Significance
Daily rhythms in behavior depend on the coordinated cycling of circadian neurons in the brain. Here, we found that a neuropeptide that is required for synchrony among circadian neurons also, surprisingly, dose-dependently reduces synchrony among circadian cells. We find this allows the system to adjust how quickly it entrains to environmental cycles. We propose that treatments that enhance this signaling pathway could reduce jet lag associated with shift work and travel across time zones.
Keywords: circadian oscillator, biological clock, vasopressin, vasoactive intestinal peptide, period gene
Abstract
Shift work or transmeridian travel can desynchronize the body's circadian rhythms from local light–dark cycles. The mammalian suprachiasmatic nucleus (SCN) generates and entrains daily rhythms in physiology and behavior. Paradoxically, we found that vasoactive intestinal polypeptide (VIP), a neuropeptide implicated in synchrony among SCN cells, can also desynchronize them. The degree and duration of desynchronization among SCN neurons depended on both the phase and the dose of VIP. A model of the SCN consisting of coupled stochastic cells predicted both the phase- and the dose-dependent response to VIP and that the transient phase desynchronization, or “phase tumbling”, could arise from intrinsic, stochastic noise in small populations of key molecules (notably, Period mRNA near its daily minimum). The model also predicted that phase tumbling following brief VIP treatment would accelerate entrainment to shifted environmental cycles. We tested this using a prepulse of VIP during the day before a shift in either a light cycle in vivo or a temperature cycle in vitro. Although VIP during the day does not shift circadian rhythms, the VIP pretreatment approximately halved the time required for mice to reentrain to an 8-h shifted light schedule and for SCN cultures to reentrain to a 10-h shifted temperature cycle. We conclude that VIP below 100 nM synchronizes SCN cells and above 100 nM reduces synchrony in the SCN. We show that exploiting these mechanisms that transiently reduce cellular synchrony before a large shift in the schedule of daily environmental cues has the potential to reduce jet lag.
Circadian rhythms of living organisms entrain (synchronize) to daily environmental cues such as light and dark. Living organisms have not evolved to make large daily adjustments in their circadian timing, so it is a challenge for them to respond to changes such as those that humans are subjected to during shift work and travel across time zones. Long-term misalignment between internal circadian rhythms in mammals and environmental cycles can induce physiological and psychological abnormalities, including depression, cancer, heart problems, obesity, and increased mortality (1, 2).
The master circadian pacemaker in mammals, the bilateral suprachiasmatic nucleus (SCN), is composed of ∼20,000 neurons that synchronize their daily rhythms to each other and entrain to ambient light cycles (3, 4). Vasoactive intestinal polypeptide (VIP), released in the SCN as a function of circadian time and light intensity (5–7), plays a critical role in this circadian synchronization. In the absence of VIP or its receptor, VPAC2R, SCN neurons fail to synchronize to each other and consequently many daily rhythms of the organism are lost (8–12). The addition of VIP to SCN cultures induces the production of Period (Per) 1 and 2 (13), two genes implicated in light-induced resetting (14–16), and shifts rhythms in behavior and SCN physiology (17–21). Notably, daily addition of VIP or an agonist to the VIP receptor, VPAC2R, entrains rhythms in SCN explants and Vip−/− SCN neurons (21, 22). Thus, VIP is thought to play a role in both the synchronization between SCN cells when the organism is in the dark and the entrainment of the SCN when the organism is exposed to a light cycle.
Exposure to a brief light pulse has been shown to reduce the amplitude of circadian rhythms in humans (23), insects (24, 25), and cell lines expressing transgenic melanopsin (26, 27). Lower-amplitude oscillations have been associated with larger shifts in behavioral and physiological rhythms (28, 29) and the ability to entrain to a wider range of periods (30, 31). An explanation of the cause of this amplitude reduction was given as phase dispersion among oscillators or damping of individual circadian oscillators or both. However, whether or how circadian cells in a network modulate their amplitudes or synchrony to entrain to the environment has not been fully determined. Several of the studies mentioned here have suggested that reducing synchrony among oscillators might allow them to entrain faster. Following an unexpected finding that VIP rapidly reduced the amplitude of SCN tissue rhythms, we tested the hypotheses that (i) VIP can either increase or decrease synchrony among SCN cells, depending on its concentration and time of release, and (ii) VIP can accelerate entrainment through transient phase dispersion (“phase tumbling”) among SCN cells.
Results
VIP Reduces the Amplitude of Circadian Rhythms in the SCN by Reducing Synchrony.
