Abstract
The adipocyte-derived hormone leptin acts within the central nervous system to decrease food intake and body weight and to increase renal and thermogenic brown adipose tissue (BAT) sympathetic nerve activity (SNA). Previous studies have focused on hypothalamic brain regions, although recent findings have identified leptin receptors (ObR) in a distributed brain network, including the circumventricular subfornical organ (SFO), a forebrain region devoid of a blood-brain barrier. We tested the hypothesis that ObR in the SFO are functionally linked to leptin-induced decreases in food intake and body weight and increases in SNA. SFO-targeted microinjections of an adenovirus encoding Cre-recombinase in ObRflox/flox mice resulted in selective ablation of ObR in the SFO. Interestingly, deletion of ObR in the SFO did not influence the decreases in either food intake or body weight in response to daily systemic or cerebroventricular administration of leptin. In line with these findings, reduction in SFO ObR did not attenuate leptin-mediated increases in thermogenic BAT SNA. In contrast, increases in renal SNA induced by systemic or cerebroventricular administration of leptin were abolished in mice with SFO-targeted deletion of ObR. These results demonstrate that ObR in the SFO play an important role in leptin-induced renal sympathoexcitation, but not in the body weight, food intake or BAT SNA thermogenic effects of leptin. These findings highlight the concept of a distributed brain network of leptin action and illustrate that brain regions, including the SFO, can mediate distinct cardiovascular and metabolic responses to leptin.
Keywords: central nervous system, sympathetic nerve activity, leptin resistance, thermogenesis, energy homeostasis
INTRODUCTION
The adipokine leptin is a pleiotropic hormone that acts within the central nervous system (CNS) to regulate energy homeostasis1-3. Leptin signaling in the CNS results in a decrease in food intake and an increase in energy expenditure3, in part by enhancing sympathetic activity to thermogenic brown adipose tissue (BAT)4. In addition to its role in central energy control, leptin increases sympathetic nerve activity (SNA) to organs involved in cardiovascular regulation, including the kidney4-6.
Initially, the focus on the site of leptin signaling highlighted the mediobasal hypothalamus. Indeed, the hypothalamic arcuate nucleus is densely populated with leptin receptors (ObR)7 and arcuate lesioning attenuates the anorexic response to exogenous leptin8. In addition, direct microinjection of leptin into the arcuate nucleus of rodents increases BAT and renal SNA9, and selective ablation of ObR in the arcuate nucleus blunts leptin-induced sympathetic activation5.
In addition to a key role for the arcuate nucleus in the central neural actions of leptin, there is mounting evidence for a distributed brain network of leptin receptors and signaling. This network includes regions involved in sympathetic regulation such as the ventromedial (VMH) and dorsomedial (DMH) hypothalamus and brainstem regions, such as the nucleus tractus solitarii (NTS) and dorsal vagal complex, all of which have been shown to be involved in the energy balance and SNA effects induced by leptin6, 10, 11. Interestingly, the VMH, DMH and NTS are protected by the blood-brain-barrier (BBB)12, 13. In order for leptin to gain access to well-protected CNS regions like these, it likely must cross the BBB through a transporter mechanism. However, large peptides, such as leptin, may have unique access to metabolic and cardiovascular brain centers inside the BBB through sensory circumventricular organs, including the subfornical organ (SFO) - a forebrain structure devoid of a blood-brain-barrier14, 15.
Due to its unique anatomical location, the SFO is constantly exposed to a variety of circulating factors (e.g. leptin) and contains extensive efferent connections to regions inside the BBB, including hypothalamic and brainstem nuclei15, 16. In addition to its well-recognized role in fluid balance and cardiovascular regulation16, 17, there is increasing evidence that the SFO plays a role in the regulation of energy homeostasis14, 15. Smith and Ferguson14 recently identified leptin receptor mRNA in the SFO of rats, and reported that CNS administration of leptin increased phosphorylation of signal transducers and activators of transcription 3 (STAT-3) in SFO neurons and influenced the excitability of dissociated SFO cells. The role of leptin signaling in the SFO in the metabolic and sympathoexcitatory actions of leptin in vivo is not known.
