Abstract
This review summarizes recent efforts to create vascularized bone tissue in vitro and in vivo using cell-based therapy approaches. The treatment of large and recalcitrant bone wounds is a serious clinical problem, and in the United States approximately 10% of all fractures are complicated by delayed or non-union. Treatment approaches using growth factor and gene delivery have shown some promise, but results are variable and clinical complications have arisen. Cell-based therapies offer the potential to recapitulate key components of the bone healing cascade, which involves concomitant regeneration of vasculature and new bone tissue. For this reason, osteogenic and vasculogenic cell types have been combined in co-cultures to capitalize on the function of each cell type, and to promote heterotypic interactions. Experiments in both 2D and 3D systems have provided insight into the mechanisms by which osteogenic and vasculogenic cells interact to form vascularized bone, and these approaches have been translated to ectopic and orthotopic models in small animal studies. The knowledge generated by these studies will inform and facilitate the next generation of pre-clinical studies, which are needed to move cell-based orthopaedic repair strategies into the clinic. The science and application of cytotherapy for repair of large and ischemic bone defects is developing rapidly, and promises to provide new treatment methods for these challenging clinical problems.
Keywords: bone, cell therapy, orthopedics, stem cells, tissue engineering, vascularization
Introduction: The Need for Improved Bone Graft Substitutes
Large bone defects are a significant clinical problem in the United States and worldwide. According to 2006 data from the U.S. Health and Cost and Utilization Project (HCUP), there are approximately one million hospital admissions related to appendicular skeletal-tissue injuries each year in the United States (1). Bone injuries and fractures require over 500,000 grafting procedures and account for over $26 billion of healthcare costs in the U.S. annually, and skull and facial fractures contribute an additional $1.3 billion to the annual health care cost. Importantly, approximately 10% of the total fractures in the U.S. are complicated by impaired healing, non-unions or delayed unions. “Non-unions” are defined as broken bones that fail to heal while “delayed unions” are fractures that take longer than usual to heal. A main cause of delayed and non-union is tissue instability and lack of nutrient supply around the defect site, since both stability and vascularization are required for the normal bone healing process. Some bones, such as the head of the femur and the wristbones, have limited vasculature to provide necessary proteins, vitamins, and calcium required for healing (2), and these bones therefore tend to be more susceptible to non-unions.
Although the natural healing response can lead to physiological bone remodeling, non-unions and large-scale traumatic bone injuries require surgical intervention. Autografts, allografts, and xenografts are currently used as treatment options, but these are associated with complications including donor site morbidity, disease transmission, and immunological rejection. The current gold standard for large bone defect repair therefore uses a tissue autograft from the patient (3). These grafts contain the cell types, matrix, and vasculature necessary for proper bone regrowth in the injured area. However, autografts require a secondary operative procedure, which can lead to complications such as pain and donor-site morbidity. Allografts using tissue from human donors are also commonly used but are associated with an increased risk of disease transmission and failure rate over long-term use, relative to autografts (4). Xenografts involve the transplantation of bone tissue across species; however, they also present the risk of disease transmission after implantation (5). Further, xenografts must undergo sterilization processes that cause the loss of osteoinductive factors within the grafting material.
Medullary rods and internal fixation using metallic devices is sometimes an option to enhance bone healing. However, these approaches require permanent implants that are not remodeled by the patient and are susceptible to fatigue fracture over long-term use (5). Bonding of such metallic devices to adjacent bone is challenging. Attempts to modify the surfaces of implants with bioceramics and mineralized coatings have shown some success, but these surface conditioning techniques can lead to decreased durability of the device (5). Local stress shielding caused by metallic implants can also lead to a reduction in bone density and can require revision surgeries (6). Vascularization of regions supported by metallic implants is also limited (5).
The problems associated with transplanted grafts and stabilization strategies have resulted in an increasing interest in improved bone graft substitutes and bone tissue engineering solutions. Current osteobiologic approaches provide a scaffold material that is designed to allow growth and proliferation of host cells. Many approaches also include osteogenic growth factors, and in particular the bone morphogenetic proteins (BMPs), to enhance bone formation in large defects (7). The commercially available Medtronic INFUSE® Bone Graft combines recombinant human BMP-2 with an absorbable collagen sponge, and has been used widely in the clinic (8). The product is currently approved for selected spinal fusion, intramedullary fixation, and sinus augmentation procedures, and it has had a dramatic effect on treatment of particularly difficult bone healing indications. However, concerns have been raised about the degree of control of BMP-2 release from the product and potentially serious reactions when the product is used off-label such as ectopic bone formation, nerve damage, edema and inflammation (9–11). Most recently, the possibility that BMP is cancer promoting has further clouded the view of how these products are best used (12–14). The limitations and potential complications associated with these early osteobiologic treatments has driven development of even more biologically-based approaches, which included living cells to provide more refined control over bone formation.
This review summarizes the current status of cell-based approaches to creating improved bone graft substitutes. In particular, it emphasizes strategies that are aimed at generating vascularized bone tissue, through a combined targeting of osteogenesis and vasculogenesis. We first examine the physiology of bone, including the bone healing cascade, and then review the main cell types involved in this process. Recent in vitro and in vivo approaches to generating vascularized bone tissue are then summarized and discussed. Finally, we offer perspectives on the current state of the field and promising future directions.
The Physiology of Bone
Composition and Architecture at the Micro- and Macro-scale
Bone is one of the main connective tissues in the human body. It is characterized by a collagenous extracellular matrix (ECM) that is extensively mineralized with hydroxyapatite (Ca10(PO4)6(OH)2) (15), which is found as plate-like structures 20–80 nm in length (16). Hydroxyapatite contributes to the high density and strength of bone which in turn provides both support and protection to the other tissues and organs of the body. The mineral component of bone is both reactive and soluble, allowing turnover and remodeling within the bone structure. Bone tissue also contains a variety of other ionic species such as carbonate and magnesium, which are liberated to the systemic circulation as bone remodels. Bone therefore serves as an important storage depot for ions, including calcium and phosphate, which play roles in homeostatic regulation and metabolic function.
The proteinaceous ECM of bone is composed primarily of collagen type I, with lesser amounts of collagen type V and a variety of noncollagenous proteins (17). Proteoglycans found in bone include chondroitin sulfate and keratin sulfate, which consist of a core protein surrounded by glycosaminoglycans and are found throughout the bone structure. Several key bone-associated proteins such as osteonectin, osteopontin, bone sialoprotein I and II, and osteocalcin play regulatory roles in bone formation and cellular attachment. In addition, the bone tissue environment includes potent growth factors and cytokines, including insulin-like growth factors (IGF), tumor necrosis factor alpha (TNF-α), transforming growth factor-β (TGF-β), and bone morphogenetic proteins, which direct cell differentiation and proliferation.
There are two main types of bone structure: cortical (also called compact) and trabecular (also called cancellous or spongy) (18). Cortical bone is stiffer and more organized than trabecular bone and forms a compact, dense layer that surrounds the trabecular tissue in the long bones. Cortical bone consists of highly organized concentric structures called osteons that serve as the tissue’s anatomical and functional unit. Osteons are supplied with blood form the marrow through Haversian canals, whereas Volkmann’s canals move blood between osteons. Trabecular tissue is found in the interior of bones and is also highly vascular. Trabecular bone is less dense and stiff compared to compact bone, due to the large marrow cavities it contains. The red marrow within trabecular bone contains hematopoietic progenitor cells that are responsible for the production of the cells of the blood, as well as a small population of stem cells that can give rise to mesenchymal tissues, including new bone. The marrow itself is also highly vascularized and provides nutrients to the surrounding bone.
There are four primary cell types in bone tissue: osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts (17). Osteoprogenitor cells reside in the marrow, periosteum, and bone canals. When environmental signals initiate the processes that require bone formation, such as tissue growth or repair, these progenitors migrate, proliferate, and differentiate into osteoblasts. The primary function of osteoblasts is to secrete the protein ECM of bone, which subsequently becomes mineralized to form new bone tissue. Found on the outer lining of bone, these cells can either remain inactive on the surface of bones or become osteocytes. Alkaline phosphatase (ALP) activity serves as a marker of their action (17). BMPs play an important role in the regulation of osteoblast differentiation and activity. As bone is formed, osteoblasts become trapped in the matrix, and they alter their phenotype to become osteocytes, which account for the majority of the cells in bone. These cells fill spaces called lacunae in the osteons and communicate to one another through channels called canaliculi, maintaining the bone under homeostasis. Osteoclasts are bone-resorbing cells that are activated during bone injury and remodeling, and their function is to digest bone. The balance of growth, remodeling, and repair that is important in maintaining skeletal function is maintained by the orchestrated action of these cell types.