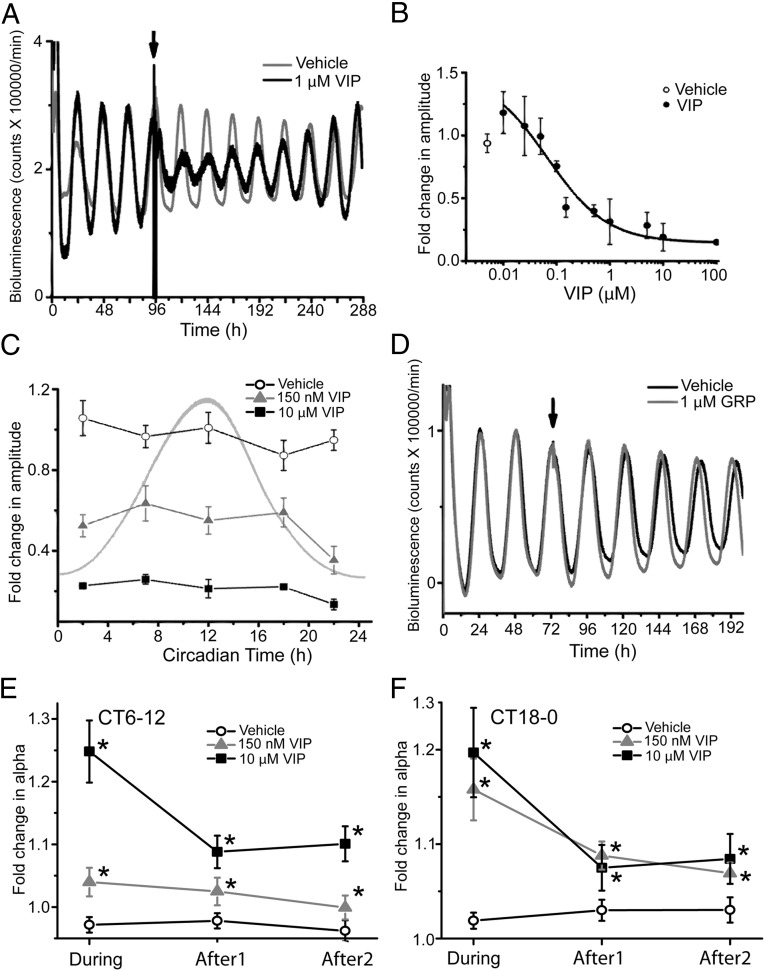
As part of a recent analysis of VIP-induced phase shifts of the SCN (21), we noted that the peak-to-trough amplitude of SCN rhythms reliably decreased and then gradually recovered after application of VIP (Fig. 1A). Replacing the medium with three full exchanges 1 h after VIP application yielded similar results, indicating that the effects persisted many days after the added VIP was gone (Fig. S1A). We found that VIP reduced the peak-to-trough amplitude of PER2 rhythms with a half-maximal response near 150 nM and saturation above 10 µM (Fig. 1B). Within this range, a 10-fold increase in VIP concentration halved the PER2 amplitude (r2 = 0.99, n = 31 explants). VIP reduced the amplitude similarly when it was applied on the rising or falling phases of the PER2 cycle, but more when PER2 levels were at their lowest around circadian time (CT) 22 (Fig. 1C). Compared with VIP, 30 µM glutamate, a major mediator of photic input to the SCN (Fig. S1B), or 1 µM gastrin-releasing peptide (GRP), another neuropeptide expressed and released in the SCN, modestly reduced PER2 amplitude on the day after application (Fig. 1D and Fig. S1B). Thus, at concentrations above 100 nM, VIP sufficed to reduce the amplitude of circadian rhythms in the SCN in a phase- and dose-dependent manner.
Fig. 1.
VIP dose-dependently reduces the amplitude of circadian rhythms in the SCN. (A) Representative detrended bioluminescence traces from PER2::LUC SCN explants were treated with 1 μM VIP (solid line) or vehicle (shaded line) delivered near the peak of PER2 expression (CT12, arrow). Note that the amplitude of the VIP-treated SCN decreased and, then, gradually recovered. Each trace was normalized to the peak before treatment. (B) The dose-dependent amplitude decrease (mean ± SEM; n = 3–5 cultures at each dose) by VIP application at CT12. Between 150 nM and 10 μM VIP, the amplitude decreased linearly with logarithmic increases in VIP concentration. Data were fitted with a logistic function (solid line). Amplitude was measured as the trough-to-peak magnitude 48 h after VIP application. (C) The amplitude reduction of PER2 cycling (mean ± SEM) was greater following 10 µM VIP (squares; n = 20) than 150 nM VIP (triangles; n = 16) at all times (P < 0.00001, F6,66 = 38.53, n = 74; two-way ANOVA with a Scheffé post hoc). Notably, 10 µM VIP delivery at CT22 had a larger effect on amplitude than at other times (P < 0.03, F4,14 = 3.87, n = 19; one-way ANOVA with a Scheffé post hoc). Vehicle (open circles) did not reduce the amplitude at any time. The shaded line corresponds to a PER2-expression rhythm peaking at CT12. (D) Representative bioluminescence traces from SCN explants treated at the peak of PER2 expression (arrow) showing that another neuropeptide, 1 μM gastrin-releasing peptide (GRP) (solid line), did not reduce amplitude compared with vehicle (shaded line) applied at CT12. (E) VIP application transiently broadens the waveform of PER2 expression. The fold change (mean ± SEM) in the duration (α) of PER2 expression is plotted relative to the PER2 duration on the day before treatment. When applied near the peak of PER2 (E), VIP dose-dependently increased the width of PER2 expression on the day of treatment and for the 2 d after (10 μM VIP, squares, n = 3; 150 nM VIP, triangles, n = 8; vehicle, circles, n = 8; P < 0.000003, F2,18 = 32.5, two-way ANOVA with a Scheffé post hoc). The peak broadening effect of VIP decreased with days after treatment (P < 0.05, F2,18 = 2.8, Two-way ANOVA with a Scheffé post hoc). Similarly, when applied near the trough of PER2 (F), 10 μM VIP (n = 12) and 150 nM VIP (n = 7) increased the width of daily PER2 expression compared with vehicle (n = 8; P < 0.0005, F2,26 = 8.3, two-way ANOVA). This effect on α persisted for the 2 d after VIP application (P = 0.006, F2,26 = 5.5, two-way ANOVA).
VIP Reduces the Synchrony of SCN Populations.
