Abstract
Haploid budding yeast has two mating types, defined by the alleles of the MAT locus, MATa and MATα. Two haploid cells of opposite mating types mate by signaling to each other using reciprocal pheromones and receptors, polarizing and growing toward each other, and eventually fusing to form a single diploid cell. The pheromones and receptors are necessary and sufficient to define a mating type, but other mating-type-specific proteins make mating more efficient. We examined the role of these proteins by genetically engineering “transvestite” cells that swap the pheromone, pheromone receptor, and pheromone processing factors of one mating type for another. These cells mate with each other, but their mating is inefficient. By characterizing their mating defects and examining their transcriptomes, we found Afb1 (a-factor barrier), a novel MATα-specific protein that interferes with a-factor, the pheromone secreted by MATa cells. Strong pheromone secretion is essential for efficient mating, and the weak mating of transvestites can be improved by boosting their pheromone production. Synthetic biology can characterize the factors that control efficiency in biological processes. In yeast, selection for increased mating efficiency is likely to have continually boosted pheromone levels and the ability to discriminate between partners who make more and less pheromone. This discrimination comes at a cost: weak mating in situations where all potential partners make less pheromone.
Keywords: Afb1, mating, pheromone signaling, robustness, genetic engineering
BIOLOGICAL processes are typically defined by the genes that are necessary and sufficient for function. However, in many cases, this minimal gene set does not encompass all the proteins involved in a process, and additional proteins promote biological efficiency. Finding these additional proteins may require the detection of subtle phenotypes, making it hard to know if all the genes involved in a process have been identified. One way to answer this question is to reengineer a pathway and ask whether the synthetic version fully mimics the natural function. Here, we show that this form of synthetic biology illuminates how cells of the budding yeast Saccharomyces cerevisiae mate efficiently.
Budding yeast can be stably maintained as haploids or diploids. Haploids mate when two cells of opposite mating types signal to each other using reciprocal pheromones and receptors, polarize and grow toward each other, and eventually fuse to form a single diploid. Yeast has two mating types, a and α (Figure 1A), determined by two alternative alleles at the MAT locus, MATa and MATα, which encode different transcription factors (Herskowitz 1988). These factors regulate the expression of mating-type-specific genes, many of which are involved with the production and detection of the pheromones that yeast cells use to signal to one another. The pheromones (a- and α-factor) are detected by G-protein-coupled receptors. MATa cells express a-factor (Betz and Duntze 1979), which is secreted through an ATP binding cassette transporter (Ste6) (McGrath and Varshavsky 1989) and the α-factor receptor (Ste2) (Blumer et al. 1988; Dohlman and Thorner 2001). MATα cells express α-factor (Kurjan and Herskowitz 1982; Singh et al. 1983) and the a-factor receptor (Ste3) (Hagen et al. 1986; Dohlman and Thorner 2001). Pheromone binding activates a signaling pathway that produces three responses: cell polarization, cell cycle arrest in G1, and increased transcription of pheromone response genes (Bardwell 2005).
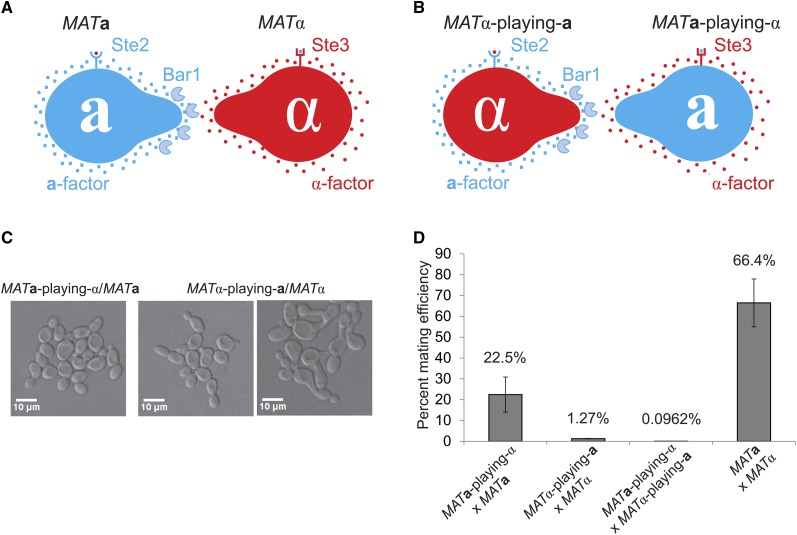
Figure 1.
Yeast cells expressing the pheromone and receptor of the opposite mating type are capable of forming zygotes with cells of their original mating type. (A) MATa cells express the pheromone a-factor, the α-factor receptor Ste2, and the α-factor protease Bar1. MATα cells express the pheromone α-factor and the a-factor receptor Ste3. (B) MATα-playing-a cells are MATα cells that express a-factor instead of α-factor, Ste2 instead of Ste3, and the α-factor protease Bar1. MATa-playing-α cells are MATa cells that express α-factor instead of a-factor, Ste3 instead of Ste2, and lack Bar1 (bar1Δ). (C) The indicated diploid strains were grown in YPD, and pictures were taken using DIC with ×20 magnification. (Right) Abnormal morphologies indicative of cells secreting pheromones that they can respond to. (D) Mating efficiency of the indicated crosses. Mating efficiencies are the percentage of diploids that form colonies on double dropout plates relative to the number of colonies formed on single dropout plates. Error bars are standard deviations. Matings were performed as described in Materials and Methods.
Bender and Sprague (1989) used mutations that alter pheromone and receptor expression to show that a cell’s mating type is determined by which pheromones and receptors it expresses. Although pheromone secretion and detection are the essential elements for mating, additional, mating-type-specific genes make mating more efficient. One of these is the MATa-specific α-factor protease, Bar1 (Sprague and Herskowitz 1981; MacKay et al. 1988), which helps MATa cells detect an α-factor gradient and polarize toward MATα partners (Jackson and Hartwell 1990; Barkai et al. 1998). Yeast cells also express mating-type-specific agglutinins, which help cells attach to mating partners (Cappellaro et al. 1991) in liquid but individually have little effect on mating efficiency on solid media (Lipke et al. 1989; Roy et al. 1991; de Nobel et al. 1995). Evidence for the final, characterized MATa-specific gene was produced by Bender and Sprague (1989) who noted that cells expressing MATa-specific proteins and Ste3 were unable to mount a pheromone response. The gene responsible for this was later identified as ASG7, which terminates pheromone signaling after mating has occurred and allows diploid cells to escape from the G1 arrest of their parental haploid cell (Kim et al. 2000; Roth et al. 2000).
Bender and Sprague (1989) used mutations at MAT and exogenous promoters to manipulate pheromone and receptor expression. As a result, any quantitative defects in mating could reflect incorrect levels of pheromone and receptor expression or the accessory role of other genes in mating. To distinguish these possibilities, we constructed “transvestite” strains: genetically engineered strains that have a wild-type allele at MAT but express the pheromone, pheromone receptor, and proteins responsible for secreting or processing pheromones that are normally induced by the other MAT allele (Figure 1B). These strains should mate well if we have swapped all the genes required for efficient mating and expressed them at the right level. Mating defects in these engineered cells indicate the presence of additional, uncharacterized, mating-type-specific proteins or incorrect expression of the known mating genes.
By studying these genetically engineered cells, we learned more about the requirements for efficient mating. MATa-playing-α cells (MATa cells that express α-factor and Ste3) mate threefold less efficiently than genuine MATα cells. Their main defect is low α-factor secretion: increasing α-factor production makes them mate almost as well as genuine MATα cells. In contrast, MATα-playing-a cells (MATα cells that express a-factor, Ste6, Ste2, and Bar1) mate 60-fold less efficiently than genuine MATa cells. These transvestites have two defects: they express a novel, MATα-specific a-factor blocker, which we named Afb1 (a-factor barrier), and they show a transient as opposed to a prolonged arrest when exposed to α-factor. Our manipulations reveal that mating is not robust to reduced levels of pheromone production.