The microenvironment in bone comprises a complex combination of physical and chemical cues that orchestrate tissue function in both health and disease. Both bone resorption and formation are intertwined processes that are dependent on loading and mechanical demands induced by compressive and tensile strain as well as interstitial fluid flow (18). A comprehensive treatment of the mechanobiology of bone is beyond the scope of this review, but the reader is referred to recent focused reviews on the topic (19, 20). In general, it is well established that the cellular components of bone can be stimulated and guided by mechanical forces. In particular, osteocytes have been suggested to be the mechanosensory cells of bone. In response to mechanical stimuli, these cells produce and secrete proteins that form new extracellular matrix, and thereby regulate the function of local osteoblasts and osteoclasts (21). However osteoblast function has also been shown to be regulated by mechanical forces as fluid flow induces OPN production (21). Taken together, it is clear that mechanical environment in bone is a potent regulator of cell function, though the precise role of mechanobiology in bone regeneration is not fully understood.
The Vascular Supply to Bone
Bone is a highly metabolic tissue requiring an abundant vascular supply throughout its structure for homeostasis, growth and remodeling. It is estimated that bone tissue uses approximately 10 to 20% of resting cardiac output (22). A dual blood supply exists in both flat and long bones, through major arteries surrounding the bones (23). Long bones have a nutrient foramen in both the diaphysis (midsection of bone) and epiphysis (end of long bone), which are openings in the hard tissue to allow blood vessels to pass through and reach the marrow cavities. Smaller epiphyseal and metaphyseal arteries arising from surroundings joints connect with capillaries throughout the diaphysis. In the outer areas of cortical bone, capillaries that run through the Volkmann’s canals split into smaller arterioles that enter Haversian canals and in turn connect with surrounding skeletal muscle (23). Blood and waste drainage through the venous supply closely follows the nutrient arteries through bone. As flat bones do not contain diaphyses, metaphyses, or epiphyses, the dual blood supply runs adjacent to the plates of flat bones providing areas for nutrient and waste exchange along the bone structure (23). The highly vascularized structure of bone allows the high demand of nutrient, waste, and ion transfer to be satisfied throughout the tissue and maintains normal development, growth, and remodeling of bone.
The Bone Healing Process
Injury to bone results in a cascade of events that allows the tissue to regenerate in a manner in which functionally developed tissue is recreated. These processes are triggered when skeletal integrity and local vasculature are disrupted at the defect site. Tissue damage initiates bone healing which encompasses an initial inflammatory response, followed by endochondral bone formation, and finally bone remodeling (Figure 1).
Figure 1.
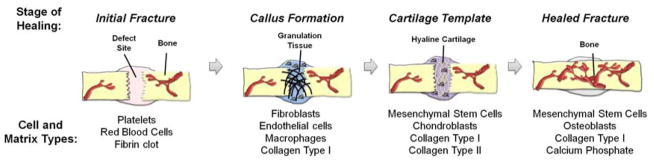
Schematic of the process of bone fracture healing, showing the major cell and matrix types involved at each stage.
Angiogenesis is the formation of new blood vessels and is a critical component of the bone healing process, as it is required for the transport of nutrients, wastes, and cells to and from the injured site. Lack of angiogenesis has been cited as one of the primary causes of delayed- and non-unions. The capillary networks formed during the inflammatory phase of healing are typically transient in nature and are incapable of forming the functional vasculature required to heal non-unions. Evidence of this observation has been provided in a rat distraction osteogenesis model where administration of anti-angiogenic drugs during osteogenesis caused fibrous tissue formation and resulted in non-union (24).
Immediately after a bone fracture, ruptured blood vessels in the injured area constrict and a clot is formed to prevent further bleeding (17). The resulting lack of blood supply causes a local hypoxic environment. Fibroblasts migrate towards the injured site to deposit initial extracellular matrix and generate granulation tissue (17). The lack of oxygen acts as a signal for local endothelial cells to proliferate and chondroblasts to differentiate from bone marrow stem cells, forming a bridge of hyaline cartilage between the ends of the injury site (17). The chondroblasts subsequently become hypertrophic and express pro-angiogenic factors, including vascular endothelial growth factors (VEGF) and fibroblast growth factors (FGF), causing blood vessels to further extend into the cartilaginous matrix (17). The presence of new blood vessels allows osteoprogenitor and hematopoietic cells to be transported to the wounded area, where they differentiate in the ossification center to form bone and bone marrow, respectively. In the final stages of healing, the local osteoprogenitor cells differentiate into osteoblasts to produce functional bone.
Cell Types Used in Engineering of Vascularized Bone Tissue
The general approach to creating vascularized bone tissue is to combine an osteogenic cell type with an endothelial cell type, as shown schematically in Figure 2. For regeneration of defects, 3D hydrogel- or solid scaffold-based approaches are often used, though 2D co-culture models have been used to study the healing process. There are now a range of possible osteogenic cell sources, including bone marrow-derived mesenchymal stem cells, adipose-derived stem cells, or mature osteoblasts. Similarly, a variety of endothelial cell types or their progenitors can be used. The cell types most commonly applied are described briefly below.
Figure 2.
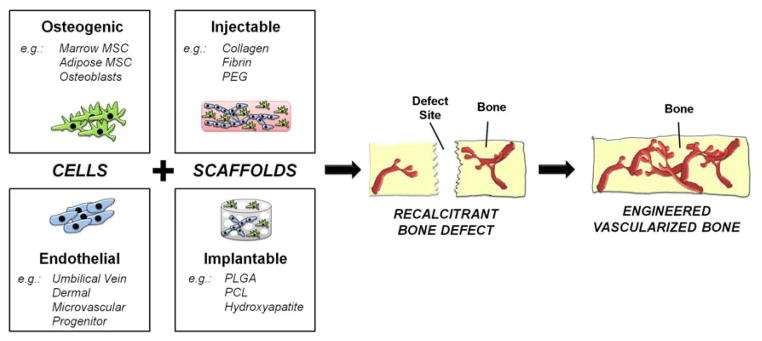
Schematic of cell-based approaches to engineering vascularized bone tissue. Cells and scaffolds are combined to treat large and recalcitrant defects.
Osteogenic Cell Types
Mesenchymal stem cells (MSC) are a multipotent cell type found in numerous tissues of the body. They are characterized as stem cells based on their ability to proliferate in an undifferentiated state and their potential to differentiate into various mesenchymal cell lineages (25, 26). MSC are commonly isolated from bone marrow and adipose tissue, though they have also been extracted from a variety of other tissues, including umbilical cord, placental tissue, cord blood, amniotic fluid, and the periosteum (27–29). These different tissues yield MSC that have different proliferative and multipotency profiles (27, 28). Furthermore, age and disease stage are critical factors that can affect MSC function and potential (27, 28).
MSC differentiation can be controlled by exogenous factors including hormones, growth factors, and extracellular matrix molecules. In vitro, bone marrow-derived mesenchymal stem cells (bmMSC) have been shown to differentiate towards bone, cartilage, muscle, adipose tissue, and tendon (30). In particular, osteogenesis of bmMSC is commonly induced in vitro by adding dexamethasone, beta-glyocerophosphate (β-GP), and ascorbic acid to the culture media (31–34). Dexamethasone, a glucocorticoid, induces transcription of osteogenic factors such as bone sialoprotein (35) and the α5 integrin, an activator of Runx-2, ALP, and collagen I mRNA expression (36). Ascorbic acid is an important cofactor in collagen formation, the most abundant protein in the ECM of bone, and increases ALP expression. Typically, ascorbic-2-phosphate is used in osteogenic studies as it is more stable in cell culture (pH = 7.4, 37°C, and 5% CO2) conditions (37). Cell-secreted ALP hydrolyzes supplemented β-GP to form the inorganic phosphate that aids in matrix mineralization (38). Furthermore, growth factors such as TGF-β1, TGF-β2, TGF-β3, and BMPs can be exogenously applied in specific concentrations to differentiate bmMSC towards both the osteogenic and chondrogenic lineages (31). MSC differentiation can also be directed through matrix identity, matrix stiffness mechanical stimulation, substrate stiffness and nanotopographical cues (39–43). The ability to control the phenotype of these progenitor cells makes them a valuable resource for tissue engineering approaches to bone regeneration.