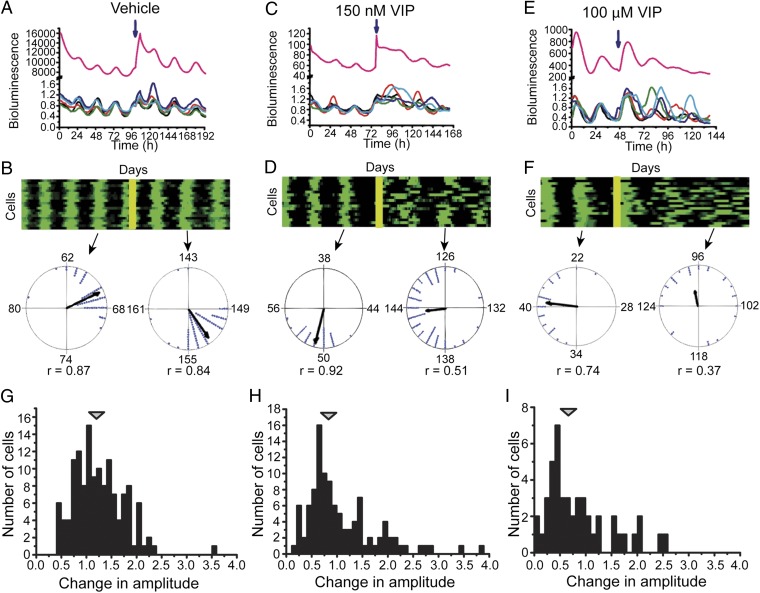
The amplitude reduction recorded from the population of SCN cells could reflect phase dispersion between rhythmic SCN cells, reduced rhythmicity of individual cells, or both. The decrease in amplitude was associated with an increase in the width of daily PER2 expression, suggesting that VIP spread the phases of SCN cells (Fig. 1 E and F). To further distinguish between these possibilities, we measured the effect of VIP on rhythms in individual neurons in SCN slices. Whereas vehicle had no measurable effect on rhythmicity or synchrony, 150 nM or 10 µM VIP reduced the synchrony among SCN neurons (Fig. 2; Raleigh test r values before and after vehicle, 0.93 ± 0.01 and 0.91 ± 0.07, n = 2 cultures; before and after 150 nM VIP, 0.84 ± 0.03 and 0.51 ± 0.07, n = 5; before and after 10 µM VIP, 0.77 ± 0.03 and 0.25 ± 0.12, n = 2) and modestly reduced the peak-to-trough amplitude of individual cells (measured two cycles after treatment: vehicle, 0.94 ± 0.04; 150 nM VIP, 0.73 ± 0.03, P < 0.05, Student’s t test; Wilcoxon–Mann–Whitney rank sum test for differences in phase clustering, vehicle, P > 0.07 in two cultures; 150 nM VIP, P < 1 × 10−3 in five cultures; 10 μM VIP, P < 0.01 in two cultures). The amplitude reduction of individual cells following 150 nM VIP accounts for less than half of the amplitude reduction seen at the population level. Therefore, VIP-induced desynchrony among SCN cells is required to explain the reduced amplitude of ensemble PER2 rhythms.
Fig. 2.
VIP dose-dependently reduces circadian synchrony among SCN cells. (A) PER2::LUC bioluminescence traces of five randomly selected cells treated with vehicle (blue arrow). Note that the cells retained their phase relationships and amplitudes so that their summed expression (purple trace) shows a circadian rhythm with sustained amplitude. (B) A raster plot shows the daily increase (green) and decrease (black) in PER2 expression from 20 representative cells in the same SCN slice treated with vehicle (yellow bar). Two Rayleigh plots show distribution of phases among cells (n = 140) in this SCN on the day before and 1 d after vehicle administration. Each dot represents the time of daily peak PER2 expression for one cell. Note that the length of the mean vector (r) did not change following the treatment, indicating that the cells remained synchronized. In contrast, treatment with VIP reduced synchrony among SCN cells depending on the concentration of VIP as illustrated by (C and E) representative PER2::LUC traces from 5 cells and (D and F) raster plots from 20 representative cells and Rayleigh plots before and after VIP administration. Note that, compared with vehicle (G), VIP-treated cells in each of these representative cultures remained rhythmic with modest effects on their peak-to-trough amplitude (H and I), but with reduced synchrony.
Constant Light Requires VIP to Reduce Circadian Amplitude in Vivo.
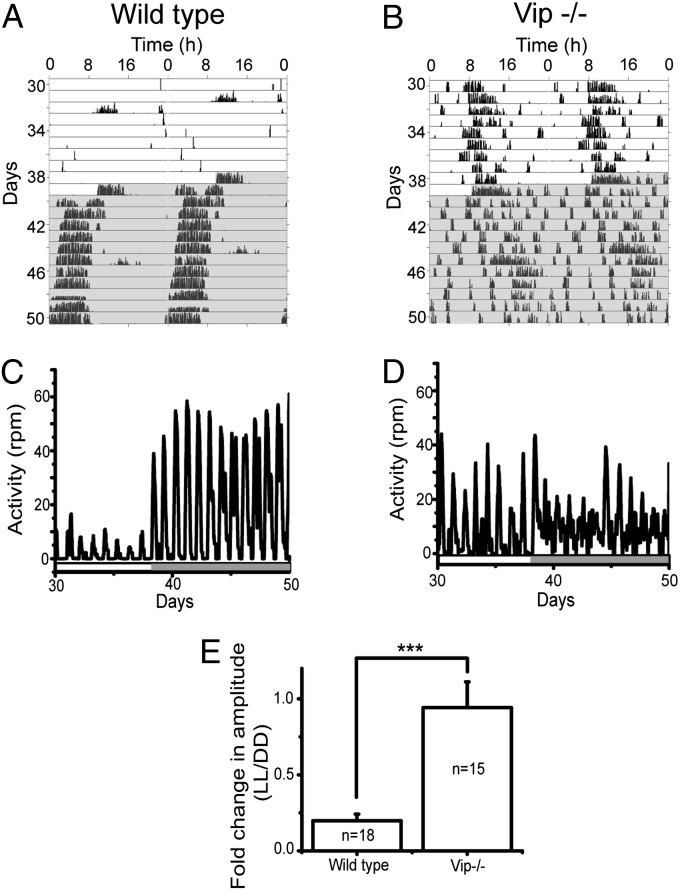
These in vitro results led us to test whether VIP plays a role in modulating circadian amplitude in vivo. Because prolonged constant light (LL) has been reported to desynchronize rhythms among SCN cells and produce arrhythmic locomotor behavior (32), we recorded wheel running from wild-type (C57BL/6, n = 18) and Vip-deficient (Vip−/−, n = 15) mice maintained in LL for 40 d followed by 10–11 d in constant darkness (DD). Control mice showed a marked reduction of their daily peak-to-trough amplitude in LL compared with DD (LL/DD ratio: 0.2 ± 0.04), whereas amplitude changed little in Vip−/− mice (LL/DD ratio: 0.94 ± 0.17, P < 0.0005, comparison of fold change in amplitude between wild type and Vip−/−, Student’s two-tailed t test; Fig. 3). Therefore, VIP plays a critical role in reducing the amplitude of locomotor activity rhythms in response to light.
