Abstract
Background: Epidemiologic evidence has shown a link between short sleep and obesity. Clinical studies suggest a role of increased energy intake in this relation, whereas the contributions of energy expenditure (EE) and substrate utilization are less clearly defined.
Objective: Our aim was to investigate the effects of sleep curtailment on 24-h EE and respiratory quotient (RQ) by using whole-room indirect calorimetry under fixed-meal conditions.
Design: Ten females aged 22–43 y with a BMI (in kg/m2) of 23.4–27.5 completed a randomized, crossover study. Participants were studied under short- (4 h/night) and habitual- (8 h/night) sleep conditions for 3 d, with a 4-wk washout period between visits. Standardized weight-maintenance meals were served at 0800, 1200, and 1900 with a snack at 1600. Measures included EE and RQ during the sleep episode on day 2 and continuously over 23 h on day 3.
Results: Short compared with habitual sleep resulted in significantly higher (±SEM) 24-h EE (1914.0 ± 62.4 compared with 1822.1 ± 43.8 kcal; P = 0.012). EE during the scheduled sleep episode (0100–0500 and 2300–0700 in short- and habitual-sleep conditions, respectively) and across the waking episode (0800–2300) were unaffected by sleep restriction. RQ was unaffected by sleep restriction.
Conclusions: Short compared with habitual sleep is associated with an increased 24-h EE of ∼92 kcal (∼5%)—lower than the increased energy intake observed in prior sleep-curtailment studies. This finding supports the hypothesis that short sleep may predispose to weight gain as a result of an increase in energy intake that is beyond the modest energy costs associated with prolonged nocturnal wakefulness. This trial was registered at clinicaltrials.gov as NCT01751581.
INTRODUCTION
Obesity has reached epidemic proportions, and recent estimates indicate that almost one-third of adults in the United States are obese (1). Body weight stability is achieved when energy intake is equal to energy expenditure (EE)5. Excess weight gain results from positive energy balance, which occurs when energy intake is increased relative to EE. Epidemiologic evidence has shown a cross-sectional association between short sleep and obesity (2), and laboratory-based intervention studies have helped define some of the mechanisms underlying the sleep-obesity relation (3). Specifically, researchers have observed an increase in food intake when sleep is restricted to ∼4 to 5 h/night compared with sleep of ∼8 to 10 h/night (4–7). Nevertheless, studies on the effects of short sleep on EE are scarce and inconsistent (8). An understanding of how short sleep affects EE in addition to intake is critical to establish sleep as a risk factor for obesity (9).
Prior studies using doubly labeled water to assess free-living EE observed no difference in total EE in short compared with habitual sleep (5, 6). Researchers also explored the relation between sleep and energy metabolism using whole-room indirect calorimetry (10–12). Jung et al (10) reported an increase in 24-h EE of ∼7% during a day of total sleep elimination compared with a baseline day, which supports a role of sleep in energy conservation. Recent studies by Klingenberg et al (11) and Markwald et al (12) observed a significant increase of ∼4 to 5% in 24-h EE measured by whole-room indirect calorimetry under short sleep [4 or 5 h time in bed (TIB)] compared with long sleep (9 h/night), mainly because of a rise in EE during the habitual nighttime period in the short-sleep condition. Measures of EE in the latter study, however, may have been influenced by increased ad libitum food intake under sleep-restriction conditions (12). The study by Klingenberg et al (11) was conducted exclusively in adolescent males (11) and may not be applicable to adults or females.
The aim of the current study was to investigate the effects of short sleep (4 h TIB) compared with habitual sleep (8 h TIB) on 24-h EE by using whole-room calorimetry in women. Because food intake was strictly controlled and matched during the experimental phases, we believe that the current report is among the first studies to clarify the consequences of short sleep per se on EE in women, who may be at a higher risk than men of both sleep disruption (13) and obesity (14).
SUBJECTS AND METHODS
Participants
Twelve females aged 21–45 y with a BMI (in kg/m2) of 22 to 29.9 were enrolled in the study. Before entry, a history of habitual sleep duration between 7 and 9 h/night was confirmed with wrist-worn actigraphy (Actiwatch; Actilife LLC) and a sleep diary for 2 wk. Inclusion criteria required a mean sleep duration during the 2-wk screening period of 7 to 9 h/night, with ≥10 nights of sleep with a duration ≥7 h and <4 nights of sleep with a duration <6 h. At the initial screening meeting, participants were asked to answer the questions “During the past month, when have you usually gone to bed?” and “During the past month, when have you usually gotten up in the morning?” Chronotype was assessed with the Horne-Ostberg Morningness-Eveningness Questionnaire (15), and individuals with extreme morning or evening chronotypes (defined as scores ≥70 and ≤30, respectively) were excluded from the study.