Here we tested the hypothesis that ObR in the SFO are functionally linked to leptin-induced decreases in food intake and body weight and increases in BAT and renal SNA. Using selective genetic targeting of ObR in mice, our findings demonstrate that SFO ObR contribute to leptin-mediated renal sympathoexcitation, but not to the body weight, food intake or the BAT thermogenic SNA effects of leptin.
METHODS
Animals
All procedures were approved by the Animal Care and Use Committees at Cornell University and the University of Iowa. Care of the mice met the standards set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, USDA regulations, and the AVMA Panel on Euthanasia. Studies were conducted in adult (8-12 wks old) male ObRflox/flox mice initially obtained from the colony of Dr. Jeffrey Friedman1 and used to establish our own colony. CAG-CATZ reporter mice were obtained from an in-house colony18. Mice were fed standard chow and water ad libitum and were housed with a 12-hour light/dark cycle.
Leptin administration
Murine leptin (R&D Systems Inc.) was diluted with sterile saline to the desired concentration and was injected either intraperitoneally (i.p.; 30 μg twice daily) or via the lateral cerebroventricle (ICV; 2 μg daily) over a three-day period, as previously described5, 19. For studies of leptin-induced sympathetic activation, leptin was administered intravenously (i.v.; 120 μg) or ICV (2 μg)5.
Adenoviral targeting of Cre to SFO and lateral ventricle cannulation
Recombinant adenoviral vectors encoding Cre-recombinase (AdCre, 4 × 1010 plaque-forming units/ml) and titer-matched AdLacZ were obtained from the Iowa Gene Transfer Vector Core. Targeting of adenoviral vectors to the SFO was performed as previously described in detail by our laboratory18, 20-23. Using this method, we have shown highly localized and robust transgene expression in the SFO by 3 days post-injection that remains stable at high levels for at least 6 weeks. Briefly, mice were anesthetized (ketamine, 150 mg/kg + xylazine, 15 mg/kg, ip) and placed in a stereotaxic device. The surface of the skull was visualized under a dissecting microscope and a small hole was drilled in the skull at the appropriate location. Viral vectors were targeted to the SFO using pulled glass pipettes. The glass pipette tip was lowered 3.2 mm ventral from the dorsal surface of the skull at 0.3 mm rostral and 1.0 mm lateral to bregma, and the adenovirus was delivered via a custom pressure injection system. For experiments involving ICV injection of leptin, mice were subsequently instrumented with an indwelling ICV cannula (PlasticsOne Inc.) for later CNS injection of leptin as previously described23. Mice were given at least 7 days for surgical recovery.
SNA recording
Mice were instrumented for anesthetized recordings of BAT and renal sympathetic SNA as previously described6, 19, 65. Mice were anesthetized i.p. (ketamine, 91 mg/kg + xylazine, 9.1 mg/kg). Intubation was performed (PE-50) to allow for spontaneous respiration of oxygen-enriched air. The right jugular vein was cannulated to maintain the level of anesthesia throughout the protocol with α-chloralose (initial dose: 25 mg/kg; supplemental dose of 6mg/kg/hr) and the left carotid artery was cannulated for continuous measurements of arterial pressure. The nerves to BAT or the left kidney were identified under a dissecting microscope. Following isolation, the nerves were then mounted on custom-made 36-gauge platinum-iridium recording electrodes (Cooner Wire Co.) and the nerve was fixed to the electrode with silicone gel (Kwik-Sil, World Precision Instruments Inc.). Following surgical procedures, the animals were allowed to stabilize prior to obtaining BAT and renal SNA measurements before and for up to 4 hours following intravenous or ICV administration of leptin. Body temperature was maintained at 37°C throughout surgical and recording procedures. SNA data were acquired and analyzed as previously described619, 65. The nerve electrodes were attached to a high-impedance probe (HIP-511, Grass Instruments Co.), amplified (105) with a preamplifier (Grass P5 AC), and filtered (100-1000Hz). The amplified, filtered neural signal was routed through an analogue-digital converter (Powerlab model 8S, AD Instruments Inc.) to a computer for continuous recording and data analysis. Background noise was determined from the residual SNA signal following euthanasia and data were expressed as the percentage change in integrated voltage relative to baseline values.