Materials and Methods
Yeast strains and culturing
Supporting Information Table S1 lists the strains that we used. All strains were derived from the W303 wild-type background (ade2-1 can1-100 his3-11,15 leu2-112 trp1-1 ura3-1) using standard genetic techniques. All media were prepared as described (Sherman et al. 1974) and contained 2% wt/vol of glucose. Cells were grown in synthetic complete (SC) media (2% glucose) or yeast extract peptone dextrose (YPD) (2% glucose) at 30° in culture tubes on roller drums or on agar plates or at room temperature (25°) for time-lapse microscopy. Mating assays used agar plates containing SC without adenine (SC−ade), SC without uracil (SC−ura), or SC without adenine and uracil (SC−ade−ura). Bovine serum albumin (BSA) was used to reduce the nonspecific absorption of α-factor to glass and plastic surfaces. A 10% wt/vol stock was prepared in deionized water and then diluted into media to 0.1% wt/vol. Synthetic α-factor (Biosynthesis, Lewisville, TX) was suspended in dimethyl sulfoxide and then diluted into either YPD + 0.1% BSA or SC + 0.1% BSA at the appropriate concentration. Yeast extract was obtained from EMD Millipore (Billerica, MA). Peptone and yeast nitrogen base were obtained from BD (Franklin Lakes, NJ). Bacto-agar was obtained from US Biological (Swampscott, MA). Unless otherwise noted, all chemicals were obtained from Sigma-Aldrich (St. Louis).
Quantitative mating assay
Quantitative mating assays were modified from Reid and Hartwell (1977). Briefly, cells were grown to log phase (∼5 × 106 cells/ml). Cells (5 × 106) were harvested from each strain, mixed at a 1:1 ratio, sonicated, and filtered onto a 0.22-µm nitrocellulose filter (Millipore). Filters were placed on a YPD plate and incubated at 30° for 5 hr. To assay for the initial ratio of the haploid cells, a 2.5 × 10−5 dilution of the initial mating mixture was plated onto SC−ade and SC−ura plates. After 5 hr, cells were washed off the filters into 1 ml of deionized water and then plated onto SC−ade, SC−ura, and SC−ade−ura plates at appropriate dilutions to produce ∼400 colonies per plate. SC dropout plates were incubated for 2 days before counting the colonies on each plate. Mating efficiencies were determined by dividing the number of colonies on the SC−ade−ura plate by the number of colonies on whichever of the SC−ade or SC−ura plates plated after the mating incubation had fewer colonies. Three technical replicates were done of each mating assay and averaged for a single biological replicate. Error bars are the standard deviation of at least five biological replicates. Statistical significance was determined using Student’s t-test.
Bioassay for α-factor production
The bioassay for α-factor production was modified from Gonçalves-Sá and Murray (2011). For details see File S1.
Shmooing index
Cells were grown to log phase (∼5 × 106 cells/ml), washed into YPD + 0.1% BSA with various concentrations of synthetic α-factor added at 5 × 105 cells/ml, and then incubated at 30° on a roller drum for 2 hr. After incubation, the cells were sonicated, fixed using 60% ethanol at −20°, and resuspended into 20% glycerol in phosphate-buffered saline (PBS). Culture tubes were BSA-coated by incubating overnight at 4° with PBS + 2% BSA. The PBS + 2% BSA was poured out immediately prior to the use of the culture tube. At least 200 cells were counted to determine the percentage of cells shmooing. Error bars are standard deviations. Statistical significance was determined using Student’s t-test.
Microscopy
Microscopy was done at room temperature using a Nikon Ti-E inverted microscope with either a ×20 Plan Apo VC 0.75NC air lens or a ×60 Plan Apo VC 1.4NA oil lens, and images were acquired with a Photometrics CoolSNAP HQ camera (Roper Scientific). Time-lapse photography was done using Metamorph 7.7 (Molecular Devices). For details see File S2.
Halo assay
Halo assays were modified from Sprague (1991). Cells whose a-factor production was to be measured were grown to saturation in YPD at 30°. For halo assays on individual strains, 4.5 × 108 cells of each strain were pelleted and resuspended in 20 µl of deionized water. For halo assays on cell mixtures, cells were mixed at a 1:8 ratio (MATa wild-type:cell type of interest) with a final cell count of 4.5 × 108. Cells were pelleted and resuspended in 20 µl of deionized water. Ten microliters of each strain or strain mix was spotted onto YPD plates and incubated overnight (∼24 hr) at 30°. Supersensitive MATα sst2Δ cells grown to stationary phase were then sprayed over the cell spots using a martini atomizer (item 900432, Oenophilia, Hillsborough, NC). Plates were incubated overnight (∼18 hr) at 30°, and pictures were taken using a Panasonic (Secaucus, NJ) Lumix DMC-TZ5 camera.
RNA isolation and sequencing
Cells were grown to log phase (5 × 106 cells/ml) in YPD + 0.1% BSA at 30°. Ten milliliters of the culture was harvested by spinning at 4°, washed in 1 ml RNase-free ice-cold water, pelleted, and flash-frozen in dry ice. α-Factor (10 nM) was added to the remaining culture, incubated for 2 hr at 30°, and harvested in the same manner. RNA was isolated as described by Collart and Oliviero (2001) and dissolved in 1 mM sodium citrate, pH 6.4. RNase-free chemicals were obtained from Invitrogen (Carlsbad, CA) except for chloroform, which was obtained from VWR (Radnor, PA).
RNA libraries were prepared using the Illumina TruSeq kit (http://www.illumina.com) and sequenced using an Illumina HiSequation 2000 with 50-bp single-end reads with 89× mean coverage across the genome.
Sequence analysis
To analyze the sequencing data, the RNA sequences were aligned to the S288C reference genome r64 (downloaded from the Saccharomyces Genome Database at http://www.yeastgenome.org) using TopHat (Trapnell et al. 2009). We then used Cufflinks (Trapnell et al. 2010) to look for genes with significantly different levels of gene expression between MATa bar1Δ cells and MATα-playing-a PBAR1-BAR1 cells. Significant differences in expression were identified using the default setting in Cufflinks, which tests the observed log-fold change in gene expression against the null hypothesis of no difference between the two samples with a false discovery rate of 0.05 (Trapnell et al. 2010). The data discussed in this publication have been deposited in Gene Expression Omnibus at the National Center for Biotechnology Information (NCBI) (Edgar et al. 2002) and are accessible through GEO series accession no. GSE49372 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE49372).
Results
Transvestite cells can mate
To find genes required for efficient mating, we constructed two types of transvestite strains. MATa-playing-α cells are MATa cells that have been engineered to produce α-factor and the a-factor receptor by replacing the open reading frame of STE2 with STE3 and the open reading frames of the two a-factor genes with those of the two α-factor genes (replacing MFA1 with MFα1 and MFA2 with MFα2) (Figure 1B). We also deleted BAR1, which encodes the α-factor protease (Sprague and Herskowitz 1981), and ASG7, which inhibits signaling from Ste3 (Roth et al. 2000). However, these cells are still MATa at the MAT locus and thus will have MATa-specific expression patterns for all genes except those we manipulated. MATα-playing-a cells are MATα at the MAT locus but have been engineered to produce a-factor and the α-factor receptor by replacing the open reading frame of STE3 with STE2, MFα1 with MFA1, and MFα2 with MFA2 (Figure 1B). We also drove the expression of BAR1 with an engineered version of the haploid-specific promoter PFUS1 (Ingolia and Murray 2007), which is expressed in both MATa and MATα cells (Trueheart et al. 1987), and the expression of the a-factor transporter STE6 with the MFα1 promoter. Since we tested mating efficiency on solid media, we did not manipulate the expression of the mating agglutinins, which function mainly when cells mate in liquid (Lipke et al. 1989; Roy et al. 1991).
We asked if the manipulated genes are the only mating-type-specific proteins required for efficient mating. Crossing these cells with wild-type cells of their original mating type (e.g., MATα-playing-a crossed to MATα) carries a potential caveat. When two wild-type cells mate, the combination of the transcription factors expressed from MATa and MATα turns off the pheromone and receptor genes of both mating types (Haber 1998), and the zygotes escape pheromone-induced G1 arrest (Roth et al. 2000). But zygotes produced by crossing a transvestite to a wild-type cell of the same mating type will keep expressing pheromones and receptors from both mating types (since both parents have the same MAT locus), raising the concern that these zygotes respond to their own pheromones and remain arrested in G1. To measure the mating efficiency of these crosses, we selected for viable diploids by crossing transvestite cells and wild-type cells with complementary nutritional requirements. We obtained both MATa-playing-α/MATa and MATα-playing-a/MATα diploids. Most of these diploids progress normally through the cell cycle and have normal cell morphology (Figure 1C). We did find an occasional population of MATα-playing-a/MATα diploids with abnormal morphology, suggesting delayed progression through G1, but even these are capable of budding (Figure 1C). The ability of these diploids to bud indicates that it is possible to measure the mating efficiency of transvestites crossed with wild-type cells.