Autologous bmMSC have advantages when designing cell-based therapies, because they avoid issues of immune rejection and can be harvested from patients using relatively simple surgical procedures. Clinical studies have been performed in which autologous bmMSC were purified from bone marrow aspirates, followed by ex vivo expansion and subsequent reimplantation as therapies for graft-versus-host-disease, liver disease, bone fractures, heart failure, and multiple sclerosis (44). Biopsy from the iliac crest is the most widely used procedure for obtaining bone marrow from which bmMSC can be purified. However, this method is associated with donor site pain and other types of morbidity. Furthermore, bone marrow aspirates contain bmMSC at a frequency of only 0.001–0.01% of total marrow cells, and therefore yield a relatively low number of bmMSC after isolation. For these reasons, other sources of MSC have been examined and developed for cell-based therapies.
Allogeneic cells and tissues typically elicit a host immune reaction upon implantation. This response can be managed through pharmacological immune suppression, but may also cause an array of undesired side effects. Interestingly, it has been suggested that bmMSC are hypoimmunogenic relative to other cell types, and therefore that they may be useful for therapeutic purposes even in the absence of immune modulation (45, 46). Studies have shown that bmMSC can inhibit T-cell proliferation through the secretion of soluble factors such as hepatocyte growth factor (HGF), TGF-β1, and interleukin-10 (IL-10) (47–49). It is thought that these growth factors and cytokines create an immunosuppressive environment around bmMSC, resulting in a delayed and attenuated immune response. Allogeneic bmMSC offer the great advantage that they could be produced in large quantities for therapeutic use, allowing more comprehensive quality control of both safety and function. The possibility of using these cells without immunosuppression makes them very attractive for cell-based therapies.
Adipose-derived stem cells (AdSC) are a subtype of MSC that are derived from fat tissue. They are considered separately here because of the great interest in their use, engendered by the ease of isolating them from adipose tissue and their associated translational potential (50). These cells can be isolated from liposuction aspirates or other biopsies (51, 52), and have been shown to differentiate into a variety of mesenchymal cell lineages including bone (53, 54), fat (55, 56), and cartilage (57–59). Similar to bmMSC, AdSC have been shown to possess immunomodulatory properties allowing for inhibition of inflammatory cytokines (60, 61).
Osteoblasts are the secretory cells that form the collagen matrix present within the bone structure. These cells also secrete non-collageneous proteins such as osteocalcin, osteopontin, and osteonectin, which participate in the mineralization process required to create mature bone. Osteoblasts also play a primary role in fracture healing. They are recruited towards fracture sites and deposit matrix to achieve the appropriate geometry required to fill the defect. In tissue engineering applications, primary osteoblasts (62, 63), osteoblast cell lines (64, 65), and pre-osteoblast cell lines (66–68) have typically been used to demonstrate efficacy in up-regulating osteogenic genes in vitro and bone formation in vivo. The use of primary osteoblasts in bone regeneration has the advantage that cell differentiation is not required, but an autologous source would be required. However, these cells have limited proliferative capacity in vitro and therefore present challenges in obtaining a sufficient quantity of cells to achieve a therapeutic effect (67).
Endothelial Cell (EC) Types
Endothelial cells (EC) line the blood vessels and are present throughout the vasculature in the human body (70, 71). These cells have the ability to self-assemble into vascular tubes when isolated and then cultured in protein materials such as collagen and fibrin (72, 73). Vessel formation can also occur under the guidance of pro-angiogenic factors such as VEGF, FGF, HGF, and platelet-derived growth factor (PDGF) (74, 75).
Endothelial cells can be extracted from numerous tissue sources, including umbilical cords, dermal tissue, and the saphenous vein (71). A commonly used macrovascular cell type is human umbilical vascular endothelial cells (HUVEC), which can be isolated from discarded umbilical cords through a facile collagenase digestion procedure. HUVEC can form capillary-like structures when co-cultured in 3D matrices with stromal cells, including fibroblasts (76), bmMSC (72, 77), and AdSC (78). Secreted factors from the MSC, such as MMPs, allow migration of HUVEC through matrices, thereby enabling the cells to combine and form tubular structures (77). Human dermal microvascular endothelial cells (HMVEC) are an alternate endothelial cell source for the engineering of vascularized tissue that can be isolated from neonatal foreskin (79) or adult skin capillaries (80). HMVEC have been shown to create vessel-like structures in vitro when co-cultured with stromal cell types such as fibroblasts (81), and have yielded perfused vessels in vivo (82). An advantage of HMVEC is that they potentially represent an autologous cell source for therapeutic neovascularization.
Endothelial progenitor cells (EPC) have also been studied for their ability to form capillary-like structures in vivo when co-cultured with stromal cells (83). EPC are a somewhat heterogeneous cell type that can be derived from a number of sources including from adult bone marrow, adult peripheral blood, and umbilical cords. There are two types of EPC which can be obtained from peripheral blood: late outgrowth and early outgrowth, which differ in their culture times, proliferative potential, and gene expression (84). Au et al. (85) showed that EPC derived from umbilical cord blood and EPC derived from adult peripheral blood can both form vessels in vivo when co-cultured with fibroblasts, but the stability and density of the vessels differed. EPC can be obtained from adult humans, and therefore they offer the potential of an autologous stem cell use in engineered tissues.
Other sources of EC such as those isolated directly from the bone marrow (bmEC) (86) and EC derived from an original progenitor cell source such as AdSC (87) or bmMSC (88) are being explored, including as sourced in vascularized bone tissue engineering applications. While these cell types are less commonly used, they have potential advantages in terms of ease of procurement and use as an allogeneic cell source. However, the methods for consistently isolating these cells are still being developed, and the full functional characterization of these sources as vasculogenic EC are not yet complete.
In Vitro Co-Culture Models of Vascularized Bone Formation
A variety of two-dimensional (2D) experimental models have been employed to study the mechanisms of both osteogenesis and angio/vasculogenesis, with the aim of understanding the relationships and interactions between various cell types in vitro (Table 1). Early co-culture studies established a synergistic relationship between endothelial cell types and osteoblastic cell types. Co-culturing these cell types together caused an upregulation of the activity of the osteogenic marker ALP in bmMSC, AdSCs, and osteoblasts (89–92). Increased ALP expression requires direct contact between the two cell types, allowing for gap junctional communication between HUVEC and osteoblasts (92). It has also been suggested that ALP mRNA is stabilized by p38 mitogen-activated protein kinase expressed by EC in the system (93). However the contribution of EC to osteogenesis is complex, and other 2D co-culture models have shown downregulation of osteogenic factors such as Runx2 and osteocalcin (94, 95).
Table 1.
