Abstract
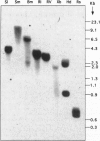
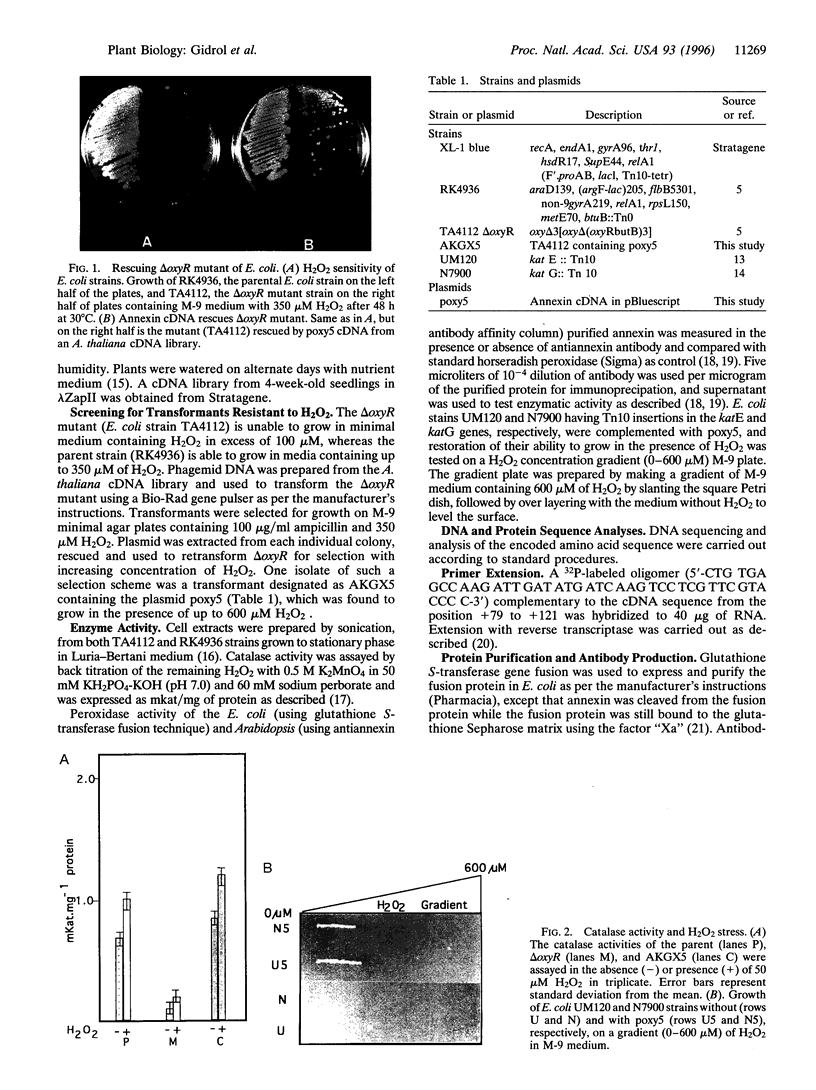
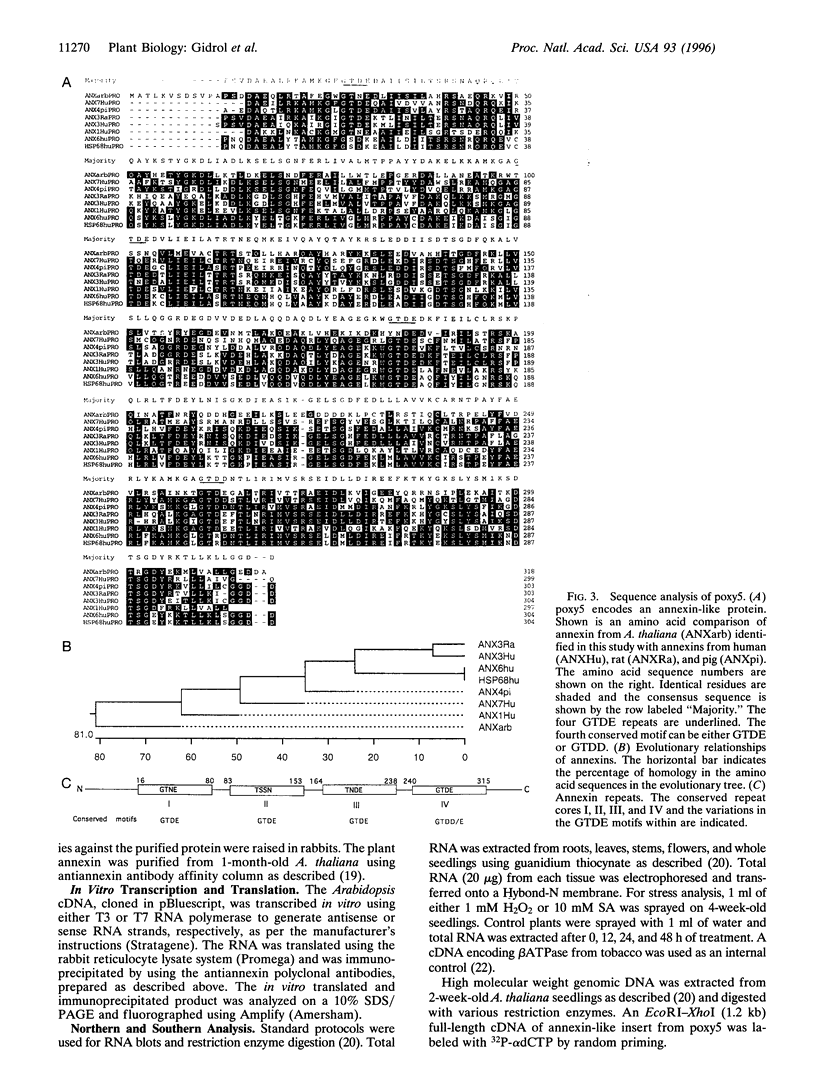
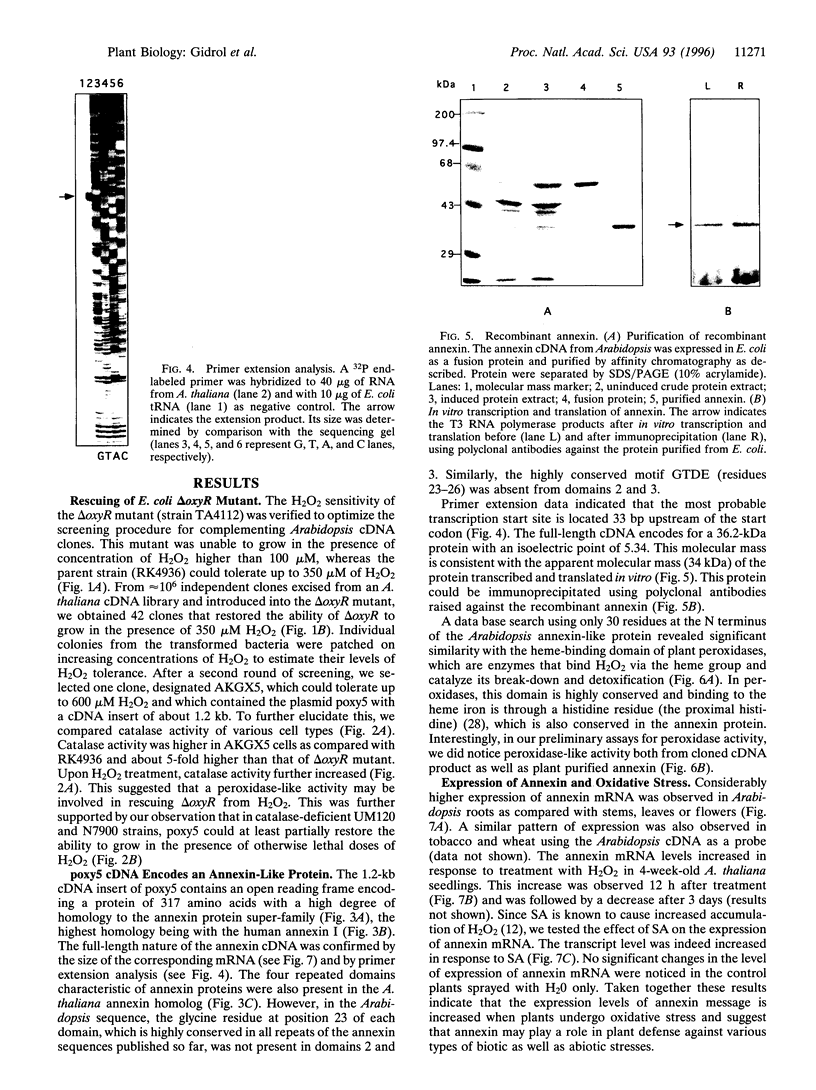
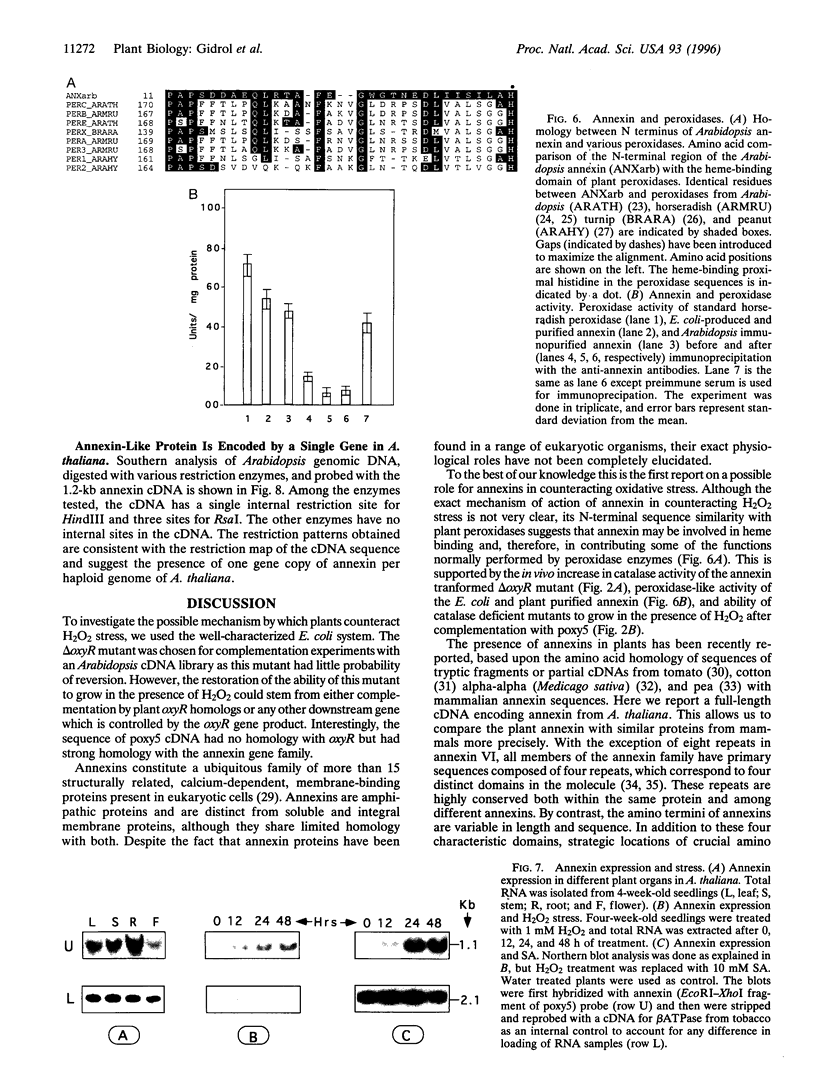
Reactive oxygen species are common causes of cellular damages in all aerobic organisms. In Escherichia coli, the oxyR gene product is a positive regulator of the oxyR regulon that is induced in response to H2O2 stress. To identify genes involved in counteracting oxidative stress in plants, we transformed a delta oxyR mutant of E. coli with an Arabidopsis thaliana cDNA library and selected for clones that restored the ability of the delta oxyR mutant to grow in the presence of H2O2. Using this approach, we isolated a cDNA that has strong homology with the annexin super-gene family. The complemented mutant showed higher catalase activity. mRNA expression of the annexin gene in A. thaliana was higher in roots as compared with other organs and was also increased when the plants were exposed to H2O2 stress or salicylic acid. Based on the results presented in this study, we propose a novel physiological role for annexin in counteracting H2O2 stress.
