness of the females, we paired wild-type CS males with females that were decapitated to minimize their behavioral responses (29). By 24 h the parental controls were courted similarly as later at 48 h. At 48 h, the CAX females were courted significantly less than either of the parental control females (Fig. 3A), indicating that they were less attractive to the males. Consistent with these results, at 48 h CS males also courted intact CAX females less than intact parental control females; by 96 h, there was no difference (Fig. S2E).
Fig. 3.
Courtship behaviors of wild-type males paired with decapitated CAX or Met or gce mutant females. (A) Courtship indices of Canton S (CS) males paired with decapitated CAX females and parental controls at 18 and 48 h after eclosion. P = 0.09 and 0.07 for CAX versus the GAL80ts; DTI and the Aug21-GAL4 > GFP parental controls, respectively, at 18 h and P = 0.026 and 0.025 for CAX versus the controls, respectively, at 48 h. Genotypes are same as in Fig. 1D. (B) Courtship indices of CS males paired with decapitated CAX females treated with 6.4 pmoles methoprene or acetone within 2 h after eclosion. P = 0.017 at 24 h, and P = 0.0037 at 48 h. Genotypes are same as in Fig. 1D. (C) Courtship indices of wild-type males paired with Met27 or gce2.5k homozygous mutant females or CS females at 18 and 48 h after eclosion. At 18 h, P = 0.99 and 0.035 for CS versus Met27 and gce2.5k, respectively. At 24 h, P = 0.2 and 0.62 for CS versus Met27 and gce2.5k, respectively, and at 48 h, P < 0.0001 and 0.13 for CS versus Met27 and gce2.5k, respectively. Genotypes as in Fig. 2 A and B. For A–C, numbers below the columns represent the number of decapitated females tested, and the values represent the average courtship index of wild-type males ± SEM. Data were compared using ANOVA followed by the Tukey multiple comparison test.
To determine if their reduced attractiveness was due to the lack of JH, we applied 6.4 pmoles methoprene at eclosion. Fig. 3 shows that JHM-treated, decapitated CAX females were courted more at both 24 and 48 h than the controls, suggesting that lack of JH caused the reduced attractiveness.
To determine whether JH acts via Met to modulate female attractiveness, we assayed decapitated Met27 null females. Surprisingly, unlike the decapitated CAX females, these females were courted more than decapitated CS females by CS males at 48 h (Fig. 3C). In contrast, intact Met27 females were courted less than were intact CS females (Fig. S2F), although the males attempted copulation more frequently with them than with the latter (14 ± 4 vs. 2.3 ± 0.7 attempts per 10 min, n = 12 each). These data taken together suggest that the Met27 females are more attractive to the males but when intact may show some subtle rejection behavior. The courtship indices (CI) for males courting Met27 females and Met27 females carrying the Met genomic rescue transgene EN71 were similar [0.43 ± 0.03 (n = 24) and 0.40 ± 0.03 (n = 35), respectively], indicating no rescue of this increased attractiveness. The reason for this lack of rescue is unknown. Because the rescued flies showed increased mating compared with the CS wild-type controls (Fig. 2A), the transgene apparently rescued the mutant behavioral rejection phenotype so that they mated when courted by the males.
A similar analysis of male courtship with the homozygous gce2.5k null decapitated females showed that loss of function of gce increased female attractiveness at 18 h but had no significant effect at 48 h (Fig. 3C). These findings suggest that Met is the primary mediator of female attractiveness by JH.
CHC Profile is Altered in the CAX Females.