Quantitative real-time PCR (qPCR)
Mice were euthanized and brains were harvested, immediately placed on dry ice and stored at −80°C. Micropunches of the SFO, paraventricular nucleus of the hypothalamus, arcuate nucleus, VMH and NTS were obtained using the coordinates of Franklin and Keith24. Tissue from two mice was pooled per biological sample. Total RNA was isolated by Trizol® (Invitrogen) extraction, reverse transcribed using random hexamer primers and template samples of 25 ng were subjected in triplicate to qPCR (ABI 7500FAST system) using Power SYBR Green (Applied Biosystems). All primers were derived from mus musculus (National Center for Biotechnology Information GenBank). Primer sequences used were:
| ObRb | F: 5′- CTATGTGGTTTTGTTACACTGG-3′ |
| R: 5′- AGGTGAGAGAAAGGAGTCATC-3′ | |
| β-actin | F: 5′-CATCCTCTTCCTCCCTGGAGAAGA-3′ |
| R: 5′-ACAGGATTCCATACCCAAGAAGGAAGG-3′ |
The average expressed isoform of the signaling form of the leptin receptor (ObRb) was expressed relative to the calibrator (β-actin) and the relative fold-change compared to the calibrator was calculated using the comparative ΔΔCt method25.
β-galactosidase staining
CAG-CATZ reporter mice were transcardially perfused with 0.9% saline follow by ice-cold 4% paraformaldehyde in PBS. The brains were removed, placed in 4% paraformaldehyde for 4 hours, followed by 30% sucrose for 24 hours at 4°C. Coronal cryosections (30μm) were obtained and sections were processed for β-galactosidase activity using 5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside (X-gal, Boehringer Mannheim) as described18. Certain sections were counterstained with eosin and mounted sections were visualized using light microscopy.
Data analysis
Data are expressed as mean ± SEM. When appropriate, a two-tailed unpaired t-test was used for comparisons between two groups. A two-way repeated measures ANOVA was used for time-course experiments. Post-hoc comparisons were performed using a Tukey’s test when appropriate. The alpha level was set at p<0.05.
RESULTS
Adenoviral targeting of the SFO for Cre-mediated deletion of ObR
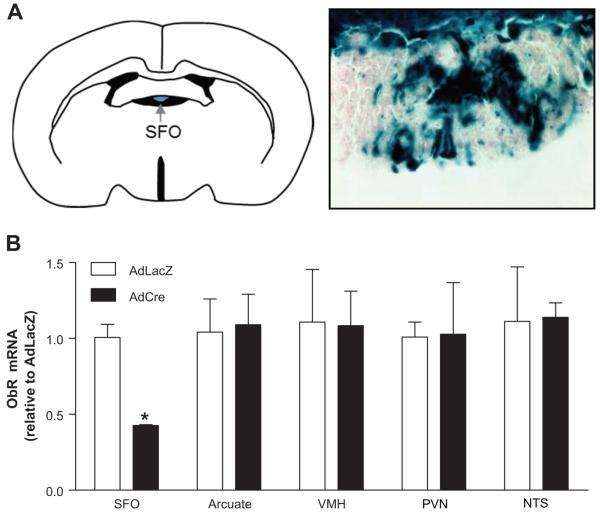
In order to evaluate the functional role of SFO ObR in leptin-induced metabolic and SNA responses, we utilized SFO-targeted microinjections of AdCre in ObRflox/flox mice. As a first step, we confirmed the efficacy and selectivity of this approach using the reporter strain CAG-CATZ in which β-galactosidase activity is induced following adenoviral delivery of Cre-recombinase (AdCre) as described18. One week following SFO-targeted injections of AdCre, robust X-gal staining was evident in the SFO (Figure 1A). Importantly, X-gal was restricted to the SFO, with no evidence of Cre-mediated β-galactosidase activation in hypothalamic regions, including the arcuate nucleus and VMH, or the brainstem NTS and area postrema (Figure S1). These findings are consistent with our previous reports18 and demonstrate the effectiveness and selectivity of AdCre-mediated recombination of loxP flanked genes in the SFO.