Fig. 3.
VIP mediates the amplitude reduction of locomotor rhythms by constant light (LL). (A and B) Representative actograms of a wild-type and a VIP-knockout (Vip−/−) mouse kept in LL for 39 d and then constant darkness (DD) for 11 d. Each line shows wheel-running activity in 6-min bins over 48 h with the last 24 h of data replotted on the line below to illustrate free-running circadian periodicity. Cage changes on days 32, 39, and 45 induced locomotor activity, showing that the mice were capable of running on their wheel. (C and D) Time series plots reveal the rapid switch from low-amplitude rhythms in LL to high-amplitude rhythms in DD of the wild-type mouse from A compared with the weak circadian rhythms in LL and DD of the Vip−/− mouse from B. The bar at the bottom of each plot shows the times of lights on (open) and off (shaded). (E) The fold change in the peak-to-trough amplitude of daily locomotion in wild-type animals was reduced dramatically in LL compared with DD, but did not change in Vip−/− mice (mean ± SEM, n indicates the number of mice; ***P < 0.0005, Student’s two-tailed t test).
VIP Speeds Photic Entrainment in Vivo.
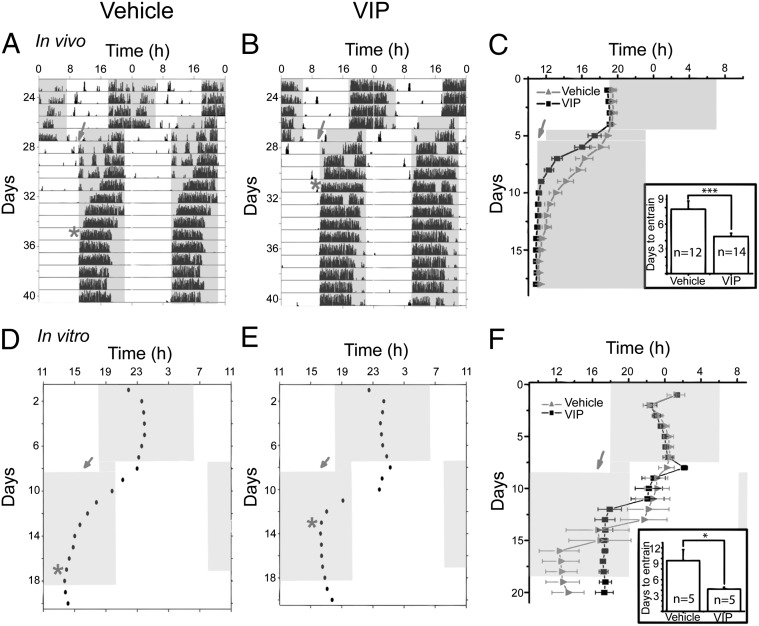
Based on simulation results using a phase-tumbling model to explain the VIP-induced desynchrony in the SCN (SI Text, Figs. S2–S4, and Tables S1–S4), we predicted that VIP-induced reduction of synchrony could accelerate entrainment to changes in the light schedule. To test this possibility, we implanted cannulae aimed at the SCN of adult mice. Because VIP has been shown to shift locomotor rhythms in vivo depending on the time of application, we chose to deliver VIP at CT3 when it does not shift circadian rhythms (17). This avoids the potential confounds of VIP inducing shifts that could speed or slow adjustment to a new schedule. We maintained wild-type mice in 12-h:12-h light:dark cycles (lights on from 7:00 AM to 7:00 PM) for 7 d before and 7 d after cannulation of the SCN. After 1 wk, mice received either 20 (n = 4) or 200 pmol (n = 10) VIP or vehicle (artificial cerebral spinal fluid; n = 12) at 10:00 AM. Lights were turned off after the injection and then turned on from 11:00 PM to 11:00 AM, corresponding to an 8-h advance in the light cycle. By using dim lights (1.0 × 1015 photons⋅s−1⋅cm−2), we reduced the confound of light suppression (masking) of locomotor activity. This had the added advantage of slowing entrainment to a shifted light schedule so that we could accurately measure the rate of entrainment. The animals injected with VIP rapidly shifted their onset of activity and required fewer days to entrain than the vehicle-injected animals (mean of VIP-injected animals, 4.5 ± 0.4 d, n = 14; vehicle-injected animals, 7.8 ± 1 d, n = 12, P < 0.005, Student’s two-tailed t test; Fig. 4 A–C). These results show that VIP sped entrainment to a large advance in the light schedule.
Fig. 4.