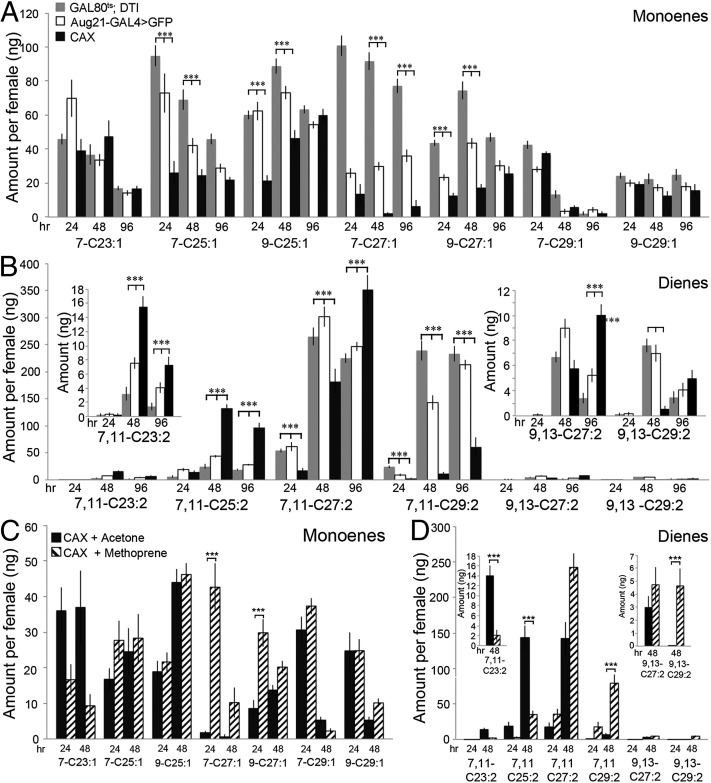
Previous studies have shown that the Drosophila CHCs are comprised primarily of long-chain alkanes, methyl alkanes, and the corresponding monoenes and dienes (14, 15). In the Canton S strain of D. melanogaster, the Z7-C23 monoene (tricosene) is prominent in the male and thought to inhibit courtship by other males and to stimulate female mating (13, 15). Two dienes [(Z,Z)-7,11-C27:2 and (Z,Z)-7,11-C29:2: the 7,11-dienes] are specific to the female and have been shown to stimulate courtship behavior by conspecific males (15–18). Also, the 7- and 9-C25 monoenes produced by the female may act as aphrodisiacs (17, 30). To elucidate the basis of the reduced attractiveness of CAX females, we examined the CHC profiles of these females at various times after eclosion. Fig. 4A shows that in the CAX females, the C25 and C27 monoenes were significantly reduced at 48 h compared with the parental controls, but by 96 h only the 7-C27 monoene remained low. The CAX females also had lower levels of the two female-specific dienes (7,11-C27 and 7,11-C29) at 48 h, with a corresponding increase in the amounts of the shorter 7,11-C23 and -C25 dienes (Fig. 4B). At 96 h the 7,11-C23, -C25, and -C27 dienes all were higher in the CAX females than in the parental controls, whereas the 7,11-C29 diene remained lower (Fig. 4B). The less abundant 9,13-C27 diene also was significantly higher at 96 h, but the 9,13-C29 diene was similar to the controls (Fig. 4 B and Inset). Thus, the loss of JH caused a significant reduction in both the monoenes and dienes thought to be important aphrodisiacs for the males.
Fig. 4.
Time course of monoene and diene profiles of CAX females. Cuticular hydrocarbons (CHC) were isolated and analyzed by gas chromatography with flame ionization detector (GC-FID) at 24, 48, and 96 h after eclosion. The amounts of monoenes and alkanes are relative to the standards included in the hexane washes. (A and B) CHCs of CAX females and the parental controls. The value for each CHC is represented as the average values of 18 hexane-washed females ± SEM from two independent experiments. Statistically significant values compared with both parental controls are shown with *** = P ≤ 0.001. Not shown are the minute levels of the 5-C23-C27and the 9-C23 monoenes. (C and D) CHCs of CAX females treated with 6.4 pmoles methoprene or acetone within 2 h after eclosion. The value for each CHC is the average value from nine females ± SEM. Statistically significant values compared with acetone-treated CAX females are shown with *** = P ≤ 0.001. (A and C) Monoenes. The position of the single double bond is indicated. (B and D) Dienes. The positions of the two double bonds are indicated. (Inset) The amount of 9,13 dienes present in low amounts.
When we applied 6.4 pmoles of the JHM methoprene to the CAX females at eclosion, only the C27 monoenes were significantly increased at 24 h in the treated females (Fig. 4C). By 48 h, the 7,11-C27 and -C29 dienes had increased (Fig. 4D) and approached levels seen in the parental controls (Fig. 4B). In contrast, the C23 and C25 dienes were significantly decreased in the JH-treated CAX females. These findings indicate that JH is important for normal timing of synthesis of select CHCs in females.
