Abstract
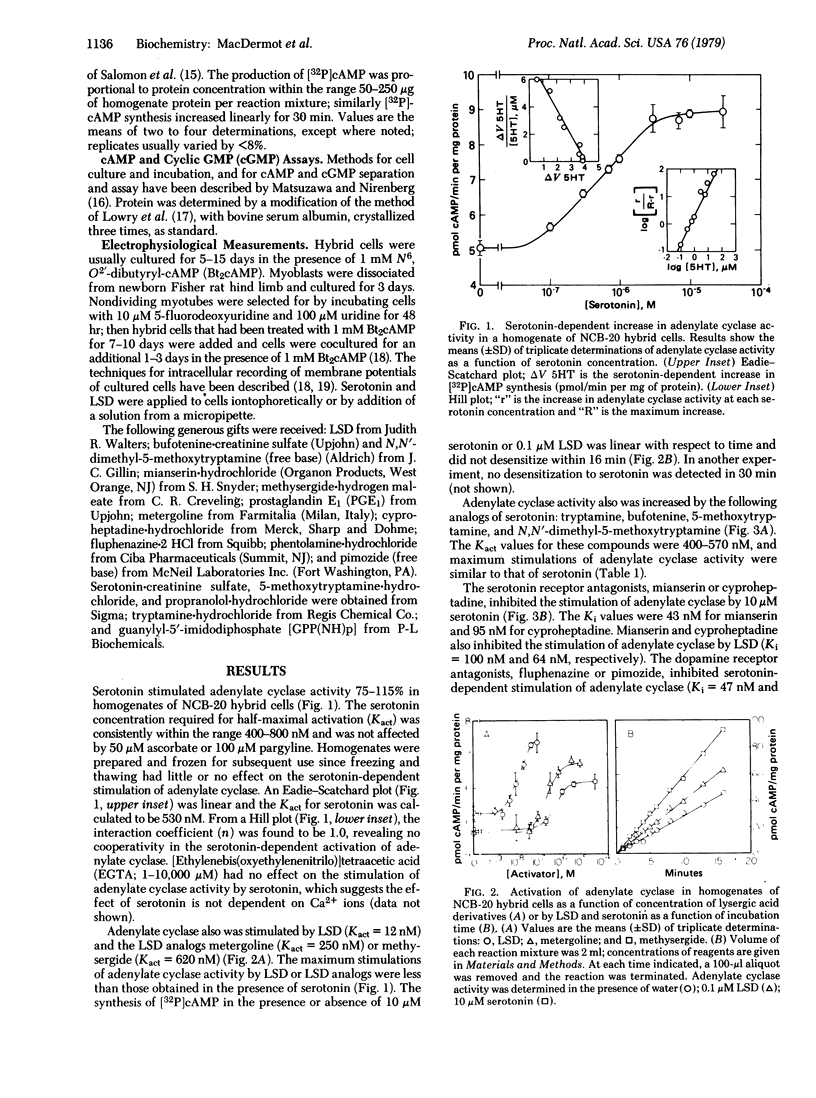
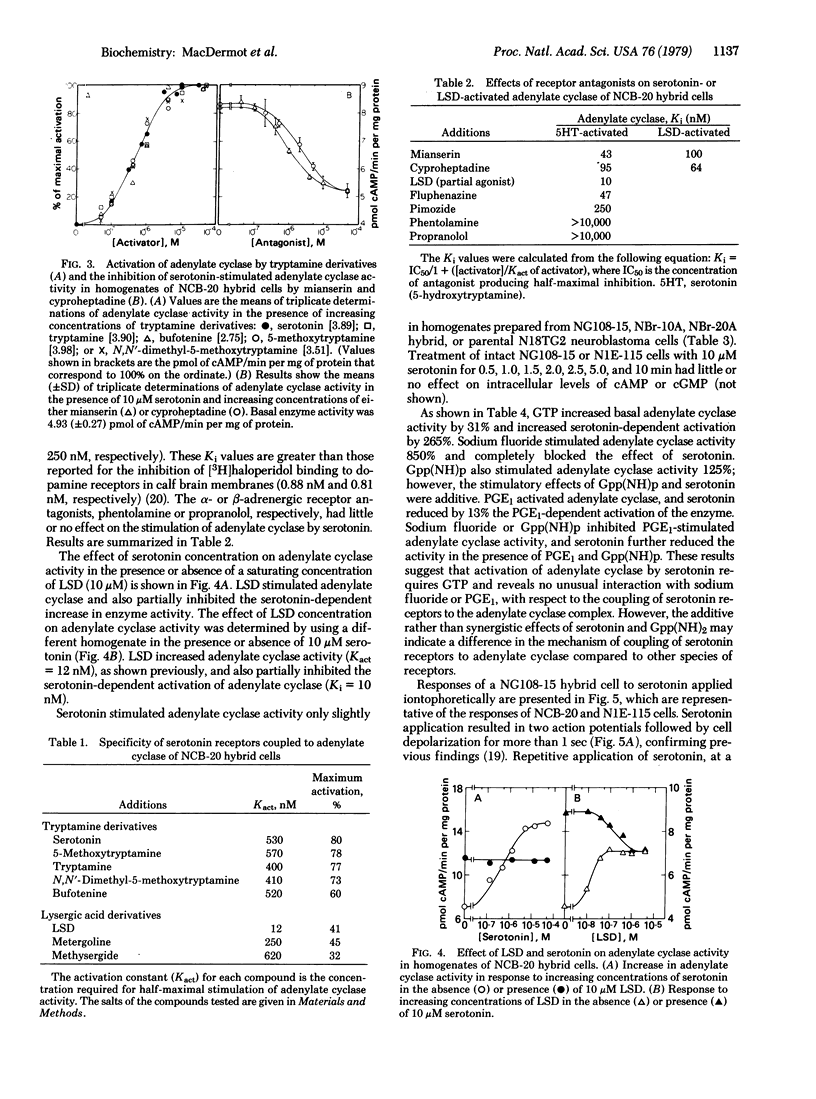
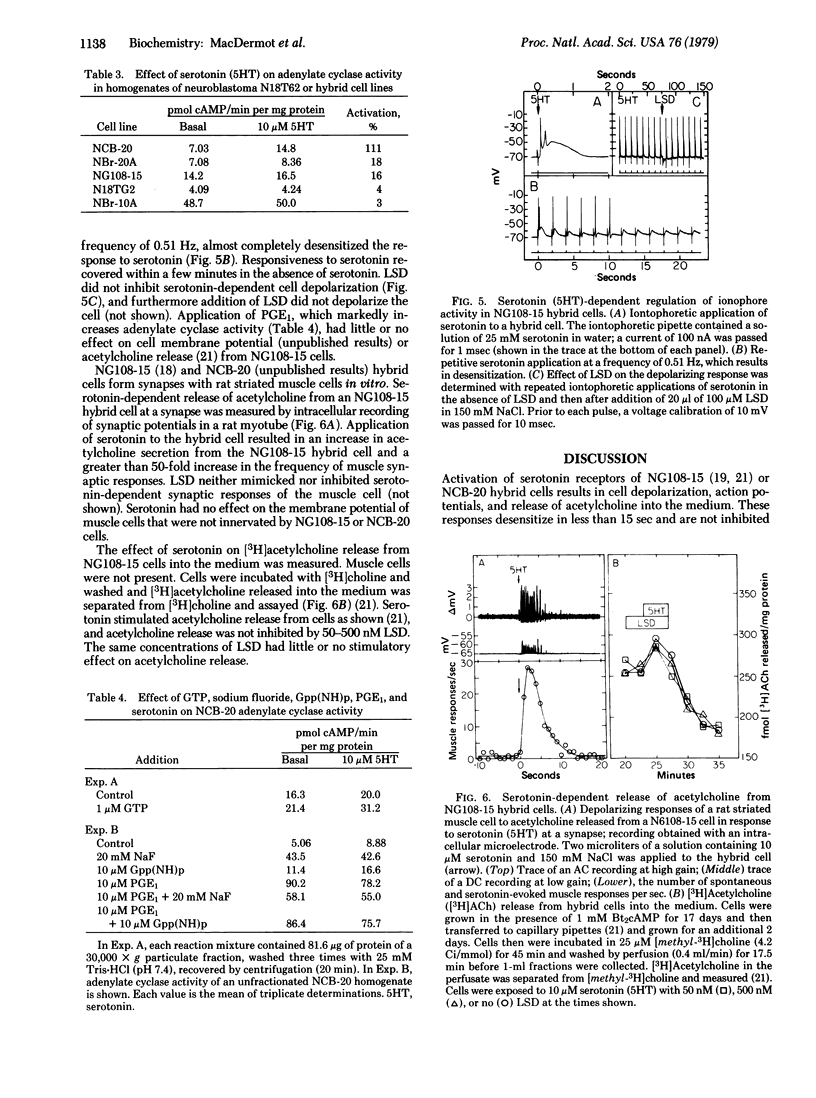
Serotonin activates adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] of NCB-20 neuroblastoma--brain hybrid cells with an activation constant of 530 nM, but has little or no effect on cellular cyclic AMP or cyclic GMP content of NIE-115 neuroblastoma or NG108-15 hybrid cells. In homogenates of NCB-20 hybrid cells, lysergic acid diethylamide stimulates adenylate cyclase activity (Kact = 12 nM) and partially inhibits (Ki = 10 nM) the stimulation of adenylate cyclase activity by serotonin. No desensitization was detected of serotonin receptors coupled to adenylate cyclase. Serotonin also depolarizes NCB-20, NG108-15, and NIE-115 cells and increases acetylcholine release. Serotonin receptors mediating depolarizing responses desensitize rapidly and reversibly, and the depolarizing effects of serotonin are neither mimicked nor inhibited by lysergic acid diethylamide. These results indicate that (i) NCB-20 cells possess at least two species of serotonin receptors, which independently regulate cellular functions, (ii) activation of adenylate cyclase does not directly affect membrane potential or acetylcholine release, and (iii) serotonin-dependent cell depolarization does not affect cyclic AMP or cyclic GMP synthesis in the cell lines tested.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Hailgler H. J. Hallucinogenic indoleamines: Preferential action upon presynaptic serotonin receptors. Psychopharmacol Commun. 1975;1(6):619–629. [PubMed] [Google Scholar]
- Amano T., Hamprecht B., Kemper W. High activity of choline acetyltransferase induced in neuroblastoma x glia hybrid cells. Exp Cell Res. 1974 Apr;85(2):399–408. doi: 10.1016/0014-4827(74)90142-6. [DOI] [PubMed] [Google Scholar]