VIP accelerates circadian synchronization to an advanced schedule in vivo and in vitro. (A and B) Representative actograms of two mice exposed to an 8-h advance in their light schedule on recording day 27. Mice received either 20 or 200 pmol VIP (B) or vehicle (A) at ZT3 before the shift (shaded arrow) and stably entrained (*) after 4 d (B) or 8 d (A) in the new light schedule. (C) The daily activity onset of all vehicle- or VIP-injected animals (mean ± SEM) and days required to entrain (mean ± SEM; Inset). (D and E) Representative actograms of two SCN cultures in a temperature cycle (shaded, 35 °C; open, 36.5 °C). Points show the daily peaks of PER2 expression before and after application of either vehicle (D) or 10 µM VIP (E) at CT3 (arrow). Note that the vehicle-treated SCN required 8 d to entrain to the new temperature cycle (*) whereas VIP-treated SCN synchronized within 5 d. (F) The daily peak of PER2 expression (mean ± SEM) of all vehicle- (n = 5) and VIP-treated SCN (n = 5). Note that the vehicle-treated SCN had greater variability in their phases at the end of the temperature cycle. Inset shows that cultures that received VIP entrained significantly faster than controls (mean ± SEM; *P < 0.05, Student’s two-tailed t test).
VIP Speeds Temperature Entrainment of the SCN in Vitro.
To determine whether VIP directly accelerates entrainment of the SCN, we measured the days required for SCN cultures to synchronize their circadian rhythm to a 10-h advanced temperature cycle. We tracked the time of peak PER2 expression in SCN explants maintained in a 12-h:12-h warm:cool cycle (36.5 °C starting at 6:00 AM and 35 °C starting at 6:00 PM) for 8 d followed by a 10-h advanced warm:cool cycle (8:00 PM warm and 8:00 AM cool) for 9 d and then for 4 d at 36.5 °C. SCN were treated with either 10 μM VIP or vehicle at 4:00 PM, 11 h before the shift in the temperature cycle. We chose to deliver VIP at the same relative time as the in vivo experiment (Zeitgeber time, ZT, 3), a time when it also produces minimal shifts in vitro (18–21). SCN were defined as entrained to the new cycle once the phase relationship between the daily warming and peak PER2 was stable for at least 48 ± 0.25 h with a phase of 17.2 ± 1 (VIP-treated cultures) or 15.5 ± 1 (vehicle-treated cultures). VIP-treated SCN cultures entrained within 4.2 ± 0.4 d to a mean phase of 17.2 ± 0.5 (Rayleigh test, P < 0.005, r = 0.8, n = 5), whereas controls took longer (9.6 ± 2.1 d, P < 0.05, Student’s two-tailed t test; Fig. 4 D–F) and had more broadly distributed peak phases (mean phase: 12.6 ± 1.7, P > 0.05, r = 0.3, n = 5). Indeed, two of the vehicle-treated cultures (40%) did not appear to be fully entrained even 13 d after the start of the shifted schedule. Thus, VIP pretreatment similarly doubled the speed of the entrainment to a new environmental cycle of behavior and of the SCN.
Discussion
We have discovered that treatment with VIP can reduce synchrony among SCN cells in vitro in a phase- and dose-dependent manner. VIP phase-dependently broadens the phase distribution of circadian cells within the SCN at concentrations above 100 nM. This concentration is ∼10- to 100-fold higher than the threshold for (and 100-fold lower than the saturating dose for) VIP-induced phase shifts and entrainment of the SCN (18, 21, 33). One hundred nanomolar VIP has been show to have physiological effects on a wide range of cell types (34–36). The degree of desynchronization is dose dependent in that VIP synchronizes at lower concentrations (21) and desynchronizes at higher concentrations. Although absolute concentrations of VIP in the SCN are unknown, VIP release is circadian and at low levels in the SCN based on in vitro and in vivo measures and, in vivo, increases as a function of light intensity (6, 37–41).
VIP administration accelerated entrainment of the SCN in vitro and locomotor behavior in vivo. What is the underlying mechanism? Initially, this may appear similar to two studies showing that blocking action potentials or synaptic transmission increased the range of entrainment of the isolated SCN (30, 31). However, VIP does not block intercellular communication. Alternatively, VIP administration might cause internalization of the VPAC2 receptor, reducing subsequent VIP-mediated signaling in the SCN. This remains a possibility although addition of VIP in vitro reduced synchrony for many days (Fig. 1), longer than has been reported for activity-induced endocytosis of VPAC2R. In addition, constant light increases VIP, but has no effect on VPAC2R, levels in the SCN (21). Instead, our experimental and modeling data (SI Text) suggest that the phase-dependent, transient desynchrony following treatment with VIP results from the variable shifts experienced by individual cells in a process that we call phase tumbling. Phase tumbling is a direct consequence of stochastic variation in small numbers of key molecules within the circadian clock. The phase-tumbling model explains the prolonged amplitude decrement of SCN rhythms and faster entrainment without explicitly weakening cell–cell communication or coupling. A recent model of the circadian clock in plants also noted that introduction of stochastic molecular noise yielded less synchrony among oscillators and faster entrainment (42).
Because constant bright light also can reduce synchrony among SCN cells (32), we tested whether constant light might act through VIP signaling and found that constant light reduced the amplitude of behavioral rhythms in wild-type mice, but not in VIP-knockout mice. This identifies a potential link between light-induced VIP release and circadian desynchrony. Constant light has been shown to increase VIP levels in the SCN (43). However, because mice lacking VIP-VPAC2R signaling express circadian rhythms in constant light (n = 15 of 17 Vip−/− mice compared with n = 18 of 18 wild-type mice, χ2 periodogram analysis, Fig. 3), entrain to light cycles, and have intact induction of c-FOS in the SCN and suppression of locomotor activity (9, 44, 45), we conclude that loss of VIP signaling does not abolish sensitivity to light. Here, we posit that VIP is unlikely to be the only endogenous signal capable of producing phase tumbling and that appropriate administration of light or other signals could mimic the desynchronizing effects of VIP. Taken together, these results highlight VIP release within the SCN as a target for potential therapies aimed at improving circadian synchronization during travel or shift work.