Exclusion criteria included smoking, type 2 diabetes by medical history, history of alcohol or substance abuse, caffeine intake >300 mg/d, shift work, trans-meridian travel within the past 4 wk, use of antidepressant medications, and the presence of any eating, sleeping, or neurological disorder. Participants were evaluated for the presence of eating, sleeping, and neurological disorders by questionnaires and medical history. Sleep disorders were further assessed by having participants fill out the Epworth Sleepiness Scale (16), the Pittsburgh Sleep Quality Index (17), and the Berlin Questionnaire for sleep apnea (18). Inclusion criteria required Epworth Sleepiness Scale scores <10, global Pittsburgh Sleep Quality Index scores <5, and being low risk of having sleep apnea based on the Berlin Questionnaire.
The habitual level of physical activity in participants before entry in the study was assessed by analyzing the wrist-actigraphy data from the 2-wk preexperimental screening period. All participants were either sedentary [1–1.5 metabolic equivalents (METs)] or had light (1.5–3 METs) physical activity levels (range: 1.07–1.65 METs). The mean (±SEM) MET rate was 1.46 ± 0.06. Participants with sedentary/light habitual physical activity levels are desirable for inclusion in an experiment such as this because the whole-room calorimeter necessarily restricts daily physical activity.
The study was approved by St Luke's–Roosevelt Institutional Review Board, and all participants provided written informed consent before enrollment. Participants were given the opportunity to ask questions about the protocol before providing informed consent.
Experimental design
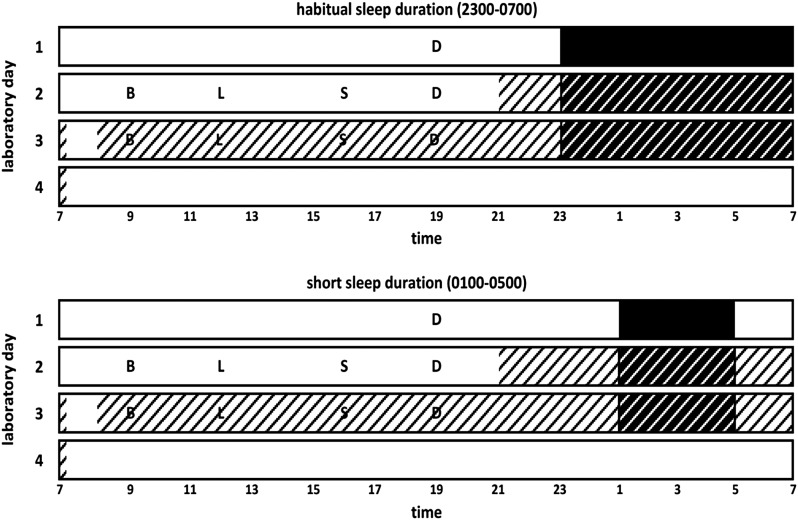
This was a laboratory-based randomized crossover study composed of 2 phases, including short- and habitual-sleep conditions (Figure 1). Each experimental phase lasted 4 d. Phases were held ∼4 wk apart to ensure recuperation from sleep deprivation and that women were studied during the same menstrual phase. Day of the menstrual cycle at laboratory entry for phase 1 was documented, and entry for the second visit occurred on the same day relative to menstruation of the subsequent menstrual cycle. Experiments took place at St Luke's–Roosevelt Hospital outpatient Clinical Research Resource of the Irving Institute for Clinical and Translational Research and of the New York Obesity Nutrition Research Center. During short sleep, participants had a 4 h/night sleep opportunity (0100–0500 h). During habitual sleep, participants had an 8 h/night sleep opportunity (2300–0700 h). Participants were fed a controlled diet based on their weight-maintenance energy requirements, estimated by the Harris-Benedict equation with an activity factor of 1.5. Meals were prepared and provided by the Bionutrition Unit at the Irving Institute for Clinical and Translational Research at Columbia University. Diets provided 30% of energy from fat, 55% from carbohydrates, and 15% from protein. Meals provided 30% of daily energy requirements, and the snack provided the remaining 10%. Meal times were fixed, with breakfast at 0900, lunch at 1200, snack at 1600, and dinner at 1900. Participants were instructed to eat all of the food presented.