We used quantitative mating assays to measure the mating efficiency of the transvestite cells. Cells of the two mating types are incubated together and then plated on media that distinguishes diploid cells from either parental haploid. When wild-type MATa cells are mated with wild-type MATα cells, 66% of haploids form diploids (Figure 1D). However, the mating efficiency of the MATa-playing-α cells crossed with MATa cells is 3-fold lower than that of a wild-type cross, and the mating efficiency of the MATα-playing-a cells crossed with MATα cells is ∼60-fold lower than the efficiency of a wild-type cross (Student’s t-test, P < 10−6) (Figure 1D). These mating defects are synergistic: crossing the transvestite strains to each other decreases mating efficiency ∼700-fold (Student’s t-test, P < 10−6) (Figure 1D). Our observation that transvestites can mate with unmanipulated strains with the same MAT locus confirms earlier work showing that pheromones and receptors define a cell’s mating type (Bender and Sprague 1989). But the low mating efficiency of the transvestite crosses implies that there are additional requirements for efficient mating.
MATa-playing-α cells produce too little α-factor
We studied the mating defects of transvestite cells. MATa-playing-α cells mate threefold less efficiently than genuine MATα cells (Figure 1D). Because the engineered genes in this strain encode the pheromone and pheromone receptor, the best candidates for this difference were the ability of the MATa-playing-α cells to respond to a-factor and to produce α-factor.
We began by testing the response to a-factor. We made mating mixtures of MATa-playing-α cells expressing YFP under the pheromone-inducible promoter PFUS1 and MATa cells expressing mCherry under the ACT1 promoter and assayed for the expression of YFP in the MATa-playing-α cells after 2.5 hr. The expression of YFP in the MATa-playing-α cells indicates that they can successfully detect a-factor using the a-factor receptor and activate pheromone-induced genes (Figure 2A). The two cell types mated to form zygotes that continue to signal to themselves, thus forming diploid cells, which express both YFP under the FUS1 promoter and mCherry under the ACT1 promoter (Figure 2A). Since the G-protein and downstream components of the pheromone-signaling pathway should be the same in MATa and MATα cells (Bardwell 2005), we asked if reduced pheromone production is the cause of the mating defect.
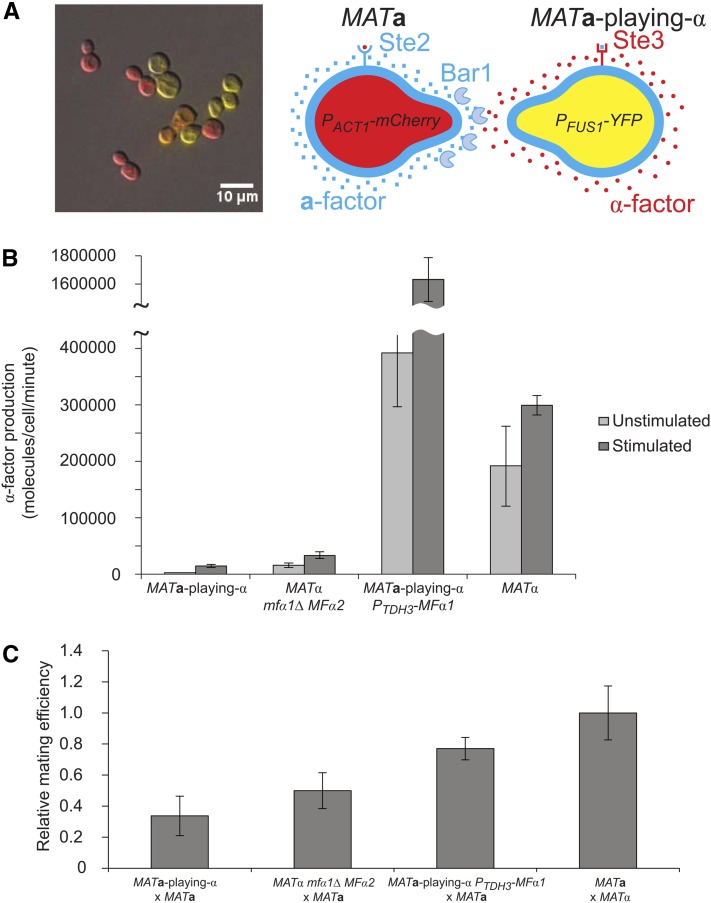
Figure 2.
Low mating efficiency of MATa-playing-α cells is due to low α-factor production. (A) MATa-playing-α PFUS1-YFP cells in a mating mixture with MATa PACT1-mCherry cells. Yellow indicates YFP expression. Red indicates mCherry expression. The orange cell is a diploid expressing both YFP and mCherry. The picture was taken 2.5 hr after mixing the cells using DIC and fluorescence at ×20 magnification. (B) α-Factor production is measured by growing cells in YPD, harvesting the supernatant, and exposing MATa bar1Δ cells to the supernatant. The shmooing index of the MATa bar1Δ cells is measured and then compared to a standard curve, produced with synthetic α-factor, to determine the amount of α-factor present in the media. Error bars are standard deviations. (C) Mating efficiency relative to a wild-type cross between MATa and MATα cells. Matings were performed as described in Materials and Methods. Error bars are standard deviations.
Pheromone production is important for zygote formation (Kurjan 1985; Michaelis and Herskowitz 1988), and MATa cells prefer the MATα cell that produces the highest amount of α-factor (Jackson and Hartwell 1990). Since we manipulated pheromone genes to make the transvestites, we measured the pheromone production of MATa-playing-α cells using a bioassay. We grew cells in rich medium, filtered out the cells, incubated the medium with MATa cells lacking the α-factor protease Bar1, measured the fraction of cells that arrest and shmoo (the shmooing index), and compared these data to a standard curve generated with synthetic α-factor. Unstimulated, MATa-playing-α cells produce ∼70 times less α-factor than MATα cells (Student’s t-test, P = 0.01) (Figure 2B). To measure the α-factor production of stimulated cells, we mixed the α-factor-producing cells in a 10:1 mixture with MATa bar1Δ cells (which produce a-factor and do not destroy α-factor) and measured the α-factor present in the supernatant. Stimulated MATa-playing-α cells produce 20-fold less α-factor than stimulated wild-type MATα cells (Student’s t-test, P = 9 × 10−6) (Figure 2B).
To test the effect of reduced pheromone production in MATα cells, we knocked out MFα1, which is the majority α-factor producer in MATα cells (Kurjan 1985). This reduces α-factor production 12-fold compared to wild-type MATα cells in unstimulated cells (Student’s t-test, P = 0.02) and 9-fold in stimulated cells (Student’s t-test, P = 10−5) (Figure 2B). We compared the mating efficiency of MATα mfα1Δ cells, which have decreased mating efficiency (Kurjan 1985), to that of MATa-playing-α cells and determined that MATα mfα1Δ cells mate only 1.5-fold more efficiently than MATa-playing-α cells (Student’s t-test, P = 0.004) (Figure 2C). This suggests that the reduced mating efficiency of the MATa-playing-α cells is due, at least in part, to low α-factor production.
To test this hypothesis, we increased α-factor production in the MATa-playing-α cells by expressing MFα1 from the TDH3 promoter. This promoter is not pheromone-regulated, but it is one of the most highly expressed promoters in the yeast genome (Krebs 1953; McAlister and Holland 1985) and should increase α-factor production to at least wild-type MATα levels. Unstimulated MATa-playing-α PTDH3-MFα1 cells secrete twice as much α-factor as unstimulated MATα cells (Student’s t-test, P = 0.04), but when stimulated, MATa-playing-α PTDH3-MFα1 cells secrete fivefold more α-factor than stimulated MATα cells (Student’s t-test, P = 10−4) (Figure 2B), suggesting that α-factor production is regulated by post-translational mechanisms, such as pheromone maturation and secretion and transcription of the pheromone genes. If low α-factor production accounts for the weak mating of MATa-playing-α cells, MATa-playing-α PTDH3-MFα1 cells should have a mating efficiency approaching that of wild-type MATα cells, which is indeed what we found (Figure 2C). This confirms previous results, which showed that sufficient α-factor production is important for efficient mating (Kurjan 1985) and shows that the principal defect of MATa-playing-α cells is insufficient α-factor production. This defect could reflect a difference in the strengths of the MFA1 vs. the MFα1 promoter or differences in the translation or processing of α-factor between MATa and MATα cells. Our analysis also shows that there are no additional MATa-specific genes, beyond those that we manipulated (STE2, MFA1, MFA2, BAR1, and ASG7), that interfere with the ability of MATα cells to mate.