Previous studies demonstrating accelerated entrainment can be reinterpreted in the context of the effects of incremented light and VIP signaling on SCN synchrony and entrainment. For example, diurnal sparrows exposed to 36 or 48 h of light before an 8-h advance or delay in their light cycle entrained more rapidly than birds subjected to darkness during the same interval (46). A number of agents including triazolam, Sildenafil, adrenalectomy, scheduled activity during usual rest, and dim light at night have been indicated to accelerate entrainment (47–52). We propose that paradigms such as these may transiently reduce synchrony among SCN neurons or perhaps other circadian oscillators and, thus, speed entrainment.
We contrast these transient effects on synchrony and entrainment with those of prolonged exposure to, for example, short days. After approximately 1 mo in a short photoperiod, nocturnal rodents shift more in response to a light pulse during the early night than rodents exposed to long days (51, 53). The isolated SCN of animals exposed to short days show a narrower peak of daily multiunit activity and, like the behavior, a larger phase shift following stimulation (54). This has been simulated qualitatively by assuming that, on short days, the circadian phases of SCN cells are more tightly clustered. Following a perturbation, the model cells from short days all shift similarly, producing a larger net shift than the perturbation delivered to the less synchronized summertime SCN (54, 55). Although VIP has been implicated in the slow adaptation to changing day length (56), the desynchronizing effects of VIP pretreatment described here function at any time of day, do not require many days to affect the phase-shifting properties of the circadian system, and appear to result from the intrinsically variable responses of different SCN cells.
We found that VIP reduces the amplitude of SCN rhythms more at CT22 than at other times and, when applied daily, entrains the SCN so that the time of VIP application falls at CT4, a time of relative phase stability. This suggests a strategy for reducing jet lag: One should arrange to arrive at the destination at a time when one will receive light exposure near CT22. At that circadian time, the dependence of the mammalian circadian clock’s period on nuclear–cytoplasmic shuttling of clock proteins and mRNA degradation (57–60) provides a sensitive point for cell–cell variation.
Our results provide a unique model of entrainment in which coupled oscillators enhance their entrainment rate through an initial reduction in their phase coherence. Via this phase-tumbling mechanism, the SCN may be able to exploit stochastic elements inherent in circadian molecular mechanisms to accelerate circadian entrainment. We found that the time to entrain to large shifts in the environmental cycle can be substantially reduced by the addition of VIP in vivo or in vitro. We conclude that rational treatments designed to transiently reduce synchrony among circadian oscillators should alleviate jet lag.
Materials and Methods
Animals.
Mice were housed in a 12-h:12-h light:dark cycle in the Danforth Animal Facility at Washington University. PER2::LUC knock-in (61) (founders generously provided by J. S. Takahashi, University of Texas Southwestern Medical Center, Dallas) and Vip−/− (founders generously provided by C. S. Colwell, University of California, Los Angeles) mice were backcrossed with C57BL/6 (purchased from Charles River Laboratories) and bred as homozygous pairs. All procedures were approved by the Animal Care and Use Committee of Washington University and followed National Institutes of Health guidelines.
Cultures and Bioluminescence Recording.
We recorded bioluminescence from 300-μm coronal SCN slices from 8- to 20-d-old homozygous PER2::LUC pups according to published methods (21). Briefly, SCN were dissected with scalpels from vibrasliced brain sections and placed on 0.4-mm membrane inserts (Millipore) in sealed 35-mm Petri dishes (BD Biosciences) with 1 mL Hepes-buffered Dulbecco’s modified medium supplemented with 10% (vol/vol) newborn calf serum (NCS) (Invitrogen) and 0.1 mM beetle luciferin (Promega) at 34 °C. Bioluminescence counts were integrated with photomultiplier tubes (HC135-11 MOD; Hamamatsu) and stored at 1-min intervals.
To record single-cell rhythms, we cultured SCN slices from postnatal day 2–5 pups for 2–5 d and then inverted the SCN onto a poly-d-lysine– and laminin-coated glass coverslip for 6–8 d in 250–400 µL of the same culture media with 10% NCS at 36 °C in 5% (vol/vol) CO2, 95% air. Slices were then imaged in a custom-built incubator (In Vivo Scientific) on an inverted microscope (TI-S/L100; Nikon) with an ultrasensitive camera (Andor iXon EMCCD, 512 × 512 pixels, 16-μm pixels; Andor Technology) in 1 mL Hepes-buffered medium with 0.1 mM luciferin and 10% NCS. Cultures were maintained at 34 °C. Photon counts were integrated in 1- or 10-min bins with Micro-Manager software (www.micro-manager.org). After recording for 4–5 d, we applied 3 μL of VIP or vehicle (culture medium) and continued the recording for 4–5 d. Images were processed with ImageJ (http://rsbweb.nih.gov/ij) so that pixel intensities of six sequential images were summed, and then the minimum value for each pixel from the resulting images was chosen to minimize the effect of cosmic rays. Finally, the pixel intensity of the five remaining images was summed again. We measured photons per hour from cells clearly distinguishable from surrounding cells.
Data Analysis—Biological Data.
We generated raster plots by normalizing bioluminescence traces to the peak and subtraction of the minimum on the cycle before VIP or vehicle application with a custom macro (Image J, “Normalize”). In this way, raster plots show the peak-to-trough bioluminescence on a scale from 0 to 1.
Rayleigh statistics of mean phase were done using Oriana software (Kovach Computing Services). We used the magnitude of the vector of the mean phase (r) as a measure of synchrony between oscillating cells where r = 0 for uniformly distributed phases and r = 1 for oscillators that peaked together each day. To measure amplitude, we detrended bioluminescence traces by subtracting a running 24-h average from a running 3-h average as described previously (21). Detrended bioluminescence was normalized to the peak before VIP or vehicle application and top-to-bottom values of each half cycle were measured. To reduce the initial culture-to-culture variations, we divided each value with an average of two to three cycles before the treatment. All of the plots and statistics except the Rayleigh statistics were done using Origin software (Origin 7.0; OriginLab).