Figure 1. Selective adenoviral targeting of the SFO and deletion of ObR.
(A) Schematic of the subfornical organ (SFO; left) and X-gal staining in the SFO following SFO-targeted delivery of an adenovirus encoding Cre-recombinase in CAG-CATZ reporter mice (right, 40×). (B) Real-time PCR measurements of ObR mRNA from micropunches of the SFO, arcuate nucleus, ventromedial hypothalamus (VMH), paraventricular nucleus of the hypothalamus (PVN) and NTS following SFO-targeted AdCre or control AdLacZ in ObRflox/flox mice. n=4; *p<0.05 vs AdLacZ.
Next, we confirmed Ad-Cre-mediated deletion of ObR at the mRNA level in mice harboring a loxP-flanked ObR allele (ObRflox/flox). As shown in Figure 1B, qPCR analysis revealed robust knockdown of ObR in mice with SFO-targeted AdCre compared to animals treated with the control vector (AdLacZ). Importantly, adenoviral delivery of Cre to the SFO did not affect ObR expression levels in the arcuate nucleus, VMH, paraventricular nucleus of the hypothalamus (PVN) or NTS (Figure 1B). These results demonstrate that ObR in the SFO can be effectively targeted for deletion without influencing ObR expression in other CNS regions that have been implicated in leptin actions.
Ablation of SFO ObR does not influence leptin-induced decreases in body weight and food intake
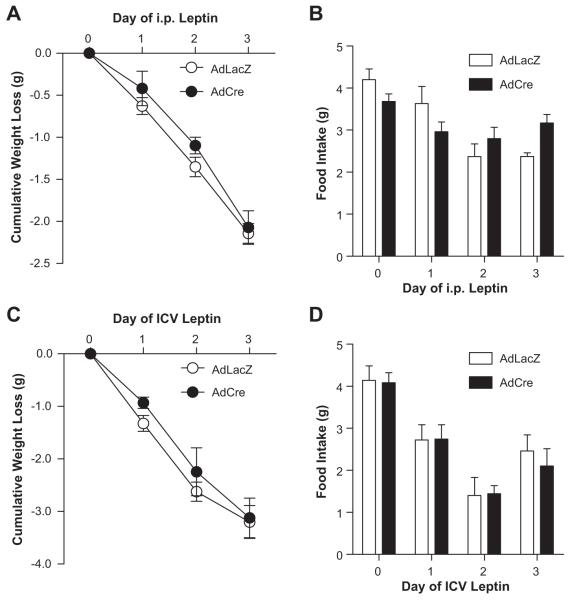
To investigate a functional role of ObR in the SFO, we first evaluated the weight-reducing and anorexic effects of daily leptin administration in ObRflox/flox mice following SFO-targeted AdCre or control AdLacZ. We used both systemic (i.p.) and central neural (ICV) dosing of leptin, each of which have been shown to activate the leptin receptor (i.e. STAT-3 phosphorylation) in the SFO14, hypothalamus5 and brainstem26. Seven days after adenoviral gene transfer, there were no differences between groups in basal body weight (22.7 ± 0.3 vs. 23.1 ± 0.3 g, AdLacZ vs. AdCre, p>0.05) and food intake (4.2 ± 0.2 vs. 4.2 ± 0.1 g, AdLacZ vs. AdCre, p>0.05). As expected, twice-daily i.p. administration of leptin resulted in a significant and progressive weight loss over a 3 day period in control animals (Δ-2.1 ± 0.1g, p<0.05 vs. baseline, Figure 2A). This decrease in body weight was accompanied by a significant reduction in daily food. Selective ablation of SFO ObR did not influence the leptin-induced decreases in body weight and food intake, with AdCre-targeted mice demonstrating a similar cumulative weight loss (Δ-2.1 ± 0.2g, p<0.05 vs. baseline) and reduction in food intake over the 3 day period compared to AdLacZ-treated mice (Figure 2A & B). Additional experiments using daily ICV administration of leptin in ObRflox/flox mice demonstrated a similar progressive decrease in body weight and food intake, and this response did not differ between AdCre and AdLacZ SFO-microinjected mice (Figure 2C & D). In line with these findings, adipose tissue mass was similar between AdLacZ and AdCre-treated mice following 3 days of i.p. or ICV leptin administration (Figure S2).