FIGURE 1.
Illustration of laboratory procedures. Participants entered the laboratory for a 4-d inpatient stay in a randomized crossover design. Experimental sleep conditions included habitual sleep duration (8 h/night sleep opportunity, from 2300 to 0700) and short sleep duration (4 h/night sleep opportunity, from 0100 to 0500). Solid black bars represent acclimatization sleep episodes occurring in the metabolic chamber without the door sealed. White hatched bars represent time spent awake inside the metabolic chamber. Black hatched bars represent time spent in bed inside the metabolic chamber. B, breakfast; D, dinner; L, lunch; S, snack.
Participants were asked to abstain from alcohol and caffeine for 24 h before the study and were instructed not to eat past 1500 on the day of entry. Participants entered the Clinical Research Resource at ∼1800 on day 1. Dinner was served at 1900, and the participants remained on site until bedtime (2300 or 0100). Sleep occurred in the whole-room calorimeter (metabolic chamber); however, during this acclimatization night, the door was not sealed and no recordings were made. Participants were awakened at either 0700 or 0500 the following morning and, on day 2, were permitted to leave the laboratory under study personnel supervision. Strenuous physical activity or exercise was not permitted, and standard meals were served at designated times. At ∼2100 on day 2, the door of the metabolic chamber was sealed, and recordings of oxygen consumption and carbon dioxide production were taken until ∼0715 h the next morning. On exiting the metabolic chamber on the morning of day 3, participants were allowed to shower before reentering at ∼0800 h for a 23-h continuous assessment of EE and respiratory quotient (RQ) (Figure 1). Daytime naps were prohibited. Study personnel ensured that participants remained awake during the duration of scheduled wakefulness by regular visual inspection through the chamber window and phone calls. Sleep duration was objectively monitored with the use of wrist-actigraphy worn during the sleep episodes while participants were in the laboratory.
Measures
Measures of EE and RQ during the sleep episode on day 2, and continuously throughout 23 h on day 3, including the day 3 sleep episode, were conducted in the chamber (Figure 1). At 1500 and 2030 h, participants performed 15 min of cycling, at 50 W, on a stationary bicycle inside the chamber. These bicycle riding sessions were included to induce some light physical activity within the confines of the calorimeter room.
The metabolic chamber at the New York Obesity Nutrition Research Center is an airtight, climate-controlled room equipped with a futon, chair, desk, television, telephone, stationary bike, toilet, and sink. The dimensions are 330 × 279 × 241 cm with an internal volume (corrected for the presence of furniture and restroom fixtures) of 21,399 L. Temperature within the whole-room indirect calorimeter chamber was maintained at 24 ± 0.5 °C unless adjusted to maintain participant comfort.
Oxygen and carbon dioxide concentrations, along with air mass flow, were measured 1×/s with the Promethion (model GA-6 and FG-1; Sable Systems) Whole-Room Indirect Calorimeter system. This is accomplished by flowing 80 L/min of fresh air through the whole-room indirect calorimeter and obtaining an air sample on the exhaust side of the system. All oxygen, carbon dioxide, and air mass flow data were corrected for the presence of water vapor pressure in kilopascals to standard-pressure dry conditions before metabolic calculations as described previously (19).
All corrected metabolic data from the instrument were amortized per minute and then EE was calculated by using the Weir equation (20). RQ was calculated as the ratio of the corrected ventilation rates of carbon dioxide production to oxygen consumption. This is an indicator of nutrient utilization (21). Physiologically normal RQ values range from 0.70, indicating mainly fat oxidation, to 1.00, indicating carbohydrate oxidation.
The accuracy and precision of the whole-room indirect calorimeter were determined through propane gas combustion. Instrument-grade propane (99.5% purity) expends 11.92 kcal/g heat energy with an RQ of 0.60. A known amount of instrument-grade propane is combusted for the same duration as that of a normal subject metabolic test. This is 23 h for the whole-room indirect calorimeter chamber. The theoretical and actual values from the propane combustion test are subtracted from one another and expressed as a delta percentage. The instrumentation is considered accurate and precise if it repeatedly measures EE and RQ of instrument-grade propane to 98–100% of the theoretical value and to 0.58–0.62, respectively.