In Vivo Microinjection.
For stereotaxic surgery, mice were anesthetized with isofluorane (Butler Animal Health Supply) mixed with continuous oxygen flow. The head was positioned flat along the Bregma to Lambda. A stainless steel guide cannula (4.0 mm, 26 gauge; Plastics One) aimed at the SCN was inserted perpendicular to the skull (0.5 mm posterior to and 0.2 mm lateral to Bregma and 4.1 mm below the surface) and fixed with a mixture of dental cement (CO-ORAL-ITE Dental MFG) and methyl methacrylate (Sigma). VIP (1.5 mM) or vehicle (artificial cerebral spinal fluid) was injected (200 nL) through polyethylene tubing with a 20-μL Hamilton syringe and a pump (Model KDS 310; Analytical West) at 50 nL/min.
Behavioral Recording.
Adult male mice were housed in individual cages with running wheels in light-tight chambers with fluorescent bulbs (General Electric). Locomotor activity was measured in 6-min bins, using Clocklab (Actimetrics). Two observers blinded to the treatments scored the days required to entrain following a shift in the light schedule. Days to entrain were defined by the number of days following the shift in the light cycle until the first of three consecutive days when the SD of activity onset varied by less than 0.1 h with a mean period of 24 h. On average, the days to entrain scored by the two observers differed by less than 10% so we report the scores from one observer.
Supplementary Material
Acknowledgments
We thank Tatiana Simon for technical support, Luciano Marpegan and other E.D.H. laboratory members, and Istvan Kiss and Hiroshi Kori for helpful discussions. We are grateful to T. Keadle and D. Piatchek of the Washington University in St. Louis Danforth Campus animal facility for their animal care. This work was supported by an Imaging Science Pathway Fellowship (T90 DA022871), the Institute for Collaborative Biotechnologies (Grant W911NF-09-0001 from the US Army Research Office), and National Institutes of Health Grants 63104 and 096873.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307088110/-/DCSupplemental.
References
- 1.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4(6):567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 2.Davidson AJ, et al. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16(21):R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8(10):790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- 4.Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152(2–3):165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinohara K, Honma S, Katsuno Y, Abe H, Honma KI. Circadian rhythms in the release of vasoactive intestinal polypeptide and arginine-vasopressin in organotypic slice culture of rat suprachiasmatic nucleus. Neurosci Lett. 1994;170(1):183–186. doi: 10.1016/0304-3940(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara K, Honma S, Katsuno Y, Abe H, Honma KI. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci USA. 1995;92(16):7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honma S, Katsuno Y, Tanahashi Y, Abe H, Honma KI. Circadian rhythms of arginine vasopressin and vasoactive intestinal polypeptide do not depend on cytoarchitecture of dispersed cell culture of rat suprachiasmatic nucleus. Neuroscience. 1998;86(3):967–976. doi: 10.1016/s0306-4522(98)00078-5. [DOI] [PubMed] [Google Scholar]
- 8.Harmar AJ, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109(4):497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 9.Colwell CS, et al. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 10.Aton SJ, Herzog ED. Come together, right...now: Synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48(4):531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25(48):11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16(6):599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002;15(3):570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama M, et al. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci. 1999;19(3):1115–1121. doi: 10.1523/JNEUROSCI.19-03-01115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci. 2000;20(20):7830–7837. doi: 10.1523/JNEUROSCI.20-20-07830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tischkau SA, Weber ET, Abbott SM, Mitchell JW, Gillette MU. Circadian clock-controlled regulation of cGMP-protein kinase G in the nocturnal domain. J Neurosci. 2003;23(20):7543–7550. doi: 10.1523/JNEUROSCI.23-20-07543.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15(8):5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877(2):361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- 19.Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13(4):839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Spasche A, Piggins HD. Vasoactive intestinal polypeptide phase-advances the rat suprachiasmatic nuclei circadian pacemaker in vitro via protein kinase A and mitogen-activated protein kinase. Neurosci Lett. 2004;358(2):91–94. doi: 10.1016/j.neulet.2003.12.114. [DOI] [PubMed] [Google Scholar]
- 21.An S, Irwin RP, Allen CN, Tsai C, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105(5):2289–2296. doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8(4):476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewett ME, Kronauer RE, Czeisler CA. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350(6313):59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- 24.Winfree AT. Unclocklike behaviour of biological clocks. Nature. 1975;253(5490):315–319. doi: 10.1038/253315a0. [DOI] [PubMed] [Google Scholar]
- 25.Enright JT. Temporal precision in circadian systems: A reliable neuronal clock from unreliable components? Science. 1980;209(4464):1542–1545. doi: 10.1126/science.7433976. [DOI] [PubMed] [Google Scholar]
- 26.Pulivarthy SR, et al. Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci USA. 2007;104(51):20356–20361. doi: 10.1073/pnas.0708877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ukai H, et al. Melanopsin-dependent photo-perturbation reveals desynchronization underlying the singularity of mammalian circadian clocks. Nat Cell Biol. 2007;9(11):1327–1334. doi: 10.1038/ncb1653. [DOI] [PubMed] [Google Scholar]