Figure 2. Deletion of ObR from the SFO does not influence leptin-induced decreases in body weight or food intake.
Food intake (A) and body weight (B) responses to intraperitoneal (i.p.) leptin over a 3 day period. Food intake (C) and body weight (D) responses to lateral cerebroventricle (ICV) leptin are also shown. n=4/group.
Loss of SFO ObR does not influence leptin-induced increases in thermogenic BAT sympathetic nerve activity
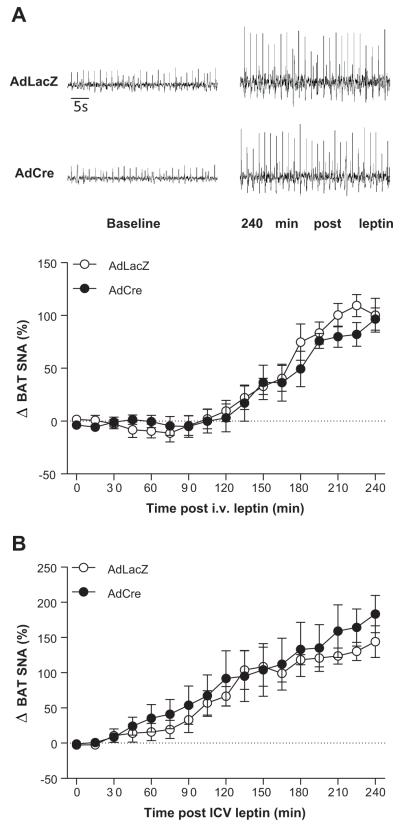
The data presented in Figure 2 suggest that ObR in the SFO are not obligatory for the weight-reducing and anorexic effects of leptin. Because leptin-induced sympathetic activation to thermogenic organs such as BAT is crucial for the weight-reducing effects of leptin27, we further sought to determine the role of SFO ObR in the BAT SNA response. In ObRflox/flox mice with SFO-targeted AdLacZ, i.v. administration of leptin caused a marked increase in BAT SNA (100 ± 16%, p<0.05 vs. baseline, Figure 3A). Interestingly, in ObRflox/flox mice with targeted deletion of ObR in the SFO, the increase in BAT SNA to i.v. leptin (97 ± 11%, p<0.05 vs. baseline, Figure 3A) was similar to that seen in the AdLacZ control mice. Analogous results were found with ICV leptin administration (Figure 3B). These data are in line with the findings presented in Figure 2 and show that ObR in the SFO are not necessary for the thermogenic BAT SNA effects of leptin.
Figure 3. Ablation of ObR in the SFO does not influence leptin-mediated increases in brown adipose tissue (BAT) SNA.
(A) Original BAT SNA recordings at baseline and 4 hours following intravenous (i.v.) leptin administration (top) and the time-course of the responses (bottom). (B) BAT SNA at baseline and 4 hours following lateral cerebroventricle (ICV) leptin administration. n=5/group.