Statistical analyses
Paired-samples t tests were used to compare daily EE (24-h EE) and mean EE and RQ during sleep between short and habitual sleep. Two-way within-subjects ANOVAs for repeated measures (factors: sleep condition and time) were used to compare repeated measures of EE and RQ throughout the night (2300–0700), sleeping episode (0100–0500 or 2300–0700), and the full 23-h recording period between short- and habitual-sleep conditions. A 3-way within-subjects ANOVA for repeated measures (factors: sleep condition, exercise session, and time) was used to compare EE and RQ throughout the 15-min bicycling sessions in the afternoon (1500–1515) and evening (2030–2045) between short- and habitual-sleep conditions. Data were analyzed with the statistical software program DATASIM (version 1.1; Drake Bradley) (22). Data are expressed as means ± SEMs.
RESULTS
Participant characteristics
A total of 12 women were enrolled in this study, and 10 completed the study. The 2 women who did not complete the study failed to return for phase 2 for unknown reasons or because of a scheduling conflict. The 2 women underwent different sleep phases for phase 1; their failure to complete was unlikely to be a result of the nature of the study. The mean age and BMI for study completers were 28.0 ± 2.3 y (range: 22–43 y) and 26.0 ± 0.47 (range: 23.4–27.5), respectively. Five women were black and 5 were white. Mean weight and height were 69.9 ± 1.8 kg (range: 59.87–78.02 kg) and 1.64 ± 0.02 m (range: 1.56–1.69 m), respectively. Five participants underwent habitual-sleep duration first, whereas the other 5 participants underwent short-sleep duration in phase 1.
The mean morningness-eveningness score on the chronotype questionnaire was 50.4 ± 3.04 (“intermediate type”). Six participants were intermediate type, 2 participants were moderate evening type, and 2 participants were moderate morning types. On the basis of questionnaires administered during the screening session, the mean habitual sleep-wake schedule was 2348–0801. Throughout the 2-wk prelaboratory ambulatory period, average sleep duration as determined by wrist-actigraphy was 430.4 ± 6.6 min (7 h 10 ± 6.6 min). During the same 2-wk ambulatory period, the mean sleep-wake schedule was 2412–0825.
Because of technical issues, overnight actigraphy data were lost for one participant during the habitual sleep phase and for 3 participants during the short-sleep phase during the in-laboratory metabolic chamber recording period (no participant had both sleep phases missing). After the data for night 1 were removed to reduce any first-night effects, the mean sleep duration of nights 2 and 3 during the habitual sleep condition was 441.3 ± 5.1 min (7 h and 21 ± 5.1 min), and the mean sleep duration of nights 2 and 3 during the short-sleep condition was 223.9 ± 2.2 min (3 h and 44 ± 2.2 min). The mean sleep onset latencies were 7.7 ± 1.2 and 5.8 ± 0.9 min for habitual and short sleep, respectively. Mean sleep efficiencies were 91.9 ± 1.1% and 93.3 ± 0.9% for habitual and short sleep, respectively.
24-h EE and RQ
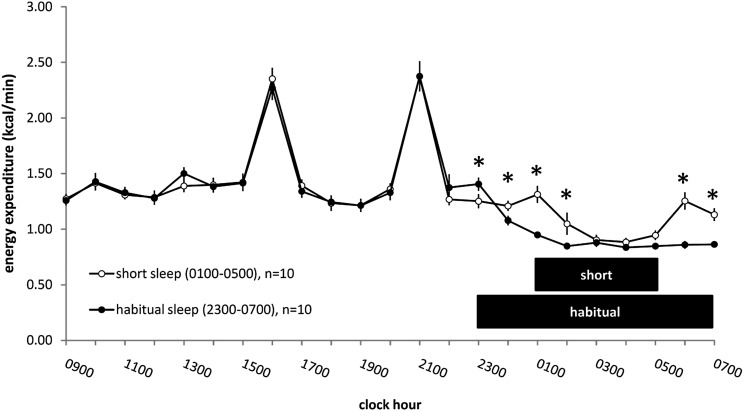
Total 24-h EE extrapolated from the 23-h metabolic chamber recording period on laboratory day 3 was 1914.0 ± 62.4 kcal for short sleep and 1822.1 ± 43.8 kcal for habitual sleep (t9 = 3.14, P = 0.012) (Table 1). This corresponds to an increased EE of ∼5% in short sleep compared with habitual sleep. The mean EE over the 23-h chamber recording period was 1.33 ± 0.04 kcal/min for short sleep and 1.27 ± 0.03 kcal/min for habitual sleep (t9 = 3.14, P = 0.012) (Table 1). When EE was examined 1×/h throughout the 23-h chamber recording period, a significant sleep condition × time interaction was seen (F22,198 = 4.51, P < 0.001) (Figure 2). The mean RQ over the full 23-h metabolic chamber recording was 0.89 ± 0.01 in short sleep and 0.88 ± 0.01 in habitual sleep (t9 = 0.09, P = 0.93) (Table 1).