Full text
PDF
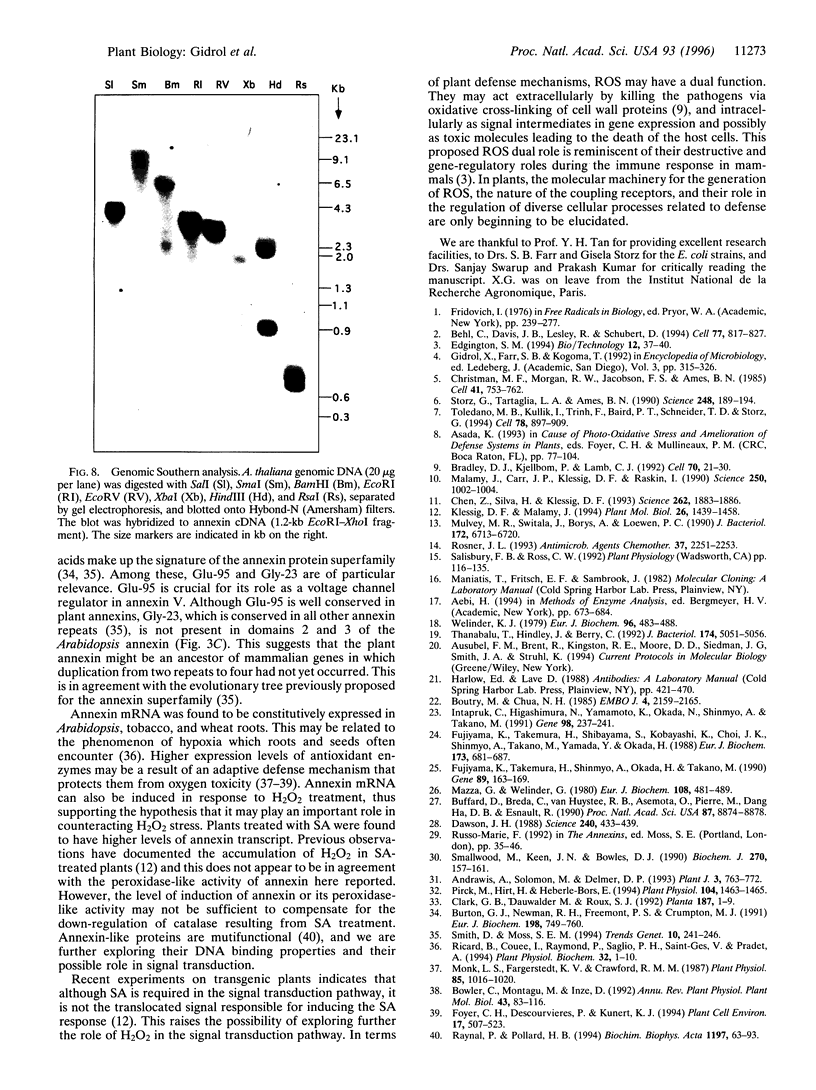
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrawis A., Solomon M., Delmer D. P. Cotton fiber annexins: a potential role in the regulation of callose synthase. Plant J. 1993 Jun;3(6):763–772. doi: 10.1111/j.1365-313x.1993.00763.x. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Newman R. H., Freemont P. S., Crumpton M. J. Amino acid sequence analysis of the annexin super-gene family of proteins. Eur J Biochem. 1991 Jun 15;198(3):749–760. doi: 10.1111/j.1432-1033.1991.tb16076.x. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. J., Kjellbom P., Lamb C. J. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992 Jul 10;70(1):21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Buffard D., Breda C., van Huystee R. B., Asemota O., Pierre M., Ha D. B., Esnault R. Molecular cloning of complementary DNAs encoding two cationic peroxidases from cultivated peanut cells. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8874–8878. doi: 10.1073/pnas.87.22.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Silva H., Klessig D. F. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993 Dec 17;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Dawson J. H. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science. 1988 Apr 22;240(4851):433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- Edgington S. M. As we live and breathe: free radicals and aging. Correlative evidence from a number of fields suggests they may be key. Biotechnology (N Y) 1994 Jan;12(1):37–40. doi: 10.1038/nbt0194-37. [DOI] [PubMed] [Google Scholar]
- Fujiyama K., Takemura H., Shibayama S., Kobayashi K., Choi J. K., Shinmyo A., Takano M., Yamada Y., Okada H. Structure of the horseradish peroxidase isozyme C genes. Eur J Biochem. 1988 May 2;173(3):681–687. doi: 10.1111/j.1432-1033.1988.tb14052.x. [DOI] [PubMed] [Google Scholar]