The CAX females also had decreased levels of the nC23, nC25, and nC27 alkanes and of 2MeC24 and 2MeC26 methyl alkanes at 24 h in comparison with parental controls (Fig. S3 A and B). By 96 h the nC27 alkane and 2MeC26 alkane were similar to the controls, whereas the shorter alkanes remained depressed, and the C24 methyl alkane increased. Methoprene application increased the level of the C27 alkane but had no significant effect on methyl alkane production (Fig. S3 C and D), indicating that JH regulates the synthesis of only certain alkanes.
JH Acts via Met and Gce to Regulate the Synthesis of Select CHCs in Females.
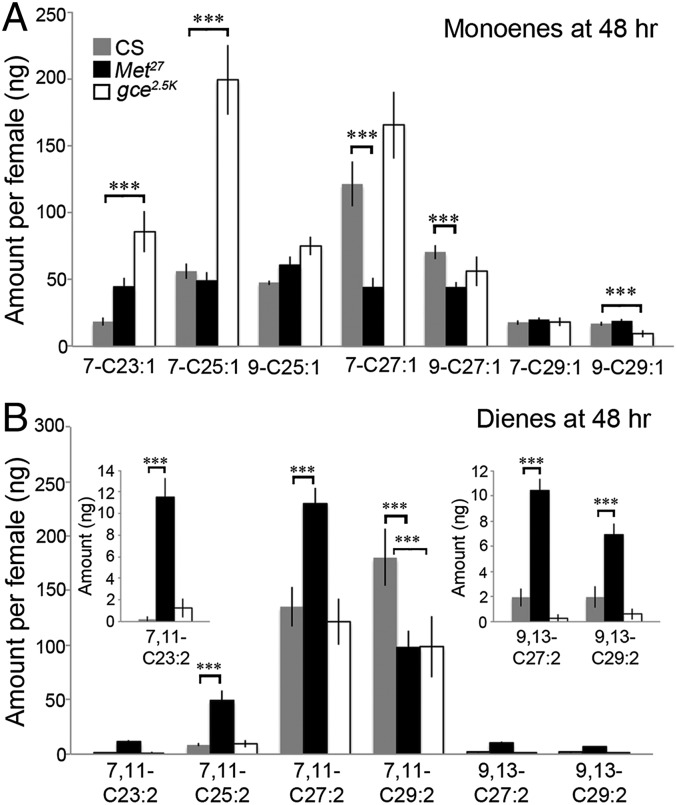
To determine the role of the two JH receptors, Met and Gce, in the synthesis of female CHCs, we examined the CHC profiles of both null mutant Met27 and gce2.5k females at 48 h, a time when the Met27 females were more attractive than Canton S controls in the courtship assay. Loss of function of Met decreased the level of the C27 monoenes but had little effect on the levels of the 7-C23 and -C25 monenes compared with the CS controls (Fig. 5A). By contrast, the levels of the 7,11-C23, -C25, and -C27 dienes were all significantly higher in the Met27 females than in the Canton S females with only the 7,11-C29 diene reduced. The minor 9,13-C27 and -C29 dienes were also higher in the Met27 null females (Fig. 5B). In addition, the C21 and C22 alkanes and the 2MeC24 alkane were significantly increased (Fig. S4). Presumably, this new blend of aphrodisiac compounds and possibly the other CHC changes are responsible for the increased attractiveness of Met27 females in the courtship assay at 48 h.
Fig. 5.
The monoene and diene profiles of JH receptor mutant females, Met27 and gce2.5k. CHC of Met27 and gce2.5k homozygous females and CS females (genotypes as in Fig. 2) were isolated at 48 h after eclosion and analyzed as in Fig. 4. (A) Monoenes. The position of the single double bond is indicated. (B) Dienes. The positions of the two double bonds are indicated. (Inset) The 9,13 dienes present in low amounts. The value for each CHC is the average value from nine females ± SEM. Statistically significant values compared with wild-type females are shown with *** = P ≤ 0.001.
In the gce2.5k null mutant females, the 7-C23 and -C25 monoenes were both increased relative to the CS controls, but the 7-C27 monoene was not significantly different (Fig. 5A). Although there was no difference between the CS control and the gce2.5k mutant in the female-specific 7,11-C27 diene, the 7,11-C29 diene in the mutant was reduced similarly to reductions seen in both the Met27 (Fig. 5B) and the CAX (Fig. 4B) females. Loss of function of gce had no significant effect on the amount of alkanes present (Fig. S4A). Of the methyl alkanes, only the 2MeC26 was reduced in the gce2.5k females (Fig. S4B), similarly to its decrease in the CAX females at 48 h (Fig. S3B). Importantly, these changes seen in the CHC blend in the absence of Gce apparently had little effect on their attractiveness to males as judged by the courtship assay (Fig. 3 and Fig. S2F).