TABLE 1.
The effects of habitual- (8 h/night; 2300–0700) and short- (4 h/night; 0100–0500) sleep durations on EE and RQ measured in a metabolic chamber1
| Habitual sleep | Short sleep | P value2 | |
| EE | |||
| Total 24-h (kcal/d) | 1822 ± 44 | 1914 ± 62 | 0.012 |
| Mean daily (kcal/min) | 1.27 ± 0.03 | 1.33 ± 0.04 | 0.012 |
| Nocturnal, night 3 (kcal/min) | 0.89 ± 0.02 | 1.09 ± 0.05 | 0.0009 |
| Daytime, day 3 (kcal/min) | 1.46 ± 0.04 | 1.46 ± 0.05 | 0.87 |
| Sleeping, night 3 (kcal/min) | 0.89 ± 0.02 | 0.94 ± 0.05 | 0.22 |
| RQ | |||
| Mean daily | 0.88 ± 0.01 | 0.89 ± 0.01 | 0.93 |
| Nocturnal, night 3 | 0.87 ± 0.01 | 0.87 ± 0.02 | 0.75 |
| Daytime, day 3 | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.98 |
| Sleeping, night 3 | 0.87 ± 0.01 | 0.87 ± 0.02 | 0.95 |
All values are means ± SEMs; n = 10. Total 24-h EE, total 23-h EE extrapolated to 24 h; mean daily, mean value throughout the 23-h recording period; nocturnal, mean value throughout times spanning the habitual sleep episode from 2300 to 0700; daytime, mean value throughout times spanning the habitual wake episode from 0800 to 2300; sleeping, mean value from 0100 to 0500 in short sleep and from 2300 to 0700 in habitual sleep. EE, energy expenditure; RQ, respiratory quotient.
Derived from paired-samples t tests.
FIGURE 2.
Mean (± SEM) energy expenditure throughout the 23-h metabolic chamber in the laboratory on day 3 during the short- and habitual-sleep duration conditions. Two-way within-subjects ANOVAs for repeated measures (factors: sleep condition and time) showed a significant sleep condition × time interaction (P < 0.001). *P < 0.05 (simple main-effects tests). Open circles represent the short-sleep condition (0100–0500), and filled circles represent the habitual-sleep condition (2300–0700). Values on the x axis are for the preceding hour. Black bars represent the sleep opportunity.
EE and RQ during the sleep/wake episode
In the short-sleep condition, the mean EE during the times spanning the habitual-sleep episode (ie, 2300–0700) was 1.09 ± 0.05 kcal/min on day 3. This was significantly higher (t9 = 4.89, P = 0.0009) than the mean EE throughout this time in the habitual-sleep condition (0.89 ± 0.02 kcal/min) (Table 1). The mean EE during times spanning the wake episode (ie, 0800–2300) on day 3 was 1.46 ± 0.05 kcal/min in the short-sleep condition and 1.46 ± 0.04 kcal/min in the habitual-sleep condition (t9 = −0.16, P = 0.87) (Table 1).
When examined 1×/h throughout the 8-h period spanning the habitual sleep opportunity, a significant sleep condition × time interaction was seen for EE (F7,63 = 8.16, P < 0.001). During short sleep, this measurement included 4 h of sleep and 4 h of nonsleep time. EE in short sleep was significantly higher than habitual sleep mainly during nonsleep times: at 0000–0100, 0100–0200, 0500–0600, and 0600–0700 (P < 0.01, simple main-effects tests).
Mean EE restricted to times spanning the scheduled sleep opportunity (ie, sleeping metabolic rate from 0100 to 0500 and from 2300 to 0700 in the short- and habitual-sleep conditions, respectively) was not significantly different between the short- and habitual-sleep conditions on day 3 (short: 0.94 ± 0.05 kcal/min compared with habitual: 0.89 ± 0.02 kcal/min; t9 = 1.33, P = 0.22) (Table 1).
Similarly, RQ during the sleep episode on laboratory day 3 was not significantly different between the short- and habitual-sleep conditions. On day 3, the mean RQ throughout the times spanning the habitual sleep episode (ie, 2300–0700) was 0.87 ± 0.02 during short sleep and 0.87 ± 0.01 during habitual sleep (t9 = 0.33, P = 0.75) (Table 1). The mean RQ throughout times spanning the habitual wake episode on day 3 was 0.89 ± 0.01 in the short-sleep condition and 0.89 ± 0.01 in the habitual-sleep condition (t9 = −0.03, P = 0.98) (Table 1).