AFB1 encodes a novel a-factor barrier protein
We examined the decreased mating efficiency of the MATα-playing-a cells. Because pheromone production is important for efficient mating (Kurjan 1985; Michaelis and Herskowitz 1988), we investigated a-factor production of MATα-playing-a cells. Both α-factor and a-factor go through several processing steps before secretion (Betz and Duntze 1979; Kurjan and Herskowitz 1982). But while α-factor is secreted as a small, unmodified peptide (Kurjan and Herskowitz 1982), mature a-factor is modified with a 15-carbon farnesyl group, causing it to be very hydrophobic (Betz and Duntze 1979; Chen et al. 1997) and hard to quantify biochemically. We therefore used a bioassay to measure the relative a-factor production of the MATα-playing-a cells: we plated patches of a-factor-producing cells and then sprayed the plates with a suspension of MATα cells that were made supersensitive to pheromone by deleting SST2, which encodes a GTPase-activating protein that reduces the duration of signaling from the pheromone-activated G protein (Chan and Otte 1982a,b; Dohlman et al. 1996; Apanovitch et al. 1998). The a-factor secreted by the patch of cells arrests the MATα sst2Δ tester cells in G1, producing a halo of growth inhibition (Chan and Otte 1982a,b); the halo’s diameter increases with the amount of a-factor produced by the cell patch (Figure 3A). The halo produced by MATα-playing-a cells is smaller than that of wild-type MATa cells, implying that MATα-playing-a cells secrete less a-factor than wild-type MATa cells (Figure 3B).
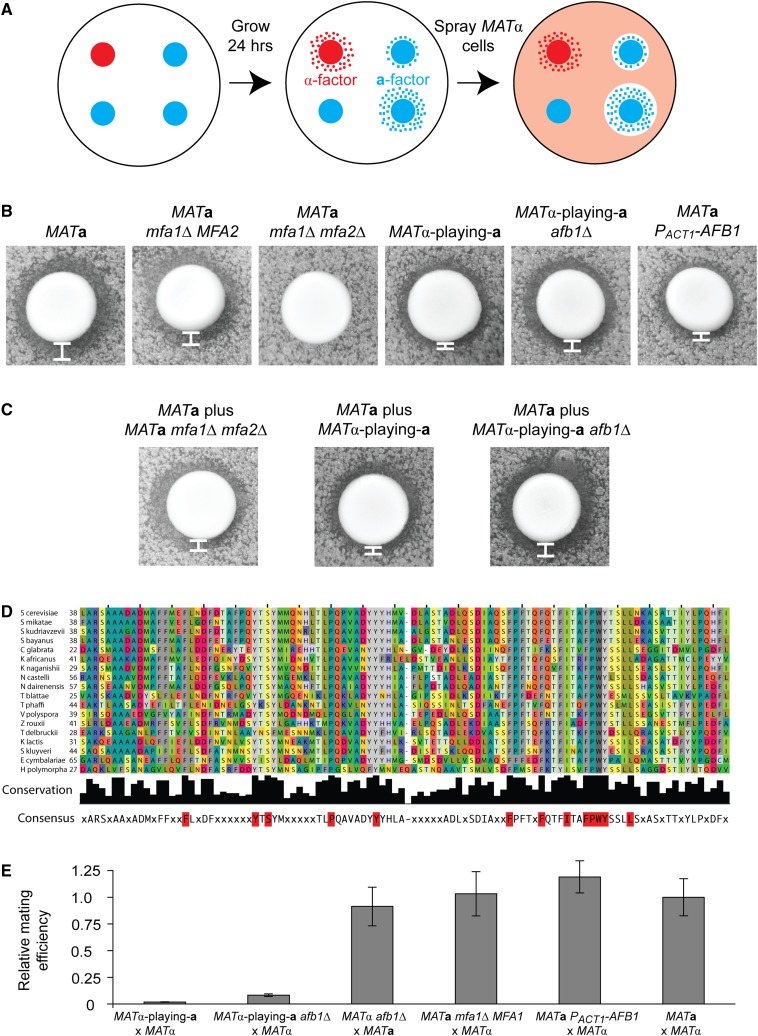
Figure 3.
AFB1 encodes a novel a-factor blocker. (A) Halo assays are done by allowing cell patches to grow on YPD overnight and then spraying supersensitive MATα sst2Δ cells over the cell patches. Where a-factor produced by the cell patches has diffused into the YPD, the MATα sst2Δ cells cannot grow, forming a halo around the cell patch with a size that corresponds to the amount of a-factor secretion. (B) Halo assays done on various cell patches containing a single cell type. MATa mfa1Δ mfa2Δ is a negative control, and MATa is a positive control. White bars indicate the width of the halo. The halo assays were repeated multiple times, and the rank order of the halo sizes is consistent. (C) Halo assays done on cell patches containing two cell types at a 1:8 ratio of MATa cells to the experimental cell of interest. White bars indicate the width of the halo. The halo assays were repeated multiple times, and the rank order of the halo sizes is consistent. (D) Sequences were obtained from the Yeast Genome Order Browser (http://ygob.ucd.ie) and Blast searches of fungal genomes at NCBI. Sequences were aligned in Jalview using the MAFFT-L-INS-I option, and the aligned core that follows the signal sequence and precedes the GPI anchorage sequence is shown. Amino acids are colored with a scheme that represents chemically similar amino acids in similar colors, and universally conserved amino acids are highlighted in red in the consensus sequence. Full species names: Saccharomyces cerevisiae, Saccharomyces mikatae, Saccharomyces kudriavzevii, Saccharomyces bayanus, Candida glabrata, Kazachstania africanus, Kazachstania naganishii, Naumovzyma castelli, Naumovzyma dairenensis, Tetraspora blattae, Tetraspora phaffi, Vanderwaltomyzoa polyspora, Zygosaccharomyces rouxii, Torulaspora delbruckii, Kluveromyces lactis, Saccharomyces kluyveri, Eremothecium cymbalariae, and Hansenula/Ogataea polymorpha. Of these, Z. rouxii, T. delbrueckii, K. lactis, S. kluyveri, E. cymbalariae, and H. polymorpha did not undergo a whole-genome duplication. (E) Mating efficiency of the indicated crosses relative to a wild-type cross between MATa and MATα cells. Matings were performed as described in Materials and Methods. Error bars are standard deviations.
We considered two explanations for the low a-factor secretion of MATα-playing-a cells: MATα-playing-a cells secrete less a-factor than MATa cells or MATα cells secrete a protein that provides a barrier to a-factor that is analogous to the MATa-specific α-factor protease, Bar1. We tested for the presence of a MATα-specific a-factor blocker secreted from MATα-playing-a cells by comparing the halo sizes of two mixtures of cells: MATa cells mixed with MATα-playing-a cells and MATa cells mixed with MATa cells that lack the genes encoding a-factor and thus produce no a-factor (MATa mfa1Δ mfa2Δ). If the MATα-playing-a cells secrete an a-factor blocker, we would expect the halo size of the MATa cells mixed with MATα-playing-a cells to be smaller than that of the MATa cells mixed with pheromone-less MATa cells because the a-factor blocker would interfere with the a-factor from both the MATα-playing-a cells and the MATa cells. However, if there is no a-factor blocker, we would expect the halo size of the MATa cells mixed with MATα-playing-a cells to be larger than that of the MATa cells mixed with pheromone-less MATa cells because both the MATa cells and the MATα-playing-a cells are capable of secreting a-factor. The halo produced by MATa cells mixed with MATα-playing-a cells is smaller than the halo produced by MATa cells mixed with pheromone-less MATa cells, indicating that MATα-playing-a cells secrete an a-factor blocker (Figure 3C).
We searched for the gene responsible for this activity by comparing the transcriptomes of MATa and MATα-playing-a cells. Although the gene expression of pheromone-stimulated MATa cells has been investigated, the extreme hydrophobicity of a-factor has made similar experiments on pheromone-stimulated MATα cells difficult (Roberts et al. 2000). The MATα-playing-a cells make it possible to study the transcriptome of pheromone-stimulated cells that are MATα at the MAT locus but are stimulated by α-factor in a controlled fashion. We chose a concentration of pheromone, 10 nM, in a regime in which MATa bar1Δ and MATα-playing-a cells with BAR1 under its endogenous promoter have a similar shmooing index (Figure 4A) to compare the transcriptomes of stimulated and unstimulated MATa and MATα-playing-a cells using RNA sequencing.
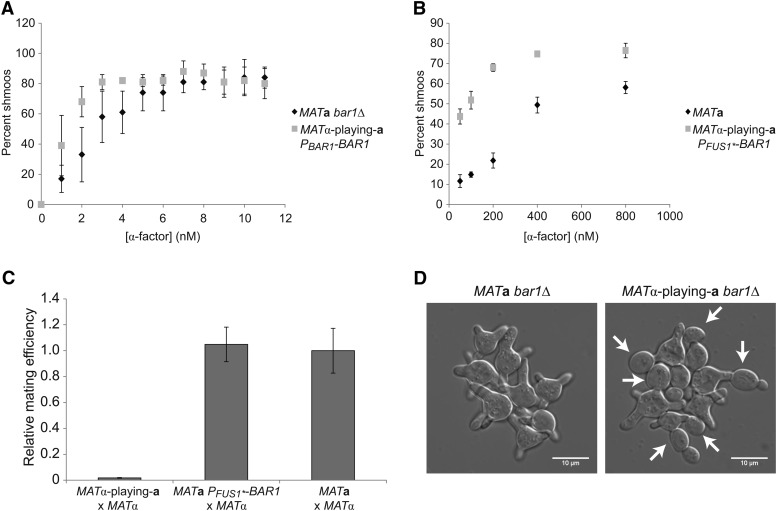
Figure 4.