Discussion
Here we have shown that JH plays a critical role in the normal timing of onset of female mating and sex pheromone production. Removal of JH through genetic ablation of the CA in the developing adult female delayed the onset of mating behaviors. This change was coupled to a decrease in male courtship, suggesting a decrease in female attractiveness. We found drastic changes in the CHC profiles in the CAX females. Some of these changes are likely due to the temperature shift regime (31) used for the genetic allatectomy. Treating CAX females with the JHM methoprene both advanced the onset of mating and increased the attractiveness of the females, apparently by increasing the production of C27 monoenes and dienes. Therefore, JH dynamically regulates the synthesis of specific CHCs, particularly the major female sex pheromones, the 7,11-C27 and 7,11-C29 dienes. These findings are consistent with previous studies showing induction of precocious mating by CA implants (6) and reduced mating in apterous mutants (10) and reduced female-specific pheromones in flies overexpressing DmP29 (19), both having reduced JH levels. Interestingly, the CAX females slowly became attractive so that by 96 h, time to onset of copulation was similar to controls. At this time the C27 monoene and the 7,11-C29 diene were still significantly reduced, whereas the C27 dienes were significantly increased (Fig. 4 A and B), suggesting that there was a change in the CHC blend. The C27 dienes at 96 h may act alone or together with other CHCs in the pheromone blend to increase female attractiveness and decrease time to copulation in the CAX females.
How does JH regulate CHC synthesis? CHCs are synthesized from fatty acids via elongation, desaturation, reduction to aldehyde, and oxidative decarbonylation reactions in oenocytes in D. melanogaster (reviewed in refs. 14, 32, and 33). The developmental appearance of the CHCs in CAX females in this study clearly shows decreases in the long-chain n-alkanes (C23-C27), the C25 and C27 monoenes, and the C27 and C29 dienes with corresponding increases in the shorter-chain dienes. Similar changes in diene profiles coupled with an increased time to copulation was seen after reduction of Elongase-F in female oenocytes (34). Reduction of Desaturase 1 in the oenocytes caused the loss of both monoenes and dienes with a large increase in the n-alkanes, whereas reduction of Desaturase-F caused a loss of the female-specific dienes with a doubling of monoenes and some increase in n-alkanes (35, 36). Both manipulations significantly increased time to mating. Thus, JH apparently influences biosynthetic enzymes important in the synthesis of the long-chain alkanes as well as Elongase-F and the desaturases. These regulatory effects of JH on pheromone synthesis are similar to its effects on aggregation pheromone synthesis in bark beetles. In these beetles, JH III regulates the transcription of many of the genes encoding the pheromone biosynthetic enzymes, especially the genes encoding geranyldiphosphate synthase/myrcene synthase (GPPS/MS), CYP9T2, and an oxidoreductase (32).
Application of 0.64 pmoles of the JHM methoprene just after eclosion rescued mating of the CAX females at 24 h after eclosion to the level of about 31% as seen in the parental controls, and a 10-fold higher dose caused about 58% to mate (Fig. S2A). After treatment with the higher dose of methoprene, the 7- and 9-C27 monoenes significantly increased at 24 h (Fig. 4C), but the two female-specific 7,11 dienes were not higher until 48 h (Fig. 4D). The increased C27 monoenes which have been implicated as aphrodisiacs (17) possibly made the CAX females more attractive at 24 h. Whether there is also an effect of the exogenous JH on the maturation of the female nervous system so that she becomes receptive to male courtship earlier is not known and warrants further study.
At least three hormones (JH, ecdysone, and pheromone biosynthesis-activating neuropeptide) have been shown to regulate sex pheromone biosynthesis in different insects (32). In this study, we have demonstrated that JH regulates sex pheromone 7,11-diene production in D. melanogaster females. Interestingly, in another dipteran, the house fly Musca domestica, the primary female sex pheromone is Z-9-tricosene (reviewed in ref. 32). Females ovariectomized immediately after eclosion do not synthesize this compound, but synthesis is restored by either ovarian implants or multiple injections of 20-hydroxyecdysone. These two families of flies are evolutionarily distant, but the basis for this difference in hormonal regulation is unknown.