When examined at hourly intervals throughout the 8-h period spanning the nocturnal time period (ie, the habitual sleep opportunity from 2300 to 0700), a significant main effect of time was seen for RQ; values declined significantly across the 2300–0700 time period (day 3: F7,63 = 120.81, P < 0.001), but no effect of sleep was observed (day 3: F1,9 = 0.11, P = 0.75). The mean RQ restricted to times only spanning the scheduled sleep opportunity (ie, 0100–0500 and 2300–0700 in the short- and habitual-sleep conditions, respectively) was 0.87 ± 0.02 during the short- and 0.87 ± 0.01 during the habitual-sleep (t9 = 0.07, P = 0.95) conditions on day 3 (Table 1).
EE and RQ during exercise
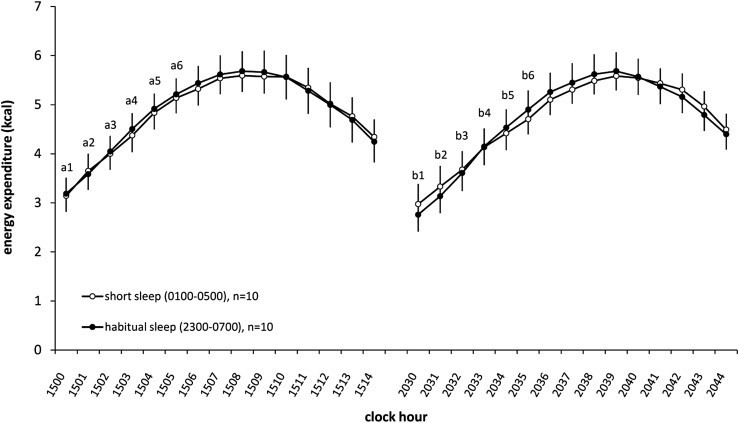
A significant exercise session × time interaction (F14,126 = 3.80, P < 0.001) was found on EE measured during the two 15-min bicycle sessions, with no effect due to sleep condition (F1,9 = 0.01, P = 0.94). Specifically, EE during minutes 1–6 was greater during the afternoon bicycling session (1500–1515) than during the corresponding minutes in the evening bicycling session (2030–2045) (Figure 3). A significant main effect of time (F14,126 = 8.01, P < 0.001) on RQ was found during the 15-min bicycle sessions, with values increasing throughout the exercise session, although no significant effects were seen for exercise session (afternoon compared with evening: F1,9 = 1.80, P = 0.21) or sleep condition (F1,9 = 0.20, P = 0.66).
FIGURE 3.
Mean (±SEM) energy expenditure during exercise sessions on laboratory day 3 during the short- and habitual-sleep duration conditions. Three-way within-subjects ANOVA for repeated measures (factors: sleep condition, exercise session, and time) showed a significant session × time interaction (P < 0.0001), with increased EE in the afternoon bicycling session (1500–1515) compared with the evening bicycling session (2030–2045) at minutes 1–6 of the 15-min exercise session. Differences between sessions at given time points are indicated by lowercase letters: a1 > b1, a2 > b2, a3 > b3, a4 > b4, a5 > b5, and a6 > b6 (P ≤ 0.05; simple main-effects tests). Open circles represent the short-sleep condition (0100–0500), and filled circles represent the habitual-sleep condition (2300–0700). EE, energy expenditure.
DISCUSSION
We investigated the effects of short sleep on 24-h EE in women under controlled feeding conditions with whole-room calorimetry. Two nights of sleep curtailment was associated with a significant increase in 24-h EE. This was mainly because of an increase in EE during nocturnal hours spent awake. In addition, mean EE during the daytime was not affected, which indicated that restricting sleep does not alter EE during normal waking hours. Finally, substrate utilization was unaffected by experimental sleep restriction under fixed-meal conditions.