MATα-playing-a cells shmoo but arrest transiently in the presence of pheromone. (A) Shmooing indices of MATa bar1Δ cells and MATα-playing-a PBAR1-BAR1 cells exposed to known concentrations of α-factor. Error bars are standard deviations. (B) Shmooing indices of MATa cells and MATα-playing-a PFUS1*-BAR1 cells exposed to known concentrations of α-factor. Error bars are standard deviations. (C) Mating efficiency relative to a wild-type cross between MATa and MATα cells. Matings were performed as described in Materials and Methods. Error bars are standard deviations. (D) MATa bar1Δ cells shmooing and MATα-playing-a bar1Δ cells shmooing and budding when incubated with SC plus 10 nM α-factor in a microfluidic chamber. Pictures were taken using DIC with ×60 magnification 8 hr after the addition of α-factor. White arrows point to buds.
Just as MATα cells do not secrete Bar1 to cleave their own α-factor (Sprague and Herskowitz 1981), we would not expect MATa cells to secrete an a-factor blocker to inhibit their own a-factor. Thus, we hypothesized that a MATα-specific a-factor blocker would be expressed more highly in both pheromone-stimulated and unstimulated MATα-playing-a than MATa cells. Ten genes fit this criterion and of these, only one, YLR040C, is annotated as encoding a secreted protein that is not already known to be important in mating (Yeast Genome Database at http://www.yeastgenome.org) (see Table S2).
YLR040C was previously identified as an α-specific gene by its reduced transcription in a MATα cell that lacked the transcription factor Matα1 (Galgoczy et al. 2004), which induces expression of α-specific genes (Strathern et al. 1981). YLR040C has also been shown to be translated by ribosome profiling (Brar et al. 2012) and localized to the cell wall (Hamada et al. 1999; Giaever et al. 2002). Deletion of YLR040C was reported as having no effect on mating (Galgoczy et al. 2004). We found that in unstimulated cells the gene is expressed 11-fold more strongly in MATα-playing-a than in MATa cells and that its transcription is not significantly induced when MATα-playing-a cells are exposed to pheromone (see Table S2). The protein is conserved in yeasts that experienced the whole-genome duplication around 100 million years ago and is also found in some yeasts, such as Hansenula polymorpha, that substantially predate this event (Wolfe and Shields 1997; Dietrich et al. 2004; Dujon et al. 2004; Kellis et al. 2004) (Figure 3D). The experiments described below demonstrate that YLR040C encodes a protein that provides an a-factor barrier function, leading us to name this gene AFB1 for a-factor barrier.
To determine whether AFB1 is indeed the a-factor blocker, we knocked it out in MATα-playing-a cells. The halos produced by MATα-playing-a afb1Δ cells are larger than those of MATα-playing-a AFB1 cells, indicating that deleting AFB1 increases the amount of pheromone secreted from a patch of MATα-playing-a cells (Figure 3B). The halo around the MATα-playing-a afb1Δ cells, however, is still smaller than the halo produced by wild-type MATa cells, suggesting that MATα-playing-a cells secrete less a-factor than wild-type MATa cells (Figure 3B). We also placed AFB1 under a strong (ACT1) promoter in MATa cells and observed a decrease in halo size compared to wild-type MATa cells, indicating that Afb1 is able to block a-factor secreted by MATa cells (Figure 3B).
To test whether Afb1 is responsible for blocking a-factor produced by other cells, we made cell mixtures of MATa cells with MATα-playing-a afb1Δ cells and compared the halo produced by this mixture to the halo produced by the mixtures of MATa cells with MATα-playing-a cells and to the halo produced by MATa cells with MATa mfa1Δ mfa2Δ cells. As expected from our other results, the mixture of MATa cells with MATα-playing-a afb1Δ cells has a slightly larger halo than the MATa cells mixed with MATa mfa1Δ mfa2Δ cells and a significantly larger halo than the MATa cells mixed with MATα-playing-a cells (Figure 3C). This result indicates that, when Afb1 is not present in the cell mixtures, the a-factor from the wild-type MATa cells as well as that from the MATα-playing-a cells is free to interact with the supersensitive MATα cells. Taken together, our results provide strong evidence that Afb1 has an a-factor barrier function.
We asked whether the expression of AFB1 affected the mating efficiency of MATα-playing-a cells. We crossed MATα-playing-a afb1Δ cells with wild-type MATα cells and observed a fivefold increase in mating efficiency over a similar cross with MATα-playing-a cells (Student’s t-test, P < 10−6) (Figure 3E). However, deleting AFB1 from wild-type MATα cells does not reduce their mating efficiency (Figure 3E), perhaps because small changes in a-factor production do not have a large effect on mating efficiency (Michaelis and Herskowitz 1988). We tested this possibility in two ways. The first was to delete MFA1 from wild-type MATa cells. We saw a small decrease in the halo size of MATa mfa1Δ compared to that of wild-type MATa cells, but as previously reported (Michaelis and Herskowitz 1988), the mating efficiency of MATa mfa1Δ cells was statistically indistinguishable from that of MATa MFA1 cells (Figure 3, B and E). We also tested the mating efficiency of MATa cells with AFB1 placed under the ACT1 promoter. These cells produce a smaller halo than wild-type MATa cells (Figure 3B) but mate slightly better than wild-type MATa cells, implying that there is a range of a-factor production that results in efficient mating, at least in the absence of additional mutations (Student’s t-test, P = 0.03) (Figure 3E).
MATα-playing-a cells shmoo but arrest only transiently in the presence of pheromone
Although the expression of AFB1 in MATα-playing-a cells was responsible for a portion of the reduced mating efficiency of MATα-playing-a cells, MATα-playing-a afb1Δ cells still mate 12-fold less efficiently than wild-type MATa cells (Student’s t-test, P < 10−6) (Figure 3E). We hypothesized that the response of MATα-playing-a cells to pheromone could also reduce their mating efficiency.
There are three cellular responses to pheromone stimulation: altered gene expression, cell polarization, and cell cycle arrest (Bardwell 2005). We compared the transcriptomes of MATα-playing-a and MATa cells both with and without exposure to α-factor (see Table S2), excluding those genes, such as STE3 and BAR1, that had been removed during the construction of the strains. Twenty-one genes showed a more than 2-fold variation in both comparisons. Ten genes showed a more than 2-fold variation when comparing the two stimulated cell types but were not significantly different when comparing the unstimulated cells, and another 8 genes showed the opposite pattern. As expected, known α-specific genes, such as the MATα-specific agglutinin gene, SAG1, were expressed more strongly in MATα-playing-a cells than in MATa cells, and known a-specific genes, such as the a-specific agglutinin gene, AGA2, were expressed less strongly in MATα-playing-a cells than in MATa cells. Despite our attempts to engineer their expression to match the levels seen in MATa cells, three important a-specific genes—STE2, MFA1, and MFA2—are expressed at 5-fold, 7-fold, and 120-fold lower levels, respectively, in MATα-playing-a cells compared to MATa cells (see Table S2).
We assayed cell polarization (Segall 1993; Butty et al. 1998) by measuring the shmooing index of MATα-playing-a cells stimulated with known quantities of synthetic α-factor. We found that MATα-playing-a PBAR1-BAR1 cells have a similar shmooing index to MATa bar1Δ cells at low concentrations of α-factor, indicating that the MATα-playing-a cells are as sensitive to low concentrations of α-factor as MATa cells (Figure 4A) and suggesting that BAR1 is not expressed in MATα cells. Because efficient mating in MATa × MATα crosses depends on the secretion of Bar1 by the MATa cells, we investigated the pheromone response of MATα-playing-a cells that express BAR1 from a mutant version of the pheromone-induced FUS1 promoter (Trueheart et al. 1987): the mutant promoter PFUS1* was selected to have a low basal and a high pheromone-stimulated level of expression (Ingolia and Murray 2007). These cells make fewer shmoos at 50 nM α-factor than MATα-playing-a PBAR1-BAR1 cells (which lack detectable Bar1 activity) make at 2 nM α-factor, indicating that MATα-playing-a PFUS1*-BAR1 cells are able to secrete Bar1 (Student’s t-test, P = 0.02) (Figure 4, A and B). In contrast, the MATα-playing-a PFUS1*-BAR1 cells make significantly more shmoos than wild-type MATa cells at each concentration of α-factor tested (Student’s t-test, P < 0.005) (Figure 4B), suggesting that MATα-playing-a PFUS1*-BAR1 cells secrete less Bar1 than wild-type MATa cells. This difference is unlikely to be the sole remaining cause of the mating defect of MATα-playing-a PFUS1*-BAR1 cells: reducing the expression of Bar1 in MATa cells, by expressing it from the FUS1* promoter, does not reduce their mating efficiency (Figure 4C).