The duplication of the JH receptor Gce occurred in the higher Diptera (37), and the two have partially redundant functions in the larval fat body of D. melanogaster and at metamorphosis (25). Met plays a distinct role in adult optic lobe development during metamorphosis, whereas Gce has no effect (24, 38). Met is also the predominant receptor required for the effects of JH on ovarian maturation, both the normal timing and normal fecundity (25). Our behavioral analysis of female mating and attractiveness of Met and gce mutants indicates that JH is also acting primarily via Met in its regulation of mating and pheromone production. Surprisingly, in courtship assays using CS males, Met27 females lacking Met were more attractive than the wild-type CS females, whereas the CAX females were less attractive, likely due to increased C27 dienes in Met27 females and decreased 7,11-C27 and -C29 dienes in the CAX females. These findings suggest that JH acts mainly via Met in mating and pheromone synthesis similarly to the roles of Met and Gce in ovarian maturation where lack of Met delays maturation and reduces fecundity, whereas lack of Gce has relatively little effect (25).
In this study, we demonstrated that JH acting through its receptor Met plays important roles in the initiation of sex pheromone production and the maturation of female mating behavior in D. melanogaster. Further investigation into these two aspects of JH action—in the peripheral tissues involved in sex pheromone production and in the neuronal circuitry underlying the mating behavior—is necessary to elucidate the details of its critical roles in modulation of the onset of mating. An understanding at the molecular level of this coordination in this Drosophila model should shed insights into how hormones regulate pheromone production and reproductive behavior in the vertebrates.
Experimental Procedures
Fly Lines and Husbandry.
Fly lines were grown on standard cornmeal molasses food in temperature-controlled incubators under constant light (LL) and 50% relative humidity (RH) unless otherwise stated. Aug21-GAL4 UAS-EGFP (Aug21-GAL4 >GFP) (24), UAS-DTI (39), tubP-GAL80ts #20 (GAL80ts) (40), Met alleles (Met27, MetW3), Met genomic rescue transgene EN71, and gce2.5k (25–27) were described previously. Both Met27 and MetW3 carry vermilion (v1). Wild-type Canton S (CS) flies were descendants of the Jeff Hall Canton S line.
Genetic Ablation of CA in Developing Females and JH Application.
Parental lines, w; GAL80ts; UAS-DTI and w; Aug21-Gal4 > egfp/CyO, were crossed to each other or w and kept at 18 °C in 12 h light: 12 h dark (12L:12D), then puparia were collected every 12 h. Sixty hours later, pupae were transferred to 29 °C in constant light (LL) to allow diphtheria toxin expression. Virgin females were collected within a 2-h window using CO2 and kept in groups of 12 in a food vial at 25 °C, LL until assayed. For JHM application, methoprene (Wellmark) was serially diluted in acetone (Fisher Scientific, HPLC grade), and 0.2 μL was applied to a female within 2 h of eclosion. For details and for imaging of the CA, see SI Experimental Procedures.
Behavior Assays and Analysis of Mating and Courtship Behaviors.
For the single pair-mating assay, a 3- to 4-d-old CS male and a virgin female (both previously housed separately in groups of 12) were placed into separate halves of the acrylic mating chamber (16 mm diameter, 11.5 mm deep) divided by a removable metal barrier. After 1 h the metal plate was removed and behavior was video-recorded with a Sony MiniDV Camcorder for 1 h at 25 °C as described previously (41). The percent mating and time to copulation of females were determined from the recording.
For the courtship assay, CO2-anesthesized females were decapitated as described previously (29) 1 h before the assay and housed for 20–30 min in a vial with a moist Kimwipe. Each decapitated female was then placed in the mating chamber described above with a single 3- to 4-d-old CS male. Behavior was video-recorded as above for 10 min. Each video was annotated for male courtship behaviors including orientation, chasing, wing extension, attempted copulation, or copulation using LifesongX. The amount of time the male spent performing these courtship behaviors was summed to calculate the courtship index, the fraction of time a male courts a female during the 10-min observation period.
All of the behavior assays were done in the afternoon between 13:00 and 18:00 under fluorescent lighting at 25 °C and 50% RH.