Whereas prior sleep curtailment studies using doubly labeled water to assess total EE failed to detect differences between short and habitual sleep (5, 6, 23), whole-room calorimetry showed an increase in 24-h EE after sleep restriction (11, 12). Consistent with current findings, an increase of ∼4% to 5% in daily EE was observed after short compared with habitual sleep (11, 12). This corresponds to an increased EE of ∼88 kcal/d when measured under fixed-meal conditions (11) and of ∼111 kcal/d when measured under ad libitum eating conditions (12). It is important to point out that ad libitum eating conditions during measures of EE under short- and habitual-sleep conditions in the latter study could have affected their results. Assuming a theoretical thermic effect of food of 10%, the additional energy consumed during restricted relative to habitual sleep in the study by Markwald et al (12) (∼181.8 kcal) would account for ∼18 kcal of the difference in EE. This would result in an increase in EE as a result of sleep restriction of ∼93 kcal, very similar to the data reported here (92 kcal) and elsewhere (11). These increases in EE were mainly the result of the additional hours spent awake. Similar to what was reported in adolescent males (11), we observed no difference in sleeping metabolic rate between short- and habitual-sleep conditions in adult women. These findings lend support to the hypothesis that a central physiologic role of sleep in humans is the conservation of energy (10). The increase in EE of ∼90 kcal when sleep is restricted to 4–5 h/night, reported here and in the aforementioned studies (11, 12), should be considered from a research design standpoint for future studies. Specifically, energy balance state may modulate the effects of sleep curtailment, particularly because it affects hormonal and metabolic outcomes (3). Thus, researchers should factor in the additional energy costs of extended wakefulness if interested in assessing the effects of sleep curtailment under energy-balance conditions.
The metabolic costs of sleep reduction (∼92 kcal in the current study) appear to trigger a set of compensatory neuroendocrine adaptations aimed at increasing hunger, reducing satiety, and stimulating food intake (9). Increased food intake of ∼300 to 415 kcal/d was observed after restricted compared with habitual sleep (5, 6, 24). This suggests that, despite increased daily EE, sleep restriction can result in a net positive energy balance of ∼200 to 400 kcal/d. Our findings, and those of others, suggest that the high prevalence of obesity in short sleepers is attributable to greater food intake that is not offset by the small increase in EE associated with greater time awake.
Because sleep restriction has been shown to increase insulin (25) and decrease leptin (26), which would favor fat deposition over fat oxidation, it was postulated that short sleep may lead to weight gain via alterations in substrate oxidation. Moreover, 24-h RQ is positively associated with weight gain (27). Because early studies found reduced leptin after sleep curtailment (26), we expected a rise in RQ with short sleep. However, our recent findings do not support reduced leptin, at least in the context of mild negative energy balance, after restricted sleep (28). Consistent with this, sleep curtailment in the current study did not affect RQ. This is similar to what was observed by Jung et al (10) and Klingenberg et al (11), although differences in the latter were statistically significant. They suggested that this was an artifact of prolonged wakefulness in the short-sleep condition (11). Available data do not support the notion that short sleep alters substrate use in such a way as to favor weight gain (eg, a shift to higher carbohydrate oxidation/less fat oxidation and greater fat retention).
We also considered EE in response to light physical activity and observed no difference in EE during stationary bicycle riding between sleep conditions. Interestingly, however, higher EE was observed during equally intense physical exertion during the afternoon session (1500–1530) than during the evening session (2030–2045), which may imply a diurnal variation. Although the current study was not designed to test this, there is evidence that exercise performance is influenced by the circadian system (29). Furthermore, it would be interesting to determine whether short sleepers delay their exercise or physical activity. Individuals with delayed sleep timing have shorter sleep duration and also consumed more calories after 2000 and more fast food and sugar beverages compared with those with normal sleep timing and longer sleep duration (30). Thus, important relations may exist between short sleep, delayed behavioral rhythms, and energy balance, which should be pursued. Others have reported that short sleepers are more sedentary than individuals sleeping ≥8 h/d (31). Reduced physical activity would further offset the modest increases in EE associated with sleep curtailment and accentuate the positive energy balance. Other behavioral changes in response to sleep restriction, such as napping, could also affect energy balance under real-life conditions. Whereas participants in the current study were not habitual nappers, naps have the potential to induce further decreases in daily physical activity and EE. Conversely, napping, either by dissipating sleepiness or by limiting eating opportunities, could result in decreased energy intake.
This was the first study that investigated the effects of sleep restriction on 24-h EE measured with whole-room indirect calorimetry that enrolled only women. The menstrual cycle can affect sleep (32) and EE (33, 34). We did not study all women during the same menstrual phase and did not measure sex hormones. This may be relevant because some have shown increases in 24-h EE during the luteal phase compared with the follicular phase (33, 34). However, to account for intraindividual variations in menstrual cycle–related EE, we tracked participants’ menstrual cycles throughout the experiment, and testing occurred on the same postmenstruation day. A strength was that EE assessments were made under controlled feeding conditions in both sleep phases. Thus, our EE data were not confounded by differences in food intake between sleep duration conditions.