We observed α-factor-induced cell cycle arrest (Chang and Herskowitz 1990) in a microfluidic device. Pheromone stimulation arrests MATa cells in G1 through phosphorylation of Far1, a protein that binds to cyclin-dependent kinase/cyclin complexes (Chang and Herskowitz 1990; Tyers and Futcher 1993; Peter and Herskowitz 1994). When MATa bar1Δ cells are exposed to 10 nM α-factor, their cell cycle remains arrested for many hours while they form multiple successive shmoos (Figure 4D and File S3). However, even at this high α-factor concentration, MATα-playing-a bar1Δ cells form shmoos but arrest only transiently (Figure 4D and File S4).
The transient cell cycle arrest in MATα-playing-a cells could be due to a difference in the response of MATα and MATa cells to pheromone stimulation or the inhibition of Ste2 by MATα-specific proteins in the MATα-playing-a cells. We tried to find the responsible genes by looking for differential expression of genes that might have an effect on cell cycle arrest between pheromone-stimulated MATa and MATα-playing-a cells. We manipulated the expression of three candidates—PCL1, GYP8, and TOS4—which had at least a twofold difference in expression between stimulated MATa and MATα-playing-a cells and a plausible connection to cell cycle control (see Table S2). None of these manipulations altered the pheromone-induced cell cycle arrest of either MATa or MATα-playing-a cells (data not shown).
How robust is mating?
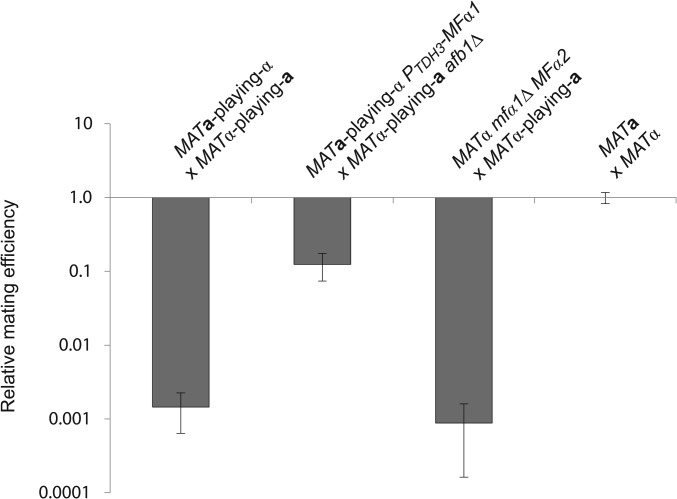
Mating would be robust to variation in pheromone levels if substantial increases or decreases in pheromone expression had no effect on mating efficiency. The mating of the transvestite strains to each other suggests that mating efficiency is not robust to variation in pheromone production. Mating MATa-playing-α cells to wild-type MATa cells reduces mating frequency 3-fold, and mating MATα-playing-a cells to wild-type MATα cells reduces mating 60-fold, relative to a wild-type MATa × MATα cross, but the mating frequency of the cross between the two transvestites is reduced 700-fold, suggesting that mating defects are synergistic (Student’s t-test, P < 10−6) (Figure 1D). If this synergism is largely due to reduced pheromone production by the transvestite strains, increasing pheromone production should increase the efficiency of the intertransvestite cross. We increased α-factor production from MATa-playing-α cells by placing MFα1 under the control of the TDH3 promoter and a-factor production from MATα-playing-a cells by deleting AFB1. When crossed to each other, these strains mate 90 times better than the cross between the original MATa-playing-α and MATα-playing-a cells. Thus, after improving pheromone production, the intertransvestite cross is only 8-fold less efficient than a standard MATa × MATα cross (Student’s t-test, P < 10−6) (Figure 5). If reduced pheromone production is the primary cause of the weak mating of the intertransvestite cross, the cross between a MATα strain making less α-factor and a MATα-playing-a cell should mimic the intertransvestite cross. The mating efficiency of the cross between MATα mfα1Δ cells, which produce less α-factor than wild-type MATα cells, and MATα-playing-a cells is statistically indistinguishable from that of the double transvestite cross (Figure 2B and Figure 5).
Figure 5.
Mating is not robust to changes in pheromone production. Mating efficiency relative to a wild-type cross between MATa and MATα cells. Matings were performed as described in Materials and Methods. Note the logarithmic scale for mating efficiency. Error bars are standard deviations.
Discussion
Our experiments show that genetic engineering can be used to investigate the factors that control the efficiency of mating in budding yeast. We tested the idea that previous research had found all the genes that control mating efficiency by engineering transvestite strains that switch the mating genes of one mating type for those that are normally expressed in its partner. The behavior of these strains led to two conclusions: there are still more genes that control mating, such as the a-factor barrier protein Afb1, and mating is not robust to reductions in pheromone production.
Engineering efficient maters
Investigating the pheromone production of the transvestite strains allowed us to account for a significant portion of their mating defects. Unstimulated MATa-playing-α cells secrete 70-fold less α-factor than wild-type MATα cells, and stimulated MATa-playing-α cells secrete 20-fold less α-factor than stimulated MATα cells. Increasing the α-factor production of the MATa-playing-α cells increased their mating efficiency to nearly that of wild type, showing that the main defect of the MATa-playing-α cells is low α-factor production and that the level of α-factor secretion is important for efficient mating. The observation that α-factor secretion is still pheromone-inducible, even when α-factor expression is driven by a strong, constitutive promoter, demonstrates that pheromone processing and export respond to pheromone stimulation. Indeed, Ste13, a protein required for the maturation of α-factor (Julius et al. 1983), is pheromone-induced (Achstetter 1989).
The mating defects of MATα-playing-a cells are more complex. We determined that these cells do not make as much a-factor as wild-type MATa cells and that at least part of this is due to the expression of the novel a-factor blocker Afb1. Increasing the a-factor production of MATα-playing-a cells by deleting AFB1 causes a fivefold increase in their mating efficiency, indicating that sufficient a-factor expression is important for efficient mating as a MATa cell. We were unable to engineer MATα cells to mate efficiently as MATa cells. There are two possible explanations for the remaining defect: even after the removal of Afb1, the MATα-playing-a cells make less a-factor than MATa cells, and MATα-playing-a cells arrest only transiently in response to α-factor. We suspect that both contribute to the reduced mating of MATα-playing-a cells.
Similar pheromone and receptor swaps have been done on other fungi, including Cryptococcus neoformans (Stanton et al. 2010) and Ustilago maydis (Bölker et al. 1992). Like the strains we constructed, the engineered versions of these organisms could mate to cells that bore the same genes at the mating-type locus. In Candida albicans, a-a or α-α matings can be induced by enhancing autocrine signaling or the presence of the opposite pheromone (Alby et al. 2009). Studying the mating defects of engineered transvestites in other fungi should identify additional genes involved in their mating pathways.
AFB1 encodes a novel MATα-specific a-factor barrier protein
Studies on pheromone-induced genes in MATα cells were hampered by the difficulties in working with a-factor. We avoided these by looking at the pattern of gene expression in MATα-playing-a cells, which would still express α-specific genes but would increase their expression in response to α- rather than a-factor. We argued that novel α-specific genes would be identified by higher expression in MATα-playing-a than in MATa cells. Mixing experiments suggested that MATα-playing-a cells produced an extracellular factor that interfered with the action of a-factor, prompting us to look for the secreted product of a MATα-specific gene. This computational sieve produced a single gene, YLR040C, which had previously been identified as a gene regulated by the MATα-specific transcription factor, Matα1 (Galgoczy et al. 2004). Removing YLR040C increased a-factor production from MATα-playing-a cells and the mating efficiency of MATα-playing-a cells, leading us to rename YLR040CAFB1 for a-factor barrier. There have been previous searches for a protein with an a-factor barrier function. The first reported a supersensitive MATα mutant, which mapped to a location on chromosome XII >600 kilobases away from AFB1 (Steden et al. 1989). The second reported the detection of MATα-specific a-factor endopeptidase activity, but the gene responsible for this was not identified, and the protein was not purified (Marcus et al. 1991). Without being able to manipulate the genes involved in these studies, it is impossible to assess their effect on a-factor activity or stability or their relationship to AFB1.