In Vitro Co-culture Models.
| Study | Osteogenic Cell | Vasculogenic Cell | Culture Method |
|---|---|---|---|
| Villars et al (89) | bmMSC | HUVEC | 2D |
| Laranjeira MS et al (90) | bmMSC | HMVEC | 2D |
| Wang J et al (91) | AdSC | HUVEC | 2D |
| Stahl A et al (92) | Osteoblasts | HUVEC | 2D |
| Hager S et al (93) | Osteoblasts | HUVEC | 2D |
| Xue Y et al (94) | bmMSC | HUVEC | 2D |
| Guillotin B et al (95) | bmMSC | HUVEC | 2D |
| Leszczynska J et al (96) | bmMSC | HUVEC | 2D |
| Steiner D et al (97) | bmMSC/Osteoblasts | HUVEC | 2D |
| Kaigler D et al (98) | bmMSC | HMVEC | 2D |
| Grellier M et al (99) | bmMSC | HUVEC | 2D |
| Dohle E et al (100) | Osteoblasts | EPC | 2D |
| Hoch AI et al (101) | bmMSC | EPC | 2D/Matrigel coatings |
| Bidarra SJ et al (102) | bmMSC | HUVEC | 2D |
| Ma J et al (103) | bmMSC | HUVEC | 2D/3D Cell Pellets |
| Kolbe M et al (104) | bmMSC | EPC | 2D Fibronectin coatings |
| Pedersen TO et al (105) | bmMSC | HUVEC | 2D |
| Thébaud NB et al (106) | bmMSC | HUVECs/EPC | 2D |
| Saleh FA et al (107) | bmMSC | HUVEC | 3D Spheroid |
| Santos MI et al (108) | Osteoblasts | HMVEC | PCL scaffolds |
| Kang Y et al (109) | bmMSC | HUVEC | β-TCP scaffolds |
| Nukavarapu SP et al (110) | bmMSC | EPC | PLGA scaffolds |
| Choong CSN et al (86) | bmMSC | bmEC | PCL scaffolds |
| Buschmann J et al (111) | Osteoblasts | Thoracic artery EC | PEU scaffolds |
Abbreviations: bmMSC – bone marrow Mesenchymal Stem Cells; AdSC – Adiposed-derived Stem Cells; HUVEC – Human Umbilical Vein Endothelial Cells; HMVEC – Human Microvascular Endothelial Cells; EPC – Endothelial Progenitor Cells; bmEC – bone marrow Endothelial Cells; EC – endothelial cells; 2D – Two-Dimensional; 3D – Three-Dimensional; PCL – polycaprolactone; β-TCP - Beta-tricalcium phosphate; PLGA - Poly(lactide-co-glycolide); PEU - polyester-urethane
Other work has demonstrated a positive effect of EC on both bmMSC and osteoblast proliferation (96), putatively through inactivation of the pro-apoptotic protein BAD (97). Conversely, MSC and osteoblasts secrete pro-angiogenic factors such as VEGF (98), causing upregulation of the VEGF receptor in EC, which in turn increases ALP expression in bmMSC and osteoblasts (99). The sonic hedgehog pathway is implicated as one of the main signaling pathways that control both angiogenesis and osteogenesis in these co-culture models (100). The secretion of pro-angiogenic factors from bmMSC has also been suggested to be differentiation-state dependent, such that osteogenically induced bmMSC show reduced secretion of VEGF and FGF-2, leading to a decrease in EPC chemotaxis (101). Other studies have investigated the effects of modulating cell ratio, cell type, and culture medium in order to optimize both osteogenic and angiogenic conditions of the two cell types (102–106).
Three-dimensional (3D) co-culture systems using a variety of natural and synthetic biomaterials have also been employed as systems to study concurrent angio-/vasculogenesis and osteogenesis. Three-dimensional spheroid co-culture of bmMSC and HUVEC was shown to produce well-organized 3D vascular structures in vitro (107). Further, the authors observed an increase in ALP expression in the bmMSC/HUVEC co-culture system compared to a control (bmMSC/fibroblast) co-culture system. These effects were attributed to enhanced activation of Wnt signaling as evidenced by β-catenin expression, as well as upregulation of BMP signaling through elevated pSmad 1/5/8 expression (107).
Similarly, 3D solid scaffold-based co-culture systems have been investigated. Santos et al cultured HMVEC with osteoblasts on fiber-mess scaffolds composed of a blend of corn starch and polycaprolactone (PCL) scaffolds and observed alignment of EC and expression of collagen IV, an endothelial basement membrane protein, after 21 days of culture (108). Further gene expression analysis showed upregulation of key osteogenic and angiogenic genes such as collagen I, VEGF, ALP, and VCAM-1. Direct cell-cell contact between the two cell types promoted increased VEGF secretion and high expression of the gap junction protein connexin 43 was detected at the osteoblast-HUVEC interface. These data suggest that heterotypic intercellular crosstalk between the two cell types impacts their respective gene expression profiles. Beta-tricalcium phosphate (β-TCP) scaffolds were assessed for their ability to support both HUVEC and bmMSC co-cultures as shown in Figure 3 (109). In this study, the authors investigated the effects of mono- or co-cultured bmMSC and HUVEC at various ratios (bmMSC:HUVEC ratios of 5:1, 1:1, and 5:1). This system was permissive to both bmMSC and HUVEC proliferation, vessel-like structure formation by the HUVEC, and upregulation of ALP. Poly(lactide-co-glycolide) (PLGA) scaffolds have also been employed as scaffolds to support co-cultures of bmMSC and EPC. Nukavarapu et. al observed increases in BMP-2 and VEGF gene expression as well as ALP expression on macro-porous scaffolds fabricated from PLGA microspheres (110). Moreover, other scaffolding materials such as PCL (86) and polyester-urethane (111) can support co-cultures of bmMSC and EC.
Figure 3.
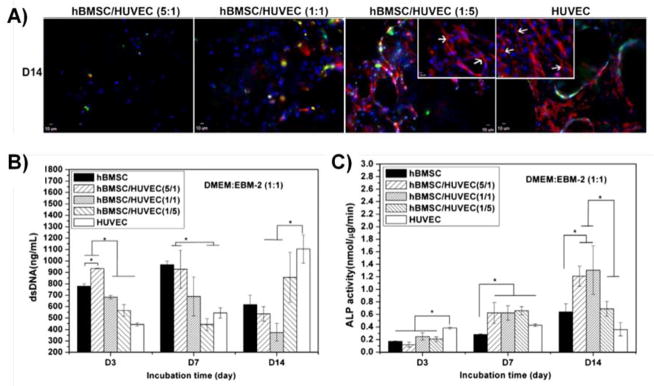
(A) Kang et al showed endothelial network formation by HUVEC on β-tricalcium phosphate scaffolds after 14 days of co-culture with bmMSC. CD31 is labeled red and cell nuclei are labeled blue. Scale bar = 10 μm. (B and C) Both cell proliferation and alkaline phosphatase expression were modulated in co-culture conditions. All panels were adapted from (109). Copyright 2013 Elsevier B.V.
In Vivo Regeneration of Vascularized Bone Tissue
Combined osteogenic/angiogenic cell-based co-culture systems have been applied to generating vascularized bone tissue in both ectopic and orthotropic sites in vivo (Table 2). After demonstrating that HMVEC increase osteogenic differentiation of bmMSC through the secretion of BMP-2, Kaigler et al investigated the co-transplantation of HMVEC and bmMSC on PLGA scaffolds into the dorsal region of SCID mice and monitored ectopic bone formation after 8 weeks (112). The authors observed no differences between total blood vessel content in the implants containing both cell types, compared to implants with bmMSC alone. However, there was a significant increase in bone formation in the HMVEC+bmMSC condition, compared to the bmMSC alone implants. In another study, examination of culture conditions of HUVEC and bmMSC in vitro suggested that vasculogenesis needed to be induced prior to osteogenesis (113). The two cell types were then cultured for 6 weeks on decellularized bone grafts and implanted subcutaneously into nude mice, which resulted in bone formation.
Table 2.
In Vivo Bone Formation and Regeneration Studies.