SFO-selective deletion of ObR abolishes leptin-induced renal sympathetic activation
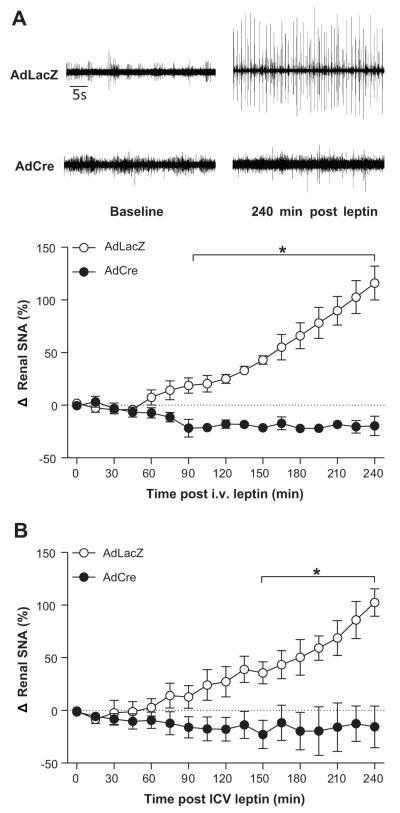
Given the well documented role of the SFO in cardiovascular sympathetic regulation16, 17, we next examined the role of leptin receptors in the SFO on leptin-mediated increases in SNA to the kidney. ObRflox/flox mice underwent SFO-targeted delivery of AdCre or AdLacZ as above and were given one week for recovery and maximal transgene expression. Intravenous administration of leptin resulted in a robust increase in renal SNA in ObRflox/flox mice treated with the control vector AdLacZ (116 ± 16%, p<0.05 vs. baseline, Figure 4A). In contrast, in ObRflox/flox mice that underwent treatment with SFO-targeted AdCre, the leptin-induced increases in renal SNA were completely abolished (−20 ± 9%, p>0.05 vs. baseline, Figure 4A). Separate studies using ICV administration of leptin demonstrated similar results (Figure 4B). Consistent with our previous findings in anesthetized mice5, 19, 28, 29, an initial small decrease in mean arterial pressure was noted within 15 minutes after i.v. and ICV) leptin administration (Figure S3). This decrease was equivalent between the two groups (i.v.: −8 ± 2 vs, −10 ± 5 mmHg, AdLacZ vs. AdCre, p>0.05; ICV: −11 ± 4 vs, 12 ± 3 mmHg, AdLacZ vs. AdCre, p>0.05), and arterial pressure remained stable at this level for the remainder of the protocol (Figure S3).
Figure 4. Ablation of ObR in the SFO abolishes leptin-induced renal sympathoexcitation.
(A) Original renal SNA recordings at baseline and 4 hours following intravenous (i.v.) leptin administration (left) and the time-course of the responses (right). (B) Renal SNA recordings at baseline and 4 hours following lateral cerebroventricle (ICV) leptin administration. n=5/group;*p<0.05 vs. AdCre.
DISCUSSION
In the current study we demonstrate a functional role for ObR within the circumventricular SFO in mediating the renal sympathetic actions of leptin. In contrast, our findings demonstrate that deletion of ObR from the SFO does not influence leptin-induced increases in BAT SNA or food intake. In line with this, leptin-mediated decreases in body weight were similar following SFO-specific loss of ObR. Collectively, these data suggest that leptin acts within the SFO to selectively increase sympathetic outflow to the kidney, without effects on thermogenic BAT SNA, food intake and body weight regulation. Our findings highlight an emerging role for the SFO in the regulation of circulating energy signals such as leptin, and further bolster the concept that brain regions, now including the SFO, can mediate distinct responses to leptin.
In order to target ObR for deletion in the SFO selectively, we used Cre-LoxP technology and site-specific targeted adenoviral microinjections18, 20, 21. Using this approach, specific and robust loss of ObR from the SFO was observed. Importantly, ObR expression remained unchanged in other brain regions implicated in the metabolic and sympathetic actions of leptin, allowing us to evaluate the unique role of ObR in the SFO on leptin’s actions within the CNS.