Deleting AFB1 increased the mating efficiency of MATα-playing-a cells. Sequence analysis shows that Afb1 is conserved in ascomycetes as evolutionarily distant as H. polymorpha and contains an N-terminal signal sequence and C-terminal motif that suggests that it is a GPI-anchored protein (Hamada et al. 1999) but lacks other detectable motifs. In particular, Afb1 shows no sequence homology with any other protease but contains a number of conserved aromatic residues (Figure 3D). Our inability to find Afb1 throughout the ascomycete fungi has two possible interpretations: either the protein evolves too rapidly to be detected by standard tools that use sequence homology to identify orthologs or the protein evolved within one branch of the ascomycete lineage, rather than in its last common ancestor. Unusually rapid evolution of a single protein or independent evolution of the same function in different lineages may also explain why the α-factor-degrading protease Bar1 in S. cerevisiae is not the closest homolog of the same protein in C. albicans (Schaefer et al. 2007).
We speculate that Afb1 acts to bind and sequester a-factor rather than to degrade it. The biological function of Afb1 may mirror that of Bar1, which promotes the efficient mating of MATa cells by keeping the α-factor concentration at the plasma membrane within the narrow range needed for accurate pheromone gradient detection (Barkai et al. 1998). Since Afb1 is predicted to be GPI-anchored, it is possible that the function of Afb1 closely mimics that of Bar1 trapped in the cell wall of MATa cells: creating a pheromone sink that makes it more likely that two cells of the same mating type will avoid each other (Jin et al. 2011) and makes it easier to distinguish between two close potential partners (Rappaport and Barkai 2012). It is also possible that Afb1 in S. cerevisiae acts like Bar1 in C. albicans (Alby et al. 2009): decreasing the threat of autocrine signaling caused by leaky repression of a-factor in MATα cells.
MATα-playing-a cells arrest only transiently in response to pheromone
In MATa cells, exposure to α-factor leads to a prolonged cell cycle arrest. In contrast, MATα-playing-a cells show only a transient arrest, even though their ability to shmoo is statistically indistinguishable from wild-type MATa cells. This result surprised us because MATa and MATα cells arrest the cell cycle in the same fashion: by signaling through Far1 (Peter and Herskowitz 1994; Bardwell 2005).
There are two possible explanations for the transient cell cycle arrest of MATα-playing-a cells. The first is that MATa and MATα cells have evolved to respond to pheromone stimulation in subtly different ways and that MATα cells shmoo but do not experience enduring arrest. Although it is important for cells to be in the same phase of the cell cycle during nuclear fusion, it is possible that transient arrest of MATα cells is sufficient to allow for the formation of zygotes, while a lasting arrest is required for MATa cells. Because α-factor is more diffusible, we suspect that initial signaling is usually from MATα to MATa cells, meaning that it is the MATa cells that arrest first and thus need to wait until the MATα cells receive a strong enough signal to arrest, implying that fusion would usually occur shortly after the arrest of the MATα cell but at a longer and more variable time after the arrest of the MATa cell.
The second possibility is that interactions between Ste2 and proteins present in the MATα-playing-a cells make the cells keep cycling, like cells that express both Ste3 and Asg7 (Bender and Sprague 1989; Roth et al. 2000). We looked for genes that might be responsible for the lack of enduring arrest in MATα-playing-a cells by focusing on genes that are differentially expressed in pheromone-stimulated MATa and MATα-playing-a cells and might not have been identified in earlier work. Although we tested the effect of deleting or overexpressing several candidate genes individually, we did not find an individual gene responsible for the transient cell cycle arrest in MATα-playing-a cells.
Robustness of mating
Characterizing the mating defects of the transvestite strains allowed us to improve our understanding of the pheromone response of MATα cells and study the robustness of mating efficiency to changes in gene expression. We investigated changing the expression levels of three proteins: Bar1, α-factor, and a-factor.
The α-factor protease Bar1 helps MATa cells to detect an α-factor gradient and choose a mating partner (Sprague and Herskowitz 1981; Jackson and Hartwell 1990; Barkai et al. 1998). Reducing Bar1 expression by using an engineered FUS1 promoter (Ingolia and Murray 2007) reduces the concentration of α-factor required to get 50% of the cells to shmoo fourfold. This change appears to have little effect on mating: expressing BAR1 under PFUS1* in MATa cells leaves mating unimpaired, suggesting that mating efficiency is robust to substantial changes in Bar1 expression.
Mating efficiency is not robust to reductions in α-factor secretion, a result that might have been predicted from work that showed that cells make graded responses to increasing levels of pheromone stimulation (Moore 1983; Takahashi and Pryciak 2008). Previous studies have shown that agglutination, shmoo formation, and pheromone-induced transcription increase with increasing α-factor concentration (Moore 1983; Takahashi and Pryciak 2008). Decreased α-factor production leads to a fusion defect and thus to a decrease in mating efficiency (Brizzio et al. 1996). We show that an ∼10-fold reduction in α-factor production in otherwise wild-type cells, such as MATα mfα1Δ cells, results in a 2-fold reduction in mating efficiency when mated to a wild-type partner. Mating MATα mfα1Δ cells to a compromised partner, such as the MATα-playing-a cells, results in a synergistic reduction in mating efficiency. Although reduced levels of a-factor production have also been shown to cause a cell fusion defect and a decrease in mating efficiency (Brizzio et al. 1996), the precise regulation of a-factor production does not appear to be as important to mating efficiency as precise regulation of α-factor production. MATa mfa1Δ cells have a mating efficiency that is indistinguishable from that of wild type (Michaelis and Herskowitz 1988), and reducing the a-factor production of MATa cells by overexpressing AFB1 actually causes a slight increase in mating efficiency, indicating that the ideal quantity of a-factor production may be less than the amount of a-factor produced by wild-type MATa cells but greater than the amount of a-factor produced by MATα-playing-a cells.
Taken together, these results argue for a molecular arms race in pheromone production. Cells prefer the partner that makes the most pheromone (Jackson and Hartwell 1990), possibly because this is the only indicator of fitness available to a potential mating partner. We speculate that both MATa and MATα cells have evolved to produce higher and higher concentrations of pheromone, resulting in the need for proteins such as Bar1 and Afb1 to improve gradient detection in dense mating mixtures. Once such functions have evolved, they imply that mutations that reduce pheromone production back to ancestral levels will decrease mating efficiency because the pheromone antagonists overwhelm the lower pheromone levels.
Supplementary Material
Acknowledgments
We thank V. Denic, R. Gaudet, C. Hunter, and members of the Murray laboratory for reading and commenting on the manuscript. We also thank J. Gonçalves-Sá for thoughtful discussion and M. Piel for strains. This work was supported by National Institute of General Medical Sciences grant P50GM068763 of the National Centers for Systems Biology Program and a National Science Foundation Graduate Research Fellowship and Ashford Fellowship to L.B.H.