| Study | Osteogenic Cell | Vasculogenic Cell | Scaffold | Animal Model |
|---|---|---|---|---|
| Kaigler D et al (112) | bmMSC | HMVEC | PLGA | Mouse SubQ |
| Correia C et al (113) | bmMSC | HUVEC | Decellularized bone grafts | Mouse SubQ |
| Mendes LF et al (114) | bmMSC | HUVEC | Cell sheet | Mouse SubQ |
| Usami K et al (115) | bmMSC | EPC | Collagen | Mouse SubQ |
| Geuze RE et al (116) | bmMSC | EPC | Biphasic CP | Goat IM |
| Fedorovich NE et al (117) | bmMSC | EPC | Matrigel | Mouse SubQ |
| Tsikgou et al (118) | bmMSC | HUVEC | PLGA/Collagen-fibronectin | Mouse SubQ |
| Kaigler D et al (119) | bmMSC | HMVEC | PLGA | Rat Cranial |
| Koob S et al (120) | bmMSC | HUVEC | Fibrin/Matrigel | Mouse Cranial |
| Kim JY et al (121) | AdSC | HUVEC | PCL/PLGA/TCP | Rat Cranial |
| He J et al (122) | bmMSC | EPC | PLGA | Rat Cranial |
| Grellier M et al (123) | bmMSC | HUVEC | Alginate | Mouse Femoral |
| Seebach C et al (124) | bmMSC | EPC | Fibronectin-coated β-TCP | Rat Femoral |
Abbreviations: bmMSC – bone marrow Mesenchymal Stem Cells; AdSC – Adiposed-derived Stem Cells; HUVEC – Human Umbilical Vein Endothelial Cells; HMVEC – Human Microvascular Endothelial Cells; EPC – Endothelial Progenitor Cells; PLGA - Poly(lactide-co-glycolide); CP – calcium phosphate; PCL – polycaprolactone; TCP - tricalcium phosphate; β-TCP - Beta-tricalcium phosphate; SubQ – subcutaneous; IM – intramuscular
Scaffold-less co-transplantation of bmMSC and HUVEC has also been investigated as a means of generating ectopic bone formation (114). A dense cell sheet was constructed by seeding bmMSC in a monolayer and inducing the cells towards the osteogenic phenotype. HUVEC were then seeded on top of the bmMSC cell layer, which created a vessel-like network within the cell sheet. After transplantation of the co-cultured cell sheet into nude mice, immunohistochemical analysis demonstrated expression of the bone marker osteocalcin, and integration of transplanted HUVEC with host vasculature. Ectoptic osteogenesis has also been evaluated through the co-transplantation of EPC and bmMSC on collagen fiber mesh scaffolds into nude mice subcutaneously (115). Neovasculature and total bone area, as measured by capillary density and histological analysis, were both increased in the co-culture group compared to the bmMSC only condition after 12 weeks of implantation. Ectopic bone formation has also been achieved in a large animal model. Geuze et al combined EPC and bmMSC on biphasic calcium phosphate scaffolds and implanted them intramuscularly into a goat model (116). The authors observed significant increases in bone formation in the co-culture condition compared to acellular controls, however there was no significant difference in bone generation compared to the bmMSC alone group. Similar results were observed by Fedorovich et al using goat bmMSC and goat blood-derived EPC in a subcutaneous ectopic bone model in nude mice (117).
An interesting approach to engineering vascularized bone grafts was demonstrated by Tsigkou et al (118). Bone marrow MSC were first seeded onto porous PLGA scaffolds and predifferentiated toward the osteogenic lineage for one week of in vitro culture (Figure 4). The scaffolds were then seeded with a collagen-fibronectin hydrogel containing HUVEC and bmMSC, and were implanted subcutaneously into SCID mice. Seeded HUVEC were shown to connect with host vasculature, and ectopic bone formation and expression of osteocalcin was achieved after 8 weeks of implantation.
Figure 4.
(A) Tsikgou et al seeded bmMSC on PLGA scaffolds and pre-differentiated them towards the osteogenic lineage prior to embedding the scaffolds in a collagen-fibronectin hydrogel containing a bmMSC and HUVEC co-culture. (B) In vitro formation of vessel-like structures after 21 days of culture. MSC (eGFP) and HUVEC (tdTomato) were transduced with a lentivirus to fluorescently label the cells. Scale bar = 50 μm. (C) Von Kossa staining after 8 weeks of subcutaneous implantation in immunodeficient mice. Calcium deposition was observed on the pore surfaces of the implanted scaffold. Scale bar = 500 μm. (D) Osteocalcin, a late marker of osteogenesis, was also present throughout the scaffold. Scale bar = 100 μm. All panels are adapted from (118). Copyright 2010 National Academy of Sciences of the United States of America.
Orthotopic models in animals have also demonstrated the potential of MSC and EC co-cultures systems to regenerate bone, but the results have been mixed. Calvarial defects in rodents are a common orthotopic model. Early studies by Kaigler et al combined bmMSC and HMVEC on PLGA scaffolds to assess bone regeneration in a rat cranial defect model (119). Bone mineral density of the bmMSC+HMVEC group was significantly higher after 6 weeks compared to the bmMSC alone condition, but was not statistically different after 12 weeks post-implantation. Conversely, bone volume was not statistically different after 6 weeks of implantation, but was significantly higher in the co-culture condition after 12 weeks. Koob et. al also used calvarial defects in SCID mice to study the effect of bmMSC+HUVEC co-cultures that were embedded in fibrin/Matrigel™ hydrogels and then seeded onto decalcified bone scaffolds (120). Human EC were successfully transplanted into the mice, as demonstrated by positive human CD31 staining. However, the dual (MSC+HUVEC) group showed no significant increase in either capillary or bone formation in the implant site, relative to controls. This result was attributed to a lack of direct contact between implanted HUVEC and bmMSC as well as the contribution of endogenous angiogenesis from the host, which enabled comparable bone formation in controls. In a separate study, pre-differentiation of AdSC toward the bone lineage and co-transplantation with HUVEC on PCL/PLGA/TCP scaffolds yielded different results (121). More rapid and more extensive bone regeneration was observed in the AdSC+HUVEC group compared to the AdSC alone condition, indicating a beneficial response to the addition of HUVEC. In a study using EPC+bmMSC co-cultures on PLGA scaffolds, no significant increase in neovascularization was observed compared to the bmMSC alone group (122). Further, the dual group did not yield improved bone regeneration compared to the bmMSC or EPC groups, which the authors attributed to low transplantation efficiency of EPC in vivo.
Femoral defects are another orthotopic model that has been used to study osteogenesis induced by bmMSC+EC co-cultures. In this model, co-transplantation of bmMSC and HUVEC embedded within alginate microspheres enhanced bone regeneration compared to the bmMSC alone condition, suggesting a synergistic response of HUVEC with transplanted bmMSC (123). Co-transplantation of EPC and bmMSC on fibronectin-coated β-TCP scaffolds also showed promising results (124). Both neovascularization and bone volume fraction were increased in the dual group compared to the cell types individually at the early time points of one and four weeks. Importantly, after 8 weeks, bone quality was significantly higher in the EPC+bmMSC group, as measured by ultimate load measurements, indicating a potential benefit of generating highly vascularized engineered bone.
Summary and Conclusions
The healing of large bone defects remains a particular clinical challenge due to the need to establish vascularization in appropriate conjunction with bone regeneration. Approaches to this problem using growth factor and gene delivery have shown some promise, but results have been variable, and consistently robust regeneration has not been achieved. Only cells can create new bone and new vasculature, and therefore cell-based therapies are particularly promising for the treatment of large bone defects where the native cellular component may be absent. Numerous cell types have been used in this application, including bone marrow mesenchymal stem cells, adipose-derived stem cells, osteoblasts, umbilical vein endothelial cells, dermal microvascular endothelial cells, and endothelial progenitor cells. Each cell type presents its own advantages and disadvantages, particularly in their capacity to be used as an autologous or allogeneic source.
In vitro 2D co-cultures models have provided a deeper mechanistic understanding of the crosstalk between MSC and EC that is critical to regenerating mature and stable tissue. For example, expression of ALP, an osteogenic protein, by MSC is increased when they are co-cultured with EC. At the same time MSC secrete VEGF, which induces local EC to form primitive tubular networks. Similarly, in vitro 3D co-cultures using various natural and synthetic biomaterials have provided proof-of-concept studies to demonstrate cell survival and maintenance of both osteogenic and angiogenic phenotypes. Furthermore, these scaffolds have served as materials to transplant cells in vivo in both ectopic bone formation and orthotopic bone regeneration models. The transplantation of MSC and EC co-cultures in vivo has shown promising results in generating vascularized bone and in regenerating higher quality bone faster compared to transplanting either cell type alone.
Cell-based approaches to the engineering of vascularized bone have the potential to promote faster, more efficient, and more complete healing of recalcitrant bone defects. A key question is what type of cells are the most appropriate for particular applications, and what phenotype of each cell type is the most conducive to achieving the desired results. One strategy that has been investigated recently is to pre-differentiate progenitor cells such as bmMSC towards the osteogenic lineage, and use them in conjunction with EC of the appropriate phenotype to create multiphase, vascularized bone tissue (118). However, other cell types and cell combinations may also add value in bone regeneration. Work in our lab has focused on creating defined cellular microenvironments in the form of 3D protein hydrogel “microbeads” that contain embedded cells. By controlling the composition of the microbeads, progenitor cell phenotype can be guided toward desired osteogenic lineages (125, 126). Another advantage of the microbead format is that different types of microenvironments (containing different cell types) can be created separately, and can subsequently be combined to form multiphase tissues (127). For example, osteogenic microbeads could be embedded within a vasculogenic matrix (73) to promote formation of endothelial networks around a nascent bone phase, and thereby achieving a dual phase osteogenic/vasculogenic tissue. These and other similar approaches are rapidly emerging as the fields of organogenesis, tissue engineering, and cell-based therapies advance toward understanding how multi-component tissues can be created and controlled.