Cuticular Hydrocarbon Extraction and Analysis.
For CHC extracts, 3 females were taken from a group of 12 in a food vial and washed with 50 μL hexane containing the C18 and C26 internal standards as described (42). Three replicate vials were set up for each time point and each genotype. The extracts were run using GC-FID (Varian CP3800) and quantified as described (42).
Statistical Analysis for Behaviors and CHCs.
For details, see SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Wilson for the Met alleles and Met genomic rescue transgene; Dr. Jiang Wang for the gce2.5k mutant; Drs. Troy Shirangi and Jon-Michael Knapp for discussions; Dr. Eric Hoopfer for help with the behavior rig; Dr. Jonathan Schneider for helpful discussions on the statistics; and Drs. Carmen Robinett, Karla Kaun, Hans Kelstrup, Dave Mellert, and Lee Henry for critical comments on early drafts of the manuscript. J.B. and L.M.R. are funded by the Howard Hughes Medical Institute. J.D.L. is funded by grants from Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council (NSERC). J.A. is funded by a postgraduate scholarship from NSERC.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318119110/-/DCSupplemental.
References
- 1.Dulac C, Kimchi T. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr Opin Neurobiol. 2007;17(6):675–683. doi: 10.1016/j.conb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt G, Davey KG. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- 3.Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol 3. Amsterdam: Elsevier Pergamon; 2005. pp. 433–491. [Google Scholar]
- 4.Barth RH, Lester LJ. Neuro-hormonal control of sexual behavior in insects. Annu Rev Entomol. 1973;18:445–472. doi: 10.1146/annurev.en.18.010173.002305. [DOI] [PubMed] [Google Scholar]
- 5.Ringo J. Sexual receptivity in insects. Annu Rev Entomol. 1996;41:473–494. doi: 10.1146/annurev.en.41.010196.002353. [DOI] [PubMed] [Google Scholar]
- 6.Manning A. Corpus allatum and sexual receptivity in female Drosophila melanogaster. Nature. 1966;211(5055):1321–1322. doi: 10.1038/2111321b0. [DOI] [PubMed] [Google Scholar]
- 7.Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- 8.Bouletreau-Merle J. Receptivite sexuelle et vitellogenese chez les femelles de Drosophila melanogaster: Effets d’une application d’hormone juvenile et de deux analogues hormonaux. C. R. Acad Sci. Paris. 1973;277:2045–2048. [Google Scholar]
- 9.Ringo J, Werczberger R, Altaratz M, Segal D. Female sexual receptivity is defective in juvenile hormone-deficient mutants of the apterous gene of Drosophila melanogaster. Behav Genet. 1991;21(5):453–469. doi: 10.1007/BF01066724. [DOI] [PubMed] [Google Scholar]
- 10.Ringo J, Talyn B, Brannan M. Effects of precocene and low protein diet on reproductive behavior in Drosophila melanogaster (Diptera: Drosophilidea) Ann Entomol Soc Am. 2005;98(4):601–607. [Google Scholar]
- 11.Yamamoto D, Fujitani K, Usui K, Ito H, Nakano Y. From behavior to development: Genes for sexual behavior define the neuronal sexual switch in Drosophila. Mech Dev. 1998;73(2):135–146. doi: 10.1016/s0925-4773(98)00042-2. [DOI] [PubMed] [Google Scholar]
- 12.Dickson BJ. Wired for sex: The neurobiology of Drosophila mating decisions. Science. 2008;322(5903):904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- 13.Ferveur J-F. Drosophila female courtship and mating behaviors: Sensory signals, genes, neural structures and evolution. Curr Opin Neurobiol. 2010;20(6):764–769. doi: 10.1016/j.conb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Wicker-Thomas C, Chertemps T. Molecular biology and genetics of hydrocarbon production. In: Blomquist GJ, Bagnères A, editors. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge, UK: Cambridge Univ Press; 2010. pp. 53–74. [Google Scholar]
- 15.Ferveur J-F. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35(3):279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 16.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461(7266):987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 17.Marcillac F, Ferveur J-F. A set of female pheromones affects reproduction before, during and after mating in Drosophila. J Exp Biol. 2004;207(Pt 22):3927–3933. doi: 10.1242/jeb.01236. [DOI] [PubMed] [Google Scholar]