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. P., Jr, Snyder S. H. Serotonin and lysergic acid diethylamide binding in rat brain membranes: relationship to postsynaptic serotonin receptors. Mol Pharmacol. 1976 May;12(3):373–389. [PubMed] [Google Scholar]
- Bloom F. E., Hoffer B. J., Siggins G. R., Barker J. L., Nicoll R. A. Effects of serotonin on central neurons: microiontophoretic administration. Fed Proc. 1972 Jan-Feb;31(1):97–106. [PubMed] [Google Scholar]
- Boakes R. J., Bradley P. B., Briggs I., Dray A. Antagonism by LSD to effects of 5-HT on single neurones. Brain Res. 1969 Oct;15(2):529–531. doi: 10.1016/0006-8993(69)90176-0. [DOI] [PubMed] [Google Scholar]
- Burt D. R., Creese I., Snyder S. H. Properties of [3H]haloperidol and [3H]dopamine binding associated with dopamine receptors in calf brain membranes. Mol Pharmacol. 1976 Sep;12(5):800–812. [PubMed] [Google Scholar]
- Christian C. N., Nelson P. G., Bullock P., Mullinax D., Nirenberg M. Pharmacologic responses of cells of a neuroblastoma X glioma hybrid clone and modulation of synapses between hybrid cells and mouse myotubes. Brain Res. 1978 May 26;147(2):261–276. doi: 10.1016/0006-8993(78)90839-9. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Bourgoin S., Hamon M., Adrien J., Bockaert J. Postsynaptic serotonin-sensitive adenylate cyclase in the central nervous system. I. Development and distribution of serotonin and dopamine-sensitive adenylate cyclases in rat and guinea pig brain. Mol Pharmacol. 1978 Jan;14(1):2–10. [PubMed] [Google Scholar]
- Enjalbert A., Hamon M., Bourgoin S., Bockaert J. Postsynaptic serotonin-sensitive adenylate cyclase in the central nervous system. II. Comparison with dopamine- and isoproterenol-sensitive adenylate cyclases in rat brain. Mol Pharmacol. 1978 Jan;14(1):11–23. [PubMed] [Google Scholar]