This review has endeavored to summarize the key cellular components and processes involved in regenerating vascularized bone tissue, and how biologists and bioengineers have attempted to mimic these processes in vitro and in vivo. It is clear that an interplay between osteogenic and vasculogenic cells is required to create vascularized bone, and the studies summarized above have provided insight into these interactions. The 2D and 3D studies that have been performed to date suggest that targeting of vasculogenesis concomitantly with osteogenesis can lead to more rapid, robust, and mature bone formation. However these early approaches need to be validated in pre-clinical large animal models, before they can be investigated in humans. The ability to generate well-vascularized bone tissue in vivo will expedite clinical translation of cell-based approaches to bone tissue engineering.
Acknowledgments
The authors are grateful to their colleagues in the field of musculoskeletal tissue engineering for their valuable insight and discussions. We apologize to those whose work could not be included because of space limitations. This work was supported in part by a National Science Foundation Graduate Research Fellowship, Grant # DGE 1256260 (to RRR).
Abbreviations
- BMPs
Bone morphogenetic proteins
- ECM
Extracellular matrix
- IGF
Insulin-like growth factor
- TNF-α
Tumor necrosis factor alpha
- TGF-β
Transforming growth factor-beta
- ALP
Alkaline phosphatase
- VEGF
Vascular endothelial growth factor
- FGF
Fibroblast growth factor
- MSC
Mesenchymal stem cells
- bmMSC
Bone marrow-derived mesenchymal stem cells
- AdSC
Adipose-derived stem cells
- β-GP
Beta-glyocerophosphate
- EC
Endothelial cells
- PDGF
Platelet-derived growth factor
- HUVEC
Human umbilical vascular endothelial cells
- HMVEC
Human dermal microvascular endothelial cells
- EPC
Endothelial progenitor cells
- bmEC
Bone marrow endothelial cells
- 2D
Two-Dimensional
- 3D
Three-Dimensional
- PCL
Polycaprolactone
- β-TCP
Beta-tricalcium phosphate
- PLGA
Poly(lactide-co-glycolide)
Footnotes
Disclosure of interest
The authors have no competing or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.HCUP Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 2.Soucacos PN, Dailiana Z, Beris AE, Johnson EO. Vascularised bone grafts for the management of non-union. Injury. 2006;37:S41–50. doi: 10.1016/j.injury.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JE, Mosheiff R. Tissue engineering approaches for bone repair: concepts and evidence. Injury. 2011;42:609–13. doi: 10.1016/j.injury.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Sayyidmousavi A, Bougherara H. Investigation of stress shielding around the Stryker Omnifit and Exeter periprosthetic hip implants using an irreversible thermodynamic-based model. J Biomed Mater Res B Appl Biomater. 2012;100:1416–24. doi: 10.1002/jbm.b.32500. [DOI] [PubMed] [Google Scholar]
- 7.Barr T, McNamara AJ, Sándor GK, Clokie CM, Peel SA. Comparison of the osteoinductivity of bioimplants containing recombinant human bone morphogenetic proteins 2 (Infuse) and 7 (OP-1) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:531–40. doi: 10.1016/j.tripleo.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 8.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–34. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreon LY, Glassman SD, Brock DC, Dimar JR, Puno RM, Campbell MJ. Adverse events in patients re-exposed to bone morphogenetic protein for spine surgery. Spine. 2008;33:391–3. doi: 10.1097/BRS.0b013e3181642a49. [DOI] [PubMed] [Google Scholar]
- 10.Shahlaie K, Kim KD. Occipitocervical fusion using recombinant human bone morphogenetic protein-2: adverse effects due to tissue swelling and seroma. Spine. 2008;33:2361–6. doi: 10.1097/BRS.0b013e318183971d. [DOI] [PubMed] [Google Scholar]
- 11.Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surg Neurol Int. 2011;2:10. doi: 10.4103/2152-7806.76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Carragee EJ, Baker RM, Benzel EC, Bigos SJ, Cheng I, Corbin TP, Deyo RA, Hurwitz EL, Jarvik JG, Kang JD, Lurie JD, Mroz TE, Oner FC, Peul WC, Rainville J, Ratliff JK, Rihn JA, Rothman DJ, Schoene ML, Spengler DM, Weiner BK. A biologic without guidelines: the YODA project and the future of bone morphogenetic protein-2 research. Spine J. 2012;12:877–80. doi: 10.1016/j.spinee.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Even J, Eskander M, Kang J. Bone morphogenetic protein in spine surgery: current and future uses. J Am Acad Orthop Surg. 2012;20:547–52. doi: 10.5435/JAAOS-20-09-547. [DOI] [PubMed] [Google Scholar]
- 15.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 (Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommerfeldt DW, Rubin CT. Biology of bone and how it orchestrates the form and function of the skeleton. Eur Spine J. 2001;10:S86–95. doi: 10.1007/s005860100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross MH, Pawlina W. Histology: A Text and Atlas. Philadelphia: Lippincott Williams & Wilkins; 2010. pp. 218–53. [Google Scholar]
- 18.Klein-Nulend J, Bacabac RG, Mullender MG. Mechanobiology of bone tissue. Pathol Biol. 2005;53:576–80. doi: 10.1016/j.patbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Allori AC, Sailon AM, Pan JH, Warren SM. Biological basis of bone formation, remodeling, and repair-part III: biomechanical forces. Tissue Eng Part B Rev. 2008;14:285–93. doi: 10.1089/ten.teb.2008.0084. [DOI] [PubMed] [Google Scholar]
- 20.Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s Law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43:108–18. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Bodle JC, Hanson AD, Loboa EG. Adipose-derived stem cells in functional bone tissue engineering: lessons from bone mechanobiology. Tissue Eng Part B Rev. 2011;17:195–211. doi: 10.1089/ten.teb.2010.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khurana JS, Safadi FF. Bone Structure, Development and Bone Biology. Essentials in Bone and Soft-Tissue Pathology. 2010:1–15. [Google Scholar]
- 23.Buck DW, 2nd, Dumanian GA. Bone biology and physiology: Part I. The fundamentals Plast Reconstr Surg. 2012;129:1314–20. doi: 10.1097/PRS.0b013e31824eca94. [DOI] [PubMed] [Google Scholar]
- 24.Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, Longaker MT. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005;20:1114–24. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- 25.Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater. 2010;20:121–33. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: A global assessment. Organogenesis. 2008;4:23–7. doi: 10.4161/org.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieback K, Wuchter P, Besser D, Franke W, Becker M, Ott M, Pacher M, Ma N, Stamm C, Klüter H, Müller A, Ho AD START-MSC consortium. Mesenchymal stromal cells (MSCs): science and f(r)iction. J Mol Med. 2012;90:773–82. doi: 10.1007/s00109-012-0915-y. [DOI] [PubMed] [Google Scholar]
- 28.Motaln H, Schichor C, Lah TT. Human mesenchymal stem cells and their use in cell-based therapies. Cancer. 2010;116:2519–30. doi: 10.1002/cncr.25056. [DOI] [PubMed] [Google Scholar]
- 29.van Gastel N, Torrekens S, Roberts SJ, Moermans K, Schrooten J, Carmeliet P, Luttun A, Luyten FP, Carmeliet G. Engineering vascularized bone: osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells. 2012;30:2460–71. doi: 10.1002/stem.1210. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Vater C, Kasten P, Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–77. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Davis HE, Rao RR, He J, Leach JK. Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. J Biomed Mater Res A. 2009;90:1021–31. doi: 10.1002/jbm.a.32169. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Stegemann JP. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31:3976–85. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Stegemann JP. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011;7:2410–7. doi: 10.1016/j.actbio.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogata Y, Yamauchi M, Kim RH, Li JJ, Freedman LP, Sodek J. Glucocorticoid regulation of bone sialoprotein (BSP) gene expression. Identification of a glucocorticoid response element in the bone sialoprotein gene promoter. Eur J Biochem. 1995;230:183–92. doi: 10.1111/j.1432-1033.1995.0183i.x. [DOI] [PubMed] [Google Scholar]
- 36.Hamidouche Z, Fromigué O, Ringe J, Häupl T, Vaudin P, Pagès JC, Srouji S, Livne E, Marie PJ. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106:18587–91. doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol Int. 2004;28:255–65. doi: 10.1016/j.cellbi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992;51:305–11. doi: 10.1007/BF00334492. [DOI] [PubMed] [Google Scholar]