Our finding that leptin signaling in the SFO mediates leptin-induced increases in sympathetic nerve activity to the kidney builds upon a large body of literature demonstrating the importance of this forebrain region in cardiovascular regulation16, 17, 30. Indeed, the SFO is well recognized in mediating cardiovascular responses to circulating signals, such as angiotensin II, and has been suggested to serve as a signal transduction “gateway” for large peptides that are too large to access sites inside the BBB16, 17, 30. Furthermore, the SFO sends efferent projections to a number of CNS regions that have been implicated in the central sympathoexcitatory responses to leptin, including the arucate nucleus and NTS. Interestingly, direct injection of leptin into the arcuate nucleus9 and NTS6 results in renal sympathetic nerve activation. Recent observations, using the ObRflox/flox model employed in the current study, further demonstrated that selective ablation of ObR from the arcuate nucleus abrogates leptin-induced increases in renal and BAT SNA5. The current results build upon and extend these previous findings by demonstrating a role for a region outside of the BBB, the circumventricular SFO, in mediating leptin-induced elevations in renal SNA. Furthermore, these findings highlight the concept of a distributed brain network in mediating the renal sympathoexcitatory responses to leptin.
To the best of our knowledge, only one previous investigation has examined a role for the SFO in mediating leptin-induced cardiovascular responses. Smith and Ferguson14 found that direct injection of leptin into the SFO of rats resulted in a small (~5mmHg), transient decrease in arterial pressure. Based on these findings, the authors hypothesized that leptin signaling in the SFO causes an early depressor response that may offset the pro-hypertensive actions of leptin signaling in other CNS nuclei. In line with these findings, we noted a slight decrease in arterial pressure (Supplemental Figure 3) during the initial 15 minutes following leptin administration. We did not, however, find a difference in the early, transient decrease in arterial pressure in the AdLacZ control mice compared with the AdCre mice with deletion of SFO ObR. This suggests that this early transient response may not be mediated by ObR in the SFO. In contrast, a clear, robust and progressive leptin-induced increase in renal SNA was seen in control mice over four hours but was absent following SFO ObR ablation. Thus, our findings suggest that the predominant effect of stimulation of leptin receptors in the SFO is renal sympathoexcitation.
One could suggest that the leptin did not reach the arcuate nucleus (or other brain regions) in our study and that this explains why arcuate ObR did not preserve the renal SNA responses to leptin when SFO ObR were deleted. However, importantly, we used a systemic dose of leptin which has recently been used to demonstrate a role for ObR in the arcuate nucleus in mediating the central sympathoexcitatory effects of leptin5. Moreover, systemic and CNS delivery of leptin activates leptin signaling in the hypothalamus31 and brainstem26, as indicated by STAT-3 phosphorylation, and this activation is apparent within 30 minutes of leptin administration. Therefore, our findings indicate that loss of ObR signaling in the SFO selectively attenuates leptin-induced renal sympathoexcitation, despite activation of ObR in other brain regions.
The magnitude of the diminution of the renal SNA response to leptin was large and was comparable to that seen with deletion of ObR in the arcuate nucleus using the same mouse model and experimental strategy5. It is not clear why deleting ObR at different sites leads to full abrogation of the renal SNA responses to leptin, but this is not the first study to suggest that sites other than the arcuate nucleus may contribute substantially to renal SNA responses to leptin. Indeed, Marsh et al32 and Mark et al6 found that direct injections of leptin into the VMH and NTS, respectively, evoked renal sympathetic activation. Morever, studies of regulation of the food intake and body weight responses to leptin have also suggested that deleting ObR or its signaling pathways at distinct sites can substantially affect food intake and body weight, despite intact ObR in other key sites10, 11, 33, 34. Interestingly, the SFO and arcuate nucleus have efferent and afferent projections to similar downstream neural regions, including but not limited to, the PVN, lateral hypothalamic areas and brainstem regions15, 17, 35, 36. Furthermore, the SFO sends direct efferent projections to the arcuate nucleus37. It is therefore possible that several brain areas, working as a distributed interconnected network and perhaps with multiplicative interactions, are critical for leptin-mediated renal SNA responses. Thus, disrupting ObR signaling at any point in this pathway may be sufficient to attenuate leptin-induced renal sympathoexcitation.