- Fujiyama K., Takemura H., Shinmyo A., Okada H., Takano M. Genomic DNA structure of two new horseradish-peroxidase-encoding genes. Gene. 1990 May 14;89(2):163–169. doi: 10.1016/0378-1119(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Intapruk C., Higashimura N., Yamamoto K., Okada N., Shinmyo A., Takano M. Nucleotide sequences of two genomic DNAs encoding peroxidase of Arabidopsis thaliana. Gene. 1991 Feb 15;98(2):237–241. doi: 10.1016/0378-1119(91)90179-f. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994 Dec;26(5):1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Mazza G., Welinder K. G. Covalent structure of turnip peroxidase 7. Cyanogen bromide fragments, complete structure and comparison to horseradish peroxidase C. Eur J Biochem. 1980 Jul;108(2):481–489. doi: 10.1111/j.1432-1033.1980.tb04745.x. [DOI] [PubMed] [Google Scholar]
- Monk L. S., Fagerstedt K. V., Crawford R. M. Superoxide Dismutase as an Anaerobic Polypeptide : A Key Factor in Recovery from Oxygen Deprivation in Iris pseudacorus? Plant Physiol. 1987 Dec;85(4):1016–1020. doi: 10.1104/pp.85.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirck M., Hirt H., Heberle-Bors E. The cDNA sequence encoding an annexin from Medicago sativa. Plant Physiol. 1994 Apr;104(4):1463–1464. doi: 10.1104/pp.104.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rosner J. L. Susceptibilities of oxyR regulon mutants of Escherichia coli and Salmonella typhimurium to isoniazid. Antimicrob Agents Chemother. 1993 Oct;37(10):2251–2253. doi: 10.1128/aac.37.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. D., Moss S. E. Structural evolution of the annexin supergene family. Trends Genet. 1994 Jul;10(7):241–246. doi: 10.1016/0168-9525(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Thanabalu T., Hindley J., Berry C. Proteolytic processing of the mosquitocidal toxin from Bacillus sphaericus SSII-1. J Bacteriol. 1992 Aug;174(15):5051–5056. doi: 10.1128/jb.174.15.5051-5056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano M. B., Kullik I., Trinh F., Baird P. T., Schneider T. D., Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell. 1994 Sep 9;78(5):897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- Welinder K. G. Amino acid sequence studies of horseradish peroxidase. Amino and carboxyl termini, cyanogen bromide and tryptic fragments, the complete sequence, and some structural characteristics of horseradish peroxidase C. Eur J Biochem. 1979 Jun 1;96(3):483–502. doi: 10.1111/j.1432-1033.1979.tb13061.x. [DOI] [PubMed] [Google Scholar]