- 39.Li D, Zhou J, Chowdhury F, Cheng J, Wang N, Wang F. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229–40. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE. Cell responses to the mechanochemical microenvironment--implications for regenerative medicine and drug delivery. Adv Drug Deliv Rev. 2007;59:1329–39. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpentier B, Layrolle P, Legallais C. Bioreactors for bone tissue engineering. Int J Artif Organs. 2011;34:259–70. doi: 10.5301/ijao.2011.6333. [DOI] [PubMed] [Google Scholar]
- 42.Mauney JR, Volloch V, Kaplan DL. Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and future prospects. Tissue Eng. 2005;11:787–802. doi: 10.1089/ten.2005.11.787. [DOI] [PubMed] [Google Scholar]
- 43.Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev. 2010;16:405–12. doi: 10.1089/ten.TEB.2009.0714. [DOI] [PubMed] [Google Scholar]
- 44.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. 2012;35:213–21. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]
- 46.Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–92. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 48.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–79. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gir P, Oni G, Brown SA, Mojallal A, Rohrich RJ. Human adipose stem cells: current clinical applications. Plast Reconstr Surg. 2012;129:1277–90. doi: 10.1097/PRS.0b013e31824ecae6. [DOI] [PubMed] [Google Scholar]
- 51.Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration--bench to bedside. J Biomed Mater Res B Appl Biomater. 2009;89:252–63. doi: 10.1002/jbm.b.31199. [DOI] [PubMed] [Google Scholar]
- 52.Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell. 2009;5:472–81. doi: 10.1016/j.stem.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bodle JC, Hanson AD, Loboa EG. Adipose-derived stem cells in functional bone tissue engineering: lessons from bone mechanobiology. Tissue Eng Part B Rev. 2011;17:195–211. doi: 10.1089/ten.teb.2010.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Correia C, Bhumiratana S, Yan LP, Oliveira AL, Gimble JM, Rockwood D, Kaplan DL, Sousa RA, Reis RL, Vunjak-Novakovic G. Development of silk-based scaffolds for tissue engineering of bone from human adipose-derived stem cells. Acta Biomater. 2012;8:2483–92. doi: 10.1016/j.actbio.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr Stem Cell Res Ther. 2010;5:116–21. doi: 10.2174/157488810791268582. [DOI] [PubMed] [Google Scholar]
- 56.Wang W, Cao B, Cui L, Cai J, Yin J. Adipose tissue engineering with human adipose tissue-derived adult stem cells and a novel porous scaffold. J Biomed Mater Res B Appl Biomater. 2013;101:68–75. doi: 10.1002/jbm.b.32816. [DOI] [PubMed] [Google Scholar]
- 57.Hildner F, Albrecht C, Gabriel C, Redl H, van Griensven M. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med. 2011;5:e36–51. doi: 10.1002/term.386. [DOI] [PubMed] [Google Scholar]
- 58.Cheng NC, Estes BT, Young TH, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19:484–96. doi: 10.1089/ten.tea.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–95. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 61.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–91. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 62.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–23. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 63.Benoit DS, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12:1663–73. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues SC, Salgado CL, Sahu A, Garcia MP, Fernandes MH, Monteiro FJ. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J Biomed Mater Res A. 2012 doi: 10.1002/jbm.a.34394. (in press) [DOI] [PubMed] [Google Scholar]
- 65.Müller U, Imwinkelried T, Horst M, Sievers M, Graf-Hausner U. Do human osteoblasts grow into open-porous titanium? Eur Cell Mater. 2006;11:8–15. doi: 10.22203/ecm.v011a02. [DOI] [PubMed] [Google Scholar]
- 66.Ignatius A, Blessing H, Liedert A, Schmidt C, Neidlinger-Wilke C, Kaspar D, Friemert B, Claes L. Tissue engineering of bone: effects of mechanical strain on osteoblastic cells in type I collagen matrices. Biomaterials. 2005;26:311–18. doi: 10.1016/j.biomaterials.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 67.Khatiwala CB, Kim PD, Peyton SR, Putnam AJ. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24:886–98. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibon E, Batke B, Jawad MU, Fritton K, Rao A, Yao Z, Biswal S, Gambhir SS, Goodman SB. MC3T3-E1 osteoprogenitor cells systemically migrate to a bone defect and enhance bone healing. Tissue Eng Part A. 2012;9–10:986–73. doi: 10.1089/ten.tea.2011.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayakumar P, Di Silvio L. Osteoblasts in bone tissue engineering. Proc Inst Mech Eng H. 2010;224:1415–40. doi: 10.1243/09544119JEIM821. [DOI] [PubMed] [Google Scholar]
- 70.Khan OF, Sefton MV. Endothelialized biomaterials for tissue engineering applications in vivo. Trends Biotechnol. 2011;29:379–87. doi: 10.1016/j.tibtech.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hendrickx B, Vranckx JJ, Luttun A. Cell-based vascularization strategies for skin tissue engineering. Tissue Eng Part B Rev. 2011;17:13–24. doi: 10.1089/ten.TEB.2010.0315. [DOI] [PubMed] [Google Scholar]
- 72.Lokmic Z, Mitchell GM. Engineering the microcirculation. Tissue Eng Part B Rev. 2008;14:87–103. doi: 10.1089/teb.2007.0299. [DOI] [PubMed] [Google Scholar]
- 73.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–64. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang G, Suggs LJ. Matrices and scaffolds for drug delivery in vascular tissue engineering. Adv Drug Deliv Rev. 2007;59:360–73. doi: 10.1016/j.addr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 75.Nomi M, Atala A, Coppi PD, Soker S. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23:463–83. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 76.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol. 2009;297:C179–87. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 77.Kachgal S, Carrion B, Janson IA, Putnam AJ. Bone marrow stromal cells stimulate an angiogenic program that requires endothelial MT1-MMP. J Cell Physiol. 2012;227:3546–55. doi: 10.1002/jcp.24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grainger SJ, Putnam AJ. Assessing the permeability of engineered capillary networks in a 3D culture. PLoS One. 2011;6:e22086. doi: 10.1371/journal.pone.0022086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davison PM, Bensch K, Karasek MA. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol. 1980;75:316–21. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- 80.Richard L, Velasco P, Detmar M. A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res. 1998;240:1–6. doi: 10.1006/excr.1998.3936. [DOI] [PubMed] [Google Scholar]
- 81.Eckermann CW, Lehle K, Schmid SA, Wheatley DN, Kunz-Schughart LA. Characterization and modulation of fibroblast/endothelial cell co-cultures for the in vitro preformation of three-dimensional tubular networks. Cell Biol Int. 2011;35:1097–110. doi: 10.1042/CBI20100718. [DOI] [PubMed] [Google Scholar]
- 82.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453–63. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 83.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–8. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 84.Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis. 2007;49:421–9. doi: 10.1016/j.pcad.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302–5. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choong CS, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521–31. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- 87.Correia C, Grayson W, Eton R, Gimble JM, Sousa RA, Reis RL, Vunjak-Novakovic G. Human adipose-derived cells can serve as a single-cell source for the in vitro cultivation of vascularized bone grafts. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1564. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang R, Gao Z, Geng W, Yan X, Chen F, Liu Y. Engineering vascularized bone graft with osteogenic and angiogenic lineage differentiated bone marrow mesenchymal stem cells. Artif Organs. 2012;36:1036–46. doi: 10.1111/j.1525-1594.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 89.Villars F, Guillotin B, Amédée T, Dutoya S, Bordenave L, Bareille R, Amédée J. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775–85. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 90.Laranjeira MS, Fernandes MH, Monteiro FJ. Reciprocal induction of human dermal microvascular endothelial cells and human mesenchymal stem cells: time-dependent profile in a co-culture system. Cell Prolif. 2012;45:320–34. doi: 10.1111/j.1365-2184.2012.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, Ye Y, Tian H, Yang S, Jin X, Tong W, Zhang Y. In vitro osteogenesis of human adipose-derived stem cells by coculture with human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2011;412:143–9. doi: 10.1016/j.bbrc.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 92.Stahl A, Wenger A, Weber H, Stark GB, Augustin HG, Finkenzeller G. Bi-directional cell contact-dependent regulation of gene expression between endothelial cells and osteoblasts in a three-dimensional spheroidal coculture model. Biochem Biophys Res Commun. 2004;322:684–92. doi: 10.1016/j.bbrc.2004.07.175. [DOI] [PubMed] [Google Scholar]