- 28.Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1(8):708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 29.Vitaterna MH, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci USA. 2006;103(24):9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham U, et al. Coupling governs entrainment range of circadian clocks. Mol Syst Biol. 2010;6:438. doi: 10.1038/msb.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8(3):267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 33.Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of colocalized neuropeptides: Functional significance in the circadian timing system. J Neurosci. 1991;11(3):846–851. doi: 10.1523/JNEUROSCI.11-03-00846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YC, Khawaja AM, Rogers DF. Effect of vasoactive intestinal peptide (VIP)-related peptides on cholinergic neurogenic and direct mucus secretion in ferret trachea in vitro. Br J Pharmacol. 1999;128(6):1353–1359. doi: 10.1038/sj.bjp.0702942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, et al. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: In vitro/in vivo evaluation. Regul Pept. 2007;144(1–3):91–100. doi: 10.1016/j.regpep.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aughton KL, et al. Pharmacological profiling of neuropeptides on rabbit vaginal wall and vaginal artery smooth muscle in vitro. Br J Pharmacol. 2008;155(2):236–243. doi: 10.1038/bjp.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shinohara K, Tominaga K, Inouye ST. Luminance-dependent decrease in vasoactive intestinal polypeptide in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;251(1):21–24. doi: 10.1016/s0304-3940(98)00491-1. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara K, Inouye ST. Photic information coded by vasoactive intestinal polypeptide and neuropeptide Y. Neurosci Biobehav Rev. 1995;19(3):349–352. doi: 10.1016/0149-7634(94)00048-6. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura W, Honma S, Shirakawa T, Honma KI. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 2001;14(4):666–674. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- 40.Laemle LK, Ottenweller JE, Fugaro C. Diurnal variations in vasoactive intestinal polypeptide-like immunoreactivity in the suprachiasmatic nucleus of congenitally anophthalmic mice. Brain Res. 1995;688(1–2):203–208. doi: 10.1016/0006-8993(95)00507-m. [DOI] [PubMed] [Google Scholar]
- 41.Francl JM, Kaur G, Glass JD. Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport. 2010;21(16):1055–1059. doi: 10.1097/WNR.0b013e32833fcba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerriero ML, et al. Stochastic properties of the plant circadian clock. J R Soc Interface. 2012;9:744–756. doi: 10.1098/rsif.2011.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.An S, Tsai C, Ronecker J, Bayly A, Herzog ED. Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol. 2012;520(12):2730–2741. doi: 10.1002/cne.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dragich JM, et al. The role of the neuropeptides PACAP and VIP in the photic regulation of gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2010;31(5):864–875. doi: 10.1111/j.1460-9568.2010.07119.x. [DOI] [PubMed] [Google Scholar]
- 45.Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24(14):3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Binkley S, Mosher K. Advancing schedules and constant light produce faster resynchronization of circadian rhythms. Chronobiol Int. 1989;6(4):305–311. doi: 10.3109/07420528909056936. [DOI] [PubMed] [Google Scholar]
- 47.Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr Biol. 2009;19(4):R156–R157. doi: 10.1016/j.cub.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Mrosovsky N, Salmon PA. A behavioural method for accelerating re-entrainment of rhythms to new light-dark cycles. Nature. 1987;330(6146):372–373. doi: 10.1038/330372a0. [DOI] [PubMed] [Google Scholar]
- 49.van Reeth O, Losee-Olson S, Turek FW. Phase shifts in the circadian activity rhythm induced by triazolam are not mediated by the eyes or the pineal gland in the hamster. Neurosci Lett. 1987;80(2):185–190. doi: 10.1016/0304-3940(87)90651-3. [DOI] [PubMed] [Google Scholar]
- 50.Agostino PV, Plano SA, Golombek DA. 2007. Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci USA 104(23):9834–9839.
- 51.Evans JA, Elliott JA, Gorman MR. Photoperiod differentially modulates photic and nonphotic phase response curves of hamsters. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R539–R546. doi: 10.1152/ajpregu.00456.2003. [DOI] [PubMed] [Google Scholar]
- 52.Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest. 2010;120(7):2600–2609. doi: 10.1172/JCI41192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Refinetti R. 2002. Compression and expansion of circadian rhythm in mice under long and short photoperiods. Integr Physiol Behav Sci 37(2):114–127.
- 54.vanderLeest HT, Rohling JH, Michel S, Meijer JH. Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE. 2009;4(3):e4976. doi: 10.1371/journal.pone.0004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bodenstein C, Gosak M, Schuster S, Marhl M, Perc M. Modeling the seasonal adaptation of circadian clocks by changes in the network structure of the suprachiasmatic nucleus. PLoS Comput Biol. 2012;8(9):e1002697. doi: 10.1371/journal.pcbi.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucassen EA, et al. Role of vasoactive intestinal peptide in seasonal encoding by the suprachiasmatic nucleus clock. Eur J Neurosci. 2012;35(9):1466–1474. doi: 10.1111/j.1460-9568.2012.08054.x. [DOI] [PubMed] [Google Scholar]
- 57.Kim TD, et al. Rhythmic serotonin N-acetyltransferase mRNA degradation is essential for the maintenance of its circadian oscillation. Mol Cell Biol. 2005;25(8):3232–3246. doi: 10.1128/MCB.25.8.3232-3246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diernfellner AC, Querfurth C, Salazar C, Höfer T, Brunner M. Phosphorylation modulates rapid nucleocytoplasmic shuttling and cytoplasmic accumulation of Neurospora clock protein FRQ on a circadian time scale. Genes Dev. 2009;23(18):2192–2200. doi: 10.1101/gad.538209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hung HC, Maurer C, Zorn D, Chang WL, Weber F. Sequential and compartment-specific phosphorylation controls the life cycle of the circadian CLOCK protein. J Biol Chem. 2009;284(35):23734–23742. doi: 10.1074/jbc.M109.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo KC, et al. Mouse period 2 mRNA circadian oscillation is modulated by PTB-mediated rhythmic mRNA degradation. Nucleic Acids Res. 2009;37(1):26–37. doi: 10.1093/nar/gkn893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoo SH, et al. 2004. Period2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101(15):5339–5346.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.