In addition to cardiovascular regulation, emerging data suggest a role for the SFO in the control of metabolism14, 15. SFO neurons have been shown to be responsive to the appetite suppressing and stimulating hormones, amylin38 and ghrelin15, respectively. More recently Ferguson and colleagues have demonstrated the presence of functional leptin receptors in the SFO14, which led us to hypothesize that the SFO ObR may play a role in the central metabolic actions of leptin. In contrast to this hypothesis, we found that deletion of ObR in the SFO did not blunt the decrease in food intake in response to systemic or centrally administered leptin. In addition, ablation of the leptin receptor in the SFO failed to influence leptin-induced increases in BAT SNA and decreases in fat mass and body weight. These findings, therefore, suggest that leptin receptor signaling in the SFO does not initiate these metabolic responses. Our study did not evaluate or exclude a role for SFO ObR in mediating other metabolic responses to leptin, such as improvement in insulin and glucose metabolism39. In addition, our results do not exclude an “indirect” role for the SFO in the neural pathways mediating leptin-induced increases in BAT SNA and decreases in food intake. Due to the strong connections from hypothalamic and brainstem regions to the SFO15, 16, this circumventricular region may be important in leptin-mediated regulation of food intake, BAT SNA and body weight responses, but our study suggests that this is likely not through direct SFO ObR signaling.
Perspectives
There is mounting evidence for a distributed brain network of leptin action and for site-specific actions of leptin. This information is particularly relevant, given that in obese conditions, the metabolic actions of leptin are limited whereas the cardiovascular sympathoexcitatory actions appear to be preserved. For example, several animal models of obesity demonstrate normal renal SNA responses to exogenously administered leptin, whereas the anorexic and weight-reducing effects of leptin are reduced19, 40-42. The evidence for CNS site-specific leptin actions, as observed in this study for the SFO, has potential relevance to selective leptin resistance in obesity as there is also evidence for brain site-specific leptin resistance that appears to involve primarily the hypothalamic arcuate nucleus31. Our findings indicate that SFO-ObR mediates the renal cardiovascular sympathetic actions of leptin but not the food intake, BAT SNA and body weight effects of leptin. We speculate that if the SFO remains leptin-sensitive in obesity, this could potentially contribute to preservation of the renal sympathetic cardiovascular actions of leptin in obese states where there is partial resistance to the food intake, BAT SNA, and body weight effects of leptin. Collectively, these findings further support a dissociation between CNS mechanisms controlling energy homeostasis and cardiovascular regulation43, and additionally highlight a selective and distributed brain network of leptin action, including the circumventricular organs.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
- What Is New?
- These findings show that leptin receptors in the brain SFO play an important role in leptin-induced renal sympathoexcitation.
- Removal of leptin receptors selectively from the SFO did not influence leptin-mediated decreases in body weight and food intake or increases in SNA to BAT.
- What Is Relevant?
- We speculate that if the SFO remains leptin-sensitive in obesity, this could potentially contribute to preservation of the renal sympathetic cardiovascular actions of leptin in obese states where there is partial resistance to the food intake, BAT SNA, and body weight effects of leptin.
- These findings highlight a selective and distributed brain network of leptin action, including the brain circumventricular organs.
- These results also support a dissociation between CNS mechanisms controlling energy homeostasis and cardiovascular regulation.
Summary: Leptin acts within the SFO to selectively increase sympathetic outflow to the kidney, without effects on thermogenic BAT SNA, food intake and body weight regulation. Our findings highlight an emerging role for the SFO in the regulation of circulating energy signals such as leptin, and further bolster the concept that brain regions, now including the SFO, can mediate distinct responses to leptin.
ACKNOWLEDGEMENTS
We thank Julie A. Horwath for her technical assistance with these studies.
FUNDING SUPPORT
This work was supported by NIH grants HL084207, HL063887, HL096571 and research funds from the Roy J. Carver Trust