- 93.Hager S, Lampert FM, Orimo H, Stark GB, Finkenzeller G. Up-regulation of alkaline phosphatase expression in human primary osteoblasts by cocultivation with primary endothelial cells is mediated by p38 mitogen-activated protein kinase-dependent mRNA stabilization. Tissue Eng Part A. 2009;15:3437–47. doi: 10.1089/ten.TEA.2009.0133. [DOI] [PubMed] [Google Scholar]
- 94.Xue Y, Xing Z, Hellem S, Arvidson K, Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online. 2009;8:34. doi: 10.1186/1475-925X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guillotin B, Bareille R, Bourget C, Bordenave L, Amédée J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone. 2008;42:1080–91. doi: 10.1016/j.bone.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 96.Leszczynska J, Zyzynska-Granica B, Koziak K, Ruminski S, Lewandowska-Szumiel M. Contribution of endothelial cells to human bone-derived cells expansion in coculture. Tissue Eng Part A. 2013;19:393–402. doi: 10.1089/ten.tea.2011.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steiner D, Lampert F, Stark GB, Finkenzeller G. Effects of endothelial cells on proliferation and survival of human mesenchymal stem cells and primary osteoblasts. J Orthop Res. 2012;30:1682–9. doi: 10.1002/jor.22130. [DOI] [PubMed] [Google Scholar]
- 98.Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95–103. doi: 10.1089/107632703762687573. [DOI] [PubMed] [Google Scholar]
- 99.Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amédée J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem. 2009;106:390–8. doi: 10.1002/jcb.22018. [DOI] [PubMed] [Google Scholar]
- 100.Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ. Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A. 2010;16:1235–7. doi: 10.1089/ten.tea.2009.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoch AI, Binder BY, Genetos DC, Leach JK. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One. 2012;7:e35579. doi: 10.1371/journal.pone.0035579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Amédée J, Granja PL. Phenotypic and proliferative modulation of human mesenchymal stem cells via crosstalk with endothelial cells. Stem Cell Res. 2011;7:186–97. doi: 10.1016/j.scr.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Ma J, van den Beucken JJ, Yang F, Both SK, Cui FZ, Pan J, Jansen JA. Coculture of osteoblasts and endothelial cells: optimization of culture medium and cell ratio. Tissue Eng Part C Methods. 2011;17:349–57. doi: 10.1089/ten.TEC.2010.0215. [DOI] [PubMed] [Google Scholar]
- 104.Kolbe M, Xiang Z, Dohle E, Tonak M, Kirkpatrick CJ, Fuchs S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng Part A. 2011;17:2199–212. doi: 10.1089/ten.TEA.2010.0474. [DOI] [PubMed] [Google Scholar]
- 105.Pedersen TO, Blois AL, Xue Y, Xing Z, Cottler-Fox M, Fristad I, Leknes KN, Lorens JB, Mustafa K. Osteogenic stimulatory conditions enhance growth and maturation of endothelial cell microvascular networks in culture with mesenchymal stem cells. J Tissue Eng. 2012;3(1):2041731412443236. doi: 10.1177/2041731412443236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thébaud NB, Siadous R, Bareille R, Remy M, Daculsi R, Amédée J, Bordenave L. Whatever their differentiation status, human progenitor derived - or mature - endothelial cells induce osteoblastic differentiation of bone marrow stromal cells. J Tissue Eng Regen Med. 2012;6:e51–60. doi: 10.1002/term.1539. [DOI] [PubMed] [Google Scholar]
- 107.Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–57. doi: 10.22203/ecm.v022a19. [DOI] [PubMed] [Google Scholar]
- 108.Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–15. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 109.Kang Y, Kim S, Fahrenholtz M, Khademhosseini A, Yang Y. Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold. Acta Biomater. 2013;9:4906–15. doi: 10.1016/j.actbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nukavarapu SP, Amini AR. Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2464–7. doi: 10.1109/IEMBS.2011.6090684. [DOI] [PubMed] [Google Scholar]
- 111.Buschmann J, Welti M, Hemmi S, Neuenschwander P, Baltes C, Giovanoli P, Rudin M, Calcagni M. Three-dimensional co-cultures of osteoblasts and endothelial cells in DegraPol foam: histological and high-field magnetic resonance imaging analyses of pre-engineered capillary networks in bone grafts. Tissue Eng Part A. 2011;17:291–9. doi: 10.1089/ten.TEA.2010.0278. [DOI] [PubMed] [Google Scholar]
- 112.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–7. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 113.Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One. 2011;6:e28352. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mendes LF, Pirraco RP, Szymczyk W, Frias AM, Santos TC, Reis RL, Marques AP. Perivascular-like cells contribute to the stability of the vascular network of osteogenic tissue formed from cell sheet-based constructs. PLoS One. 2012;7:e41051. doi: 10.1371/journal.pone.0041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, Mizuno D, Mase J, Nishiguchi H, Kagami H, Ueda M. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2009;90:730–41. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- 116.Geuze RE, Wegman F, Oner FC, Dhert WJ, Alblas J. Influence of endothelial progenitor cells and platelet gel on tissue-engineered bone ectopically in goats. Tissue Eng Part A. 2009;15:3669–77. doi: 10.1089/ten.TEA.2009.0289. [DOI] [PubMed] [Google Scholar]
- 117.Fedorovich NE, Haverslag RT, Dhert WJ, Alblas J. The role of endothelial progenitor cells in prevascularized bone tissue engineering: development of heterogeneous constructs. Tissue Eng Part A. 2010;16:2355–67. doi: 10.1089/ten.TEA.2009.0603. [DOI] [PubMed] [Google Scholar]
- 118.Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, Lin Y, Friedrich CC, Daheron L, Lin CP, Sundback CA, Vacanti JP, Neville C. Engineered vascularized bone grafts. Proc Natl Acad Sci U S A. 2010;107:3311–6. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633–7. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 120.Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011;17:311–21. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 121.Kim JY, Jin GZ, Park IS, Kim JN, Chun SY, Park EK, Kim SY, Yoo J, Kim SH, Rhie JW, Cho DW. Evaluation of solid free-form fabrication-based scaffolds seeded with osteoblasts and human umbilical vein endothelial cells for use in vivo osteogenesis. Tissue Eng Part A. 2010;16:2229–36. doi: 10.1089/ten.TEA.2009.0644. [DOI] [PubMed] [Google Scholar]
- 122.He J, Decaris ML, Leach JK. Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A. 2012;18:1520–28. doi: 10.1089/ten.tea.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R, Bourget C, Amédée J, Barbosa MA. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30:3271–8. doi: 10.1016/j.biomaterials.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 124.Seebach C, Henrich D, Kähling C, Wilhelm K, Tami AE, Alini M, Marzi I. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng Part A. 2010;16:1961–70. doi: 10.1089/ten.TEA.2009.0715. [DOI] [PubMed] [Google Scholar]
- 125.Wang L, Rao RR, Stegemann JP. Delivery of Mesenchymal Stem Cells in Chitosan/Collagen Microbeads for Orthopedic Tissue Repair. Cells Tissues Organs. 2013;3:333–43. doi: 10.1159/000348359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rao RR, Peterson AW, Stegemann JP. Osteogenic differentiation of adipose-derived and marrow-derived mesenchymal stem cells in modular protein/ceramic microbeads. J Biomed Mater Res A. 2013;101A:1531–8. doi: 10.1002/jbm.a.34611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caldwell DJ, Rao RR, Stegemann JP. Assembly of Discrete Collagen-Chitosan Microenvironments into Multiphase Tissue Constructs. Adv Healthc Mater. 2012 doi: 10.1002/adhm.201200346. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]