- Fillion G. M., Rousselle J. C., Fillion M. P., Beaudoin D. M., Goiny M. R., Deniau J. M., Jacob J. J. High-affinity binding of (3H) 5-hydroxytryptamine to brain synaptosomal membranes: comparison with (3H) lysergic acid diethylamide binding. Mol Pharmacol. 1978 Jan;14(1):50–59. [PubMed] [Google Scholar]
- GADDUM J. H., PICARELLI Z. P. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957 Sep;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Paupardin-Tritsch D. Ionic mechanisms and receptor properties underlying the responses of molluscan neurones to 5-hydroxytryptamine. J Physiol. 1974 Dec;243(2):427–456. doi: 10.1113/jphysiol.1974.sp010761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. J., Aghajanian G. K. Serotonin receptors in the brain. Fed Proc. 1977 Jul;36(8):2159–2164. [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzawa H., Nirenberg M. Receptor-mediated shifts in cGMP and cAMP levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3472–3476. doi: 10.1073/pnas.72.9.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J. D., Yavelow J., Coon H. G. Expression of phenotypes in hybrid somatic cells derived from the nervous system. Genetics. 1975 Jun;79 (Suppl):373–383. [PubMed] [Google Scholar]
- Minna J., Glazer D., Nirenberg M. Genetic dissection of neural properties using somatic cell hybrids. Nat New Biol. 1972 Feb 23;235(60):225–231. doi: 10.1038/newbio235225a0. [DOI] [PubMed] [Google Scholar]
- Nelson P., Christian C., Nirenberg M. Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):123–127. doi: 10.1073/pnas.73.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. H., Straughan D. W. Excitation and depression of cortical neurones by 5-hydroxytryptamine. J Physiol. 1967 Nov;193(2):269–294. doi: 10.1113/jphysiol.1967.sp008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hungen K., Roberts S., Hill D. F. Serotonin-sensitive adenylate cyclase activity of immature rat brain. Brain Res. 1975 Feb 7;84(2):257–267. doi: 10.1016/0006-8993(75)90980-4. [DOI] [PubMed] [Google Scholar]