- 18.Arienti M, Antony C, Wicker-Thomas C, Delbecque JP, Jallon JM. Ontogeny of Drosophila melanogaster female sex-appeal and cuticular hydrocarbons. Integr Zool. 2010;5(3):272–282. doi: 10.1111/j.1749-4877.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Li X, Prasifka JR, Jurenka R, Bonning BC. Overexpression of Drosophila juvenile hormone esterase binding protein results in anti-JH effects and reduced pheromone abundance. Gen Comp Endocrinol. 2008;156(1):164–172. doi: 10.1016/j.ygcen.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 22.Baumann A, Barry J, Wang S, Fujiwara Y, Wilson TG. Paralogous genes involved in juvenile hormone action in Drosophila melanogaster. Genetics. 2010;185(4):1327–1336. doi: 10.1534/genetics.110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009;136(12):2015–2025. doi: 10.1242/dev.033712. [DOI] [PubMed] [Google Scholar]
- 24.Riddiford LM, Truman JW, Mirth CK, Shen YC. A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development. 2010;137(7):1117–1126. doi: 10.1242/dev.037218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdou MA, et al. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem Mol Biol. 2011;41(12):938–945. doi: 10.1016/j.ibmb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Wilson TG, Ashok M. Insecticide resistance resulting from an absence of target-site gene product. Proc Natl Acad Sci USA. 1998;95(24):14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson TG, Wang S, Beno M, Farkas R. Wide mutational spectrum of a gene involved in hormone action and insecticide resistance in Drosophila melanogaster. Mol Genet Genomics. 2006;276(3):294–303. doi: 10.1007/s00438-006-0138-4. [DOI] [PubMed] [Google Scholar]
- 28.Bastock M, Manning A. The courtship of Drosophila melanogaster. Behaviour. 1955;8:85–111. [Google Scholar]
- 29.Spieth HT. Drosophilid mating behavior: The behaviour of decapitated females. Anim Behav. 1966;14(2):226–235. doi: 10.1016/s0003-3472(66)80076-3. [DOI] [PubMed] [Google Scholar]
- 30.Siwicki KK, et al. The role of cuticular pheromones in courtship conditioning of Drosophila males. Learn Mem. 2005;12(6):636–645. doi: 10.1101/lm.85605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savarit F, Ferveur J-F. Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J Exp Biol. 2002;205(Pt 20):3241–3249. doi: 10.1242/jeb.205.20.3241. [DOI] [PubMed] [Google Scholar]
- 32.Blomquist GJ, Jurenka R, Schal C, Tittiger C. Pheromone production: Biochemistry and molecular biology. In: Gilbert LI, editor. Insect Endocrinology. London: Elsevier Academic; 2012. pp. 523–567. [Google Scholar]
- 33.Qiu Y, et al. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc Natl Acad Sci USA. 2012;109(37):14858–14863. doi: 10.1073/pnas.1208650109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chertemps T, et al. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 2007;104(11):4273–4278. doi: 10.1073/pnas.0608142104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chertemps T, Duportets L, Labeur C, Ueyama M, Wicker-Thomas C. A female-specific desaturase gene responsible for diene hydrocarbon biosynthesis and courtship behaviour in Drosophila melanogaster. Insect Mol Biol. 2006;15(4):465–473. doi: 10.1111/j.1365-2583.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 36.Wicker-Thomas C, Guenachi I, Keita YF. Contribution of oenocytes and pheromones to courtship behaviour in Drosophila. BMC Biochem. 2009;10:21. doi: 10.1186/1471-2091-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baumann A, Fujiwara Y, Wilson TG. Evolutionary divergence of the paralogs Methoprene tolerant (Met) and germ cell expressed (gce) within the genus Drosophila. J Insect Physiol. 2010;56(10):1445–1455. doi: 10.1016/j.jinsphys.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Riddiford LM. How does juvenile hormone control insect metamorphosis and reproduction? Gen Comp Endocrinol. 2012;179(3):477–484. doi: 10.1016/j.ygcen.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Berdnik D, Chihara T, Couto A, Luo L. Wiring stability of the adult Drosophila olfactory circuit after lesion. J Neurosci. 2006;26(13):3367–3376. doi: 10.1523/JNEUROSCI.4941-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 41.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6(4):297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krupp JJ, et al. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18(18):1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.