In the attempt to define how short sleep duration can cause weight gain, our intervention study has determined that experimental sleep curtailment causes a moderate wake-dependent increase in 24-h EE. Although we did not concurrently assess sleepiness, energy intake, or its associated variables—including hunger/appetite ratings or appetite-regulating hormones—and other variables of energy balance likely to be affected by sleep curtailment, including physical activity, there is accumulating research by others showing increased food intake during similarly designed sleep-curtailment studies. Although not negligible, the difference in EE between short and habitual sleep observed here does not offset the consistently reported greater energy intakes associated with sleep restriction. Whereas the current report did not assess food intake or physical activity, and therefore cannot speculate on the effect of these aspects on energy balance, the findings described here suggest that altered energy metabolism is not a contributing factor in the relation between short sleep and obesity. Together, this lends support to the hypothesis that short sleep duration leads to weight gain because of an excessive increase in energy intake that is beyond the modest energy costs associated with prolonged nocturnal wakefulness. Indeed, potential other overlooked effects of sleep restriction, namely on physical activity, would further accentuate the positive energy balance induced by the rise in energy intake, forming the basis for an increased risk of obesity in short sleepers.
Acknowledgments
Special thanks to Majella O'Keeffe, Scott Wolfe, and the staff of the Bionutrition Unit at the Irving Institute for Clinical and Translational Research for their help in conducting the study and also to our research participants.
The authors’ responsibilities were as follows—M-PS and JBA: designed the research; AS and RR: conducted the research; AS, RR, JBA, and M-PS: collected the data; AS, RR, and M-PS: analyzed the data; AS and M-PS: wrote the manuscript; and M-PS: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors had any conflicts of interest to disclose.
Footnotes
Abbreviations used: EE, energy expenditure; MET, metabolic equivalent; RQ, respiratory quotient; TIB, time in bed.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med 2013;9:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 5.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M. RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingenberg L, Sjodin A, Holmback U, Astrup A, Chaput JP. Short sleep duration and its association with energy metabolism. Obes Rev 2012;13:565–77. [DOI] [PubMed] [Google Scholar]
- 9.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab 2012;97:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 2011;589:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr 2012;96:240–8. [DOI] [PubMed] [Google Scholar]
- 12.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares CN. Insomnia in women: an overlooked epidemic? Arch Women Ment Health 2005;8:205–13. [DOI] [PubMed] [Google Scholar]
- 14.Paeratakul S, White MA, Williamson DA, Ryan DH, Bray GA. Sex, race/ethnicity, socioeconomic status, and BMI in relation to self-perception of overweight. Obes Res 2002;10:345–50. [DOI] [PubMed] [Google Scholar]
- 15.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 1976;4:97–110. [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk of the sleep apnea syndrome. Ann Intern Med 1999;131:485–91. [DOI] [PubMed] [Google Scholar]
- 19.Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JR. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol 2010;298:R1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusk G. The elements of the science of nutrition. Philadelphia, PA: WB Saunders Company, 1928. [Google Scholar]
- 22.Bradley DL. Computer simulation with DATASIM. Behav Res Methods Instrum Comput 1989;21:99–112. [Google Scholar]
- 23.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med 2010;153:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008;1:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 27.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–7. [DOI] [PubMed] [Google Scholar]
- 28.St-Onge MP, O'Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 2012;35:1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly T, Waterhouse J. Sports performance: is there evidence that the body clock plays a role? Eur J Appl Physiol 2009;106:321–32. [DOI] [PubMed] [Google Scholar]
- 30.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 31.Garaulet M, Ortega FB, Ruiz JR, Rey-Lopez JP, Beghin L, Manios Y, Cuenca-Garcia M, Plada M, Diethelm K, Kafatos A, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond) 2011;35:1308–17. [DOI] [PubMed] [Google Scholar]
- 32.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep 2010;33:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb P. 24-hour energy expenditure and the menstrual cycle. Am J Clin Nutr 1986;44:614–9. [DOI] [PubMed] [Google Scholar]
- 34.Piers LS, Diggavi SN, Rijskamp J, van Raaij JM, Shetty PS, Hautvast JG. Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr 1995;61:296–302. [DOI] [PubMed] [Google Scholar]