Footnotes
Communicating editor: M. D. Rose
Literature Cited
- Achstetter T., 1989. Regulation of α-factor production in Saccharomyces cerevisiae: a-factor pheromone-induced expression of the MFα1 and STE13 genes. Mol. Cell. Biol. 9: 4507–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alby K., Schaefer D., Bennett R. J., 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apanovitch D. M., Slep K. C., Sigler P. B., Dohlman H. G., 1998. Sst2 is a GTPase-activating protein for Gpa1: purification and characterization of a cognate RGS-Gα protein pair in yeast. Biochemistry (N. Y.) 37: 4815–4822. [DOI] [PubMed] [Google Scholar]
- Bardwell L., 2005. A walk-through of the yeast mating pheromone response pathway. Peptides 26: 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkai N., Rose M. D., Wingreen N. S., 1998. Protease helps yeast find mating partners. Nature 396: 422–423. [DOI] [PubMed] [Google Scholar]
- Bender A., Sprague G., 1989. Pheromones and pheromone receptors are the primary determinants of mating specificity in the yeast Saccharomyces cerevisiae. Genetics 121: 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz R., Duntze W., 1979. Purification and partial characterization of a factor, a mating hormone produced by mating-type-a cells from Saccharomyces cerevisiae. Eur. J. Biochem. 95: 469–475. [DOI] [PubMed] [Google Scholar]
- Blumer K. J., Reneke J. E., Thorner J., 1988. The STE2 gene product is the ligand-binding component of the α-factor receptor of Saccharomyces cerevisiae. J. Biol. Chem. 263: 10836–10842. [PubMed] [Google Scholar]
- Bölker M., Urban M., Kahmann R., 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68: 441–450. [DOI] [PubMed] [Google Scholar]
- Brar G. A., Yassour M., Friedman N., Regev A., Ingolia N. T., et al. , 2012. High-resolution view of the yeast meiotic program revealed by ribosome profiling. Science 335: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V., Gammie A. E., Nijbroek G., Michaelis S., Rose M. D., 1996. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J. Cell Biol. 135: 1727–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty A. C., Pryciak P. M., Huang L. S., Herskowitz I., Peter M., 1998. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282: 1511–1516. [DOI] [PubMed] [Google Scholar]
- Cappellaro C., Hauser K., Mrśa V., Watzele M., Watzele G., et al. , 1991. Saccharomyces cerevisiae a-and α-agglutinin: characterization of their molecular interaction. EMBO J. 10: 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A., 1982a Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and α factor pheromones. Mol. Cell. Biol. 2: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A., 1982b Isolation and genetic analysis of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and α factor pheromones. Mol. Cell. Biol. 2: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Herskowitz I., 1990. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell 63: 999–1011. [DOI] [PubMed] [Google Scholar]
- Chen P., Sapperstein S. K., Choi J. D., Michaelis S., 1997. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J. Cell Biol. 136: 251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M. A., Oliviero S., 2001. Preparation of yeast RNA. Curr. Protoc. Mol. Biol. 23: 13.12.1–13.12.5. [DOI] [PubMed] [Google Scholar]
- de Nobel H., Pike J., Lipke P. N., Kurjan J., 1995. Genetics of a-agglutinin function in Saccharomyces cerevisiae. Mol. Gen. Genet. 247: 409–415. [DOI] [PubMed] [Google Scholar]
- Dietrich F. S., Voegeli S., Brachat S., Lerch A., Gates K., et al. , 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304: 304–307. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Thorner J. W., 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70: 703–754. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Song J., Ma D., Courchesne W. E., Thorner J., 1996. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein α subunit). Mol. Cell. Biol. 16: 5194–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B., Sherman D., Fischer G., Durrens P., Casaregola S., et al. , 2004. Genome evolution in yeasts. Nature 430: 35–44. [DOI] [PubMed] [Google Scholar]
- Edgar R., Domrachev M., Lash A. E., 2002. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgoczy D. J., Cassidy-Stone A., Llinás M., O’Rourke S. M., Herskowitz I., et al. , 2004. Genomic dissection of the cell-type-specification circuit in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101: 18069–18074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L., et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- Gonçalves-Sá J., Murray A., 2011. Asymmetry in sexual pheromones is not required for ascomycete mating. Curr. Biol. 21: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32: 561–599. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., McCaffrey G., Sprague G. F., Jr, 1986. Evidence the yeast STE3 gene encodes a receptor for the peptide pheromone a factor: gene sequence and implications for the structure of the presumed receptor. Proc. Natl. Acad. Sci. USA 83: 1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Terashima H., Arisawa M., Yabuki N., Kitada K., 1999. Amino acid residues in the ω-minus region participate in cellular localization of yeast glycosylphosphatidylinositol-attached proteins. J. Bacteriol. 181: 3886–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., 1988. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol. Rev. 52: 536–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia N. T., Murray A. W., 2007. Positive-feedback loops as a flexible biological module. Curr. Biol. 17: 668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L., Hartwell L. H., 1990. Courtship in S. cerevisiae: choose mating partners by responding to the strongest pheromone signal. Cell 63: 1039–1051. [DOI] [PubMed] [Google Scholar]
- Jin M., Errede B., Behar M., Mather W., Nayak S., et al. , 2011. Yeast dynamically modify their environment to achieve better mating efficiency. Sci. Signal. 4: ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J., 1983. Yeast α factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell 32: 839–852. [DOI] [PubMed] [Google Scholar]
- Kellis M., Birren B. W., Lander E. S., 2004. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 428: 617–624. [DOI] [PubMed] [Google Scholar]
- Kim J., Bortz E., Zhong H., Leeuw T., Leberer E., et al. , 2000. Localization and signaling of Gβ subunit Ste4p are controlled by a-factor receptor and the a-specific protein Asg7p. Sci. Signal. 20: 8826–8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., 1953. Yeast glyceraldehyde-3-phosphate dehydrogenase I. Electrophoresis of fractions precipitated by nucleic acid. J. Biol. Chem. 200: 471–478. [PubMed] [Google Scholar]
- Kurjan J., 1985. α-Factor structural gene mutations in Saccharomyces cerevisiae: effects on α-factor production and mating. Mol. Cell. Biol. 5: 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I., 1982. Structure of a yeast pheromone gene (MFα): a putative α-factor precursor contains four tandem copies of mature α-factor. Cell 30: 933–943. [DOI] [PubMed] [Google Scholar]
- Lipke P., Wojciechowicz D., Kurjan J., 1989. AG α1 is the structural gene for the Saccharomyces cerevisiae α-agglutinin, a cell surface glycoprotein involved in cell-cell interactions during mating. Mol. Cell. Biol. 9: 3155–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay V. L., Welch S., Insley M. Y., Manney T. R., Holly J., et al. , 1988. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc. Natl. Acad. Sci. USA 85: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Xue C., Naider F., Becker J., 1991. Degradation of a-factor by a Saccharomyces cerevisiae α-mating-type-specific endopeptidase: evidence for a role in recovery of cells from G1 arrest. Mol. Cell. Biol. 11: 1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Holland M. J., 1985. Differential expression of the three yeast glyceraldehyde-3-phosphate dehydrogenase genes. J. Biol. Chem. 260: 15019–15027. [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A., 1989. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 340: 400–404. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Herskowitz I., 1988. The a-factor pheromone of Saccharomyces cerevisiae is essential for mating. Mol. Cell. Biol. 8: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. A., 1983. Comparison of dose-response curves for α factor-induced cell division arrest, agglutination, and projection formation of yeast cells. Implication for the mechanism of α factor action. J. Biol. Chem. 258: 13849–13856. [PubMed] [Google Scholar]
- Peter M., Herskowitz I., 1994. Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1. Science 265: 1228–1231. [DOI] [PubMed] [Google Scholar]
- Rappaport N., Barkai N., 2012. Disentangling signaling gradients generated by equivalent sources. J. Biol. Phys. 38: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. J., Hartwell L. H., 1977. Regulation of mating in the cell cycle of Saccharomyces cerevisiae. J. Cell Biol. 75: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Nelson B., Marton M. J., Stoughton R., Meyer M. R., et al. , 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Sci. Signal. 287: 873–880. [DOI] [PubMed] [Google Scholar]
- Roth A. F., Nelson B., Boone C., Davis N. G., 2000. Asg7p-Ste3p inhibition of pheromone signaling: regulation of the zygotic transition to vegetative growth. Sci. Signal. 20: 8815–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Lu C., Marykwas D., Lipke P., Kurjan J., 1991. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol. Cell. Biol. 11: 4196–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D., Côte P., Whiteway M., Bennett R. J., 2007. Barrier activity in Candida albicans mediates pheromone degradation and promotes mating. Eukaryot. Cell 6: 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J. E., 1993. Polarization of yeast cells in spatial gradients of α mating factor. Proc. Natl. Acad. Sci. USA 90: 8332–8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Fink G., Lawrence C., 1974. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Singh A., Chen E. Y., Lugovoy J. M., Chang C. N., Hitzeman R. A., et al. , 1983. Saccharomyces cerevisiae contains two discrete genes coding for the α-factor pheromone. Nucleic Acids Res. 11: 4049–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr, 1991. Assay of yeast mating reaction. Methods Enzymol. 194: 77–93. [DOI] [PubMed] [Google Scholar]
- Sprague G. F., Jr, Herskowitz I., 1981. Control of yeast cell type by the mating type locus. I. Identification and control of expression of the a-specific gene BAR1. J. Mol. Biol. 153: 305–321. [DOI] [PubMed] [Google Scholar]
- Stanton B. C., Giles S. S., Staudt M. W., Kruzel E. K., Hull C. M., 2010. Allelic exchange of pheromones and their receptors reprograms sexual identity in Cryptococcus neoformans. PLoS Genet. 6: e1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steden M., Betz R., Duntze W., 1989. Isolation and characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by the mating hormone a-factor. Mol. Gen. Genet. 219: 439–444. [DOI] [PubMed] [Google Scholar]
- Strathern J., Hicks J., Herskowitz I., 1981. Control of cell type in yeast by the mating type locus: the α1-α2 hypothesis. J. Mol. Biol. 147: 357–372. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Pryciak P. M., 2008. Membrane localization of scaffold proteins promotes graded signaling in the yeast MAP kinase cascade. Curr. Biol. 18: 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., et al. , 2010. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J., Boeke J. D., Fink G. R., 1987. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol. Cell. Biol. 7: 2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M., Futcher B., 1993. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol. Cell. Biol. 13: 5659–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K. H., Shields D. C., 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387: 708–712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.