Abstract
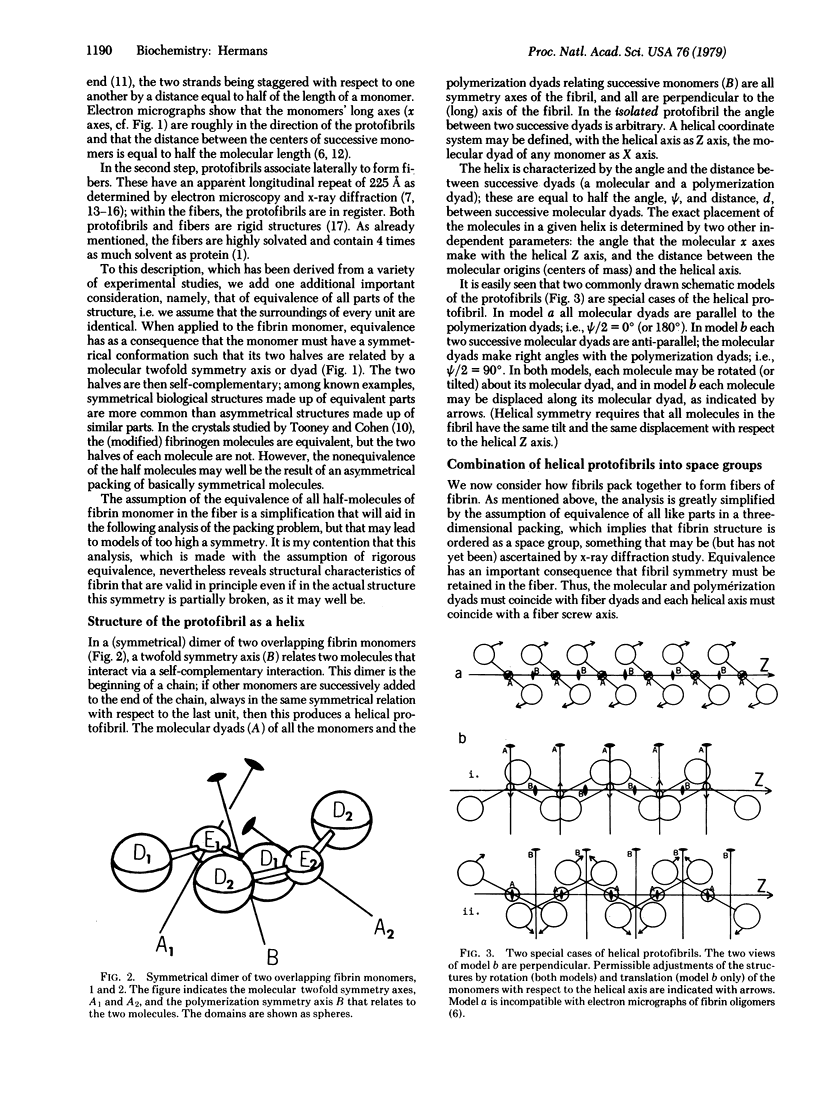
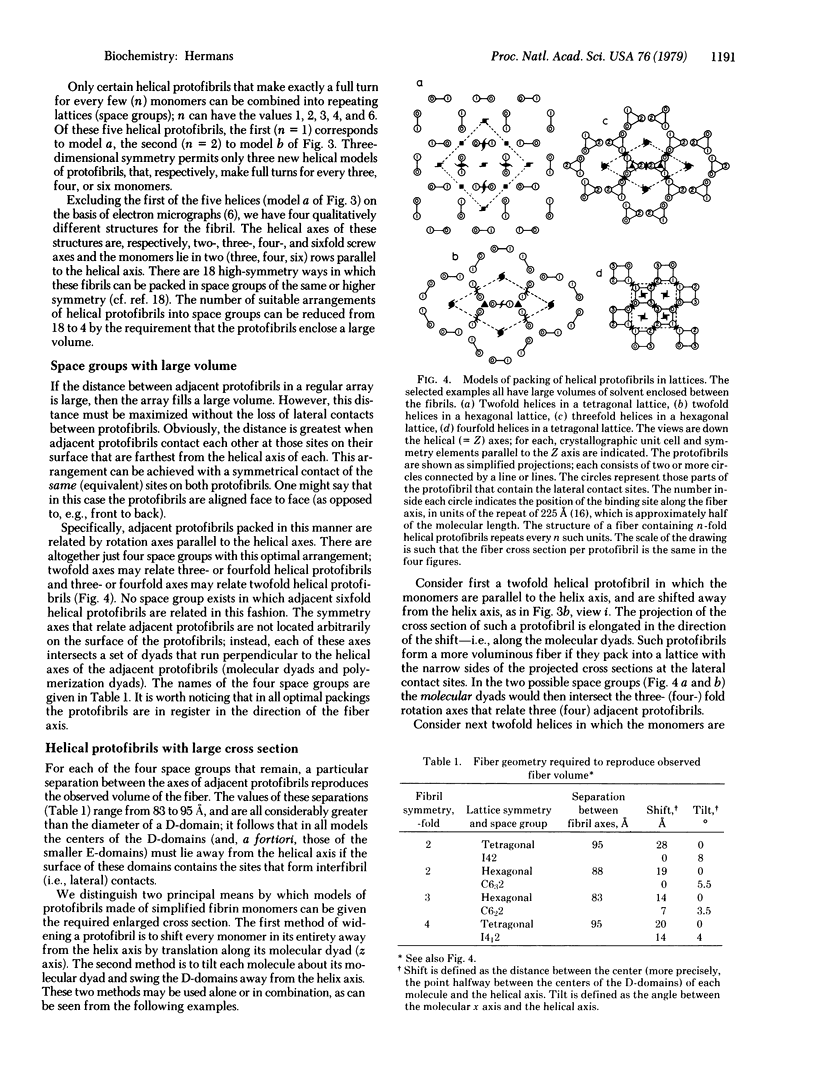
High-symmetry models of the fibrin fiber are proposed that reproduce the experimentally observed high specific volume of the fiber. The models meet the following criteria: fibrin monomers have the three-domain Hall and Slayter structure; the monomers are arranged lengthwise into strands (protofibrils) in which successive monomers half overlap; the monomers' alignment is nearly parallel to the fiber axis; and the monomers make adequate longitudinal and lateral contacts, as required by observed fiber properties and the high affinity of monomers for one another. All the models contain helical protofibrils related to each other by rotation axes parallel to the fiber axis; as a consequence the protofibrils are in register in the fiber direction. The protofibrils may contain two, three, or four monomers per helical turn and can be packed in four different symmetries (space groups). A large specific volume is achieved only if the D-domain (which are presumed to contain the lateral polymerization sites) are somewhat displaced from the helical axes of the protofibrils. This displacement may involve either a lateral shift of the monomers away from the helix axis or a tilt of the monomers, which swings the D-domains away from the helix axis. It is shown that the fiber containing tilted monomers is more highly interconnected; the two D-domains of each tilted monomer form lateral contacts with different adjacent protofibrils, whereas the two D-domains of each nontilted monomer contact the same adjacent protofibril(s).
Full text
PDF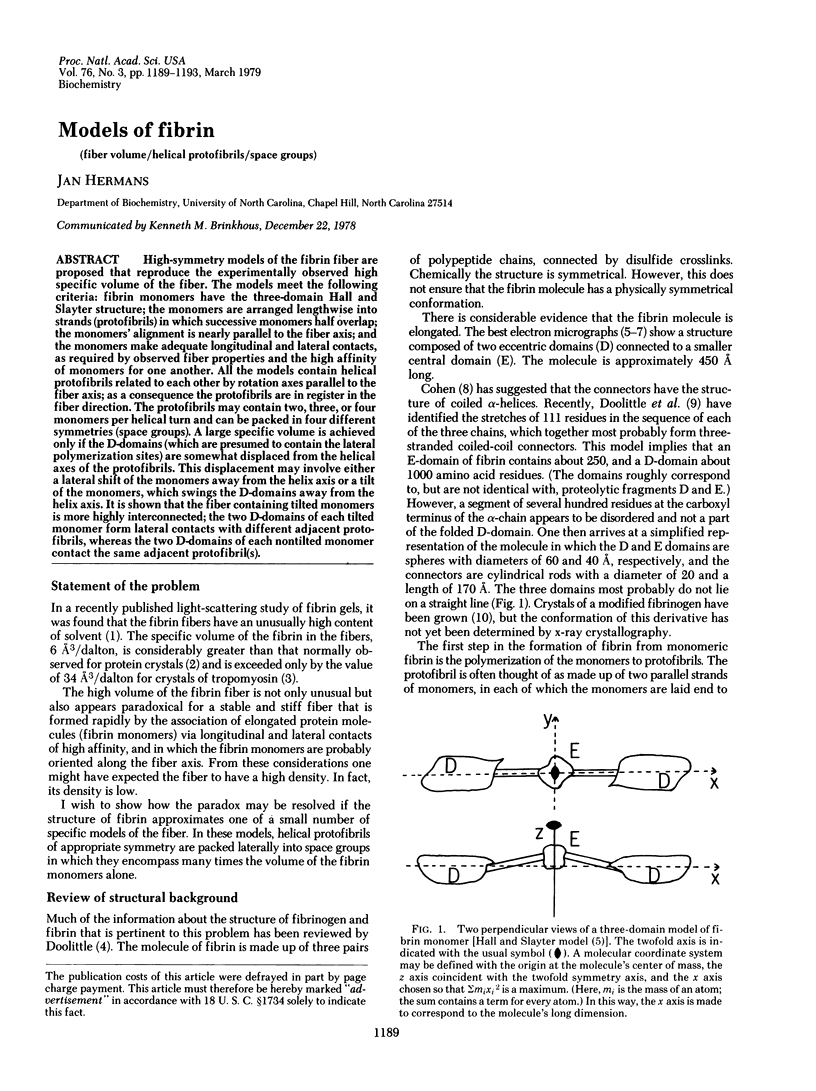


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carr M. E., Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules. 1978 Jan-Feb;11(1):46–50. doi: 10.1021/ma60061a009. [DOI] [PubMed] [Google Scholar]
- Cohen C., Caspar D. L., Parry D. A., Lucas R. M. Tropomyosin crystal dynamics. Cold Spring Harb Symp Quant Biol. 1972;36:205–216. doi: 10.1101/sqb.1972.036.01.028. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Goldbaum D. M., Doolittle L. R. Designation of sequences involved in the "coiled-coil" interdomainal connections in fibrinogen: constructions of an atomic scale model. J Mol Biol. 1978 Apr 5;120(2):311–325. doi: 10.1016/0022-2836(78)90070-0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Ferry J. D. The Mechanism of Polymerization of Fibrinogen. Proc Natl Acad Sci U S A. 1952 Jul;38(7):566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL C. E., SLAYTER H. S. The fibrinogen molecule: its size, shape, and mode of polymerization. J Biophys Biochem Cytol. 1959 Jan 25;5(1):11–16. doi: 10.1083/jcb.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D., Cuddigan B. J. The fine structure of fibrin. Br J Haematol. 1967 May;13(3):341–347. doi: 10.1111/j.1365-2141.1967.tb08749.x. [DOI] [PubMed] [Google Scholar]
- Krakow W., Endres G. F., Siegel B. M., Scheraga H. A. An electron microscopic investigation of the polymerization of bovine fibrin monomer. J Mol Biol. 1972 Oct 28;71(1):95–103. doi: 10.1016/0022-2836(72)90403-2. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Nestler F. H., Schrag J. L., Ferry J. D. Infinite-dilution viscoelastic properties of fibrinogen and intermediate fibrin polymer. Biopolymers. 1977 Sep;16(9):1957–1969. doi: 10.1002/bip.1977.360160910. [DOI] [PubMed] [Google Scholar]
- STRYER L., COHEN C., LANGRIDGE R. Axial period of fibrinogen and fibrin. Nature. 1963 Feb 23;197:793–794. doi: 10.1038/197793a0. [DOI] [PubMed] [Google Scholar]
- Tooney N. M., Cohen C. Crystalline states of a modified fibrinogen. J Mol Biol. 1977 Feb 25;110(2):363–385. doi: 10.1016/s0022-2836(77)80077-6. [DOI] [PubMed] [Google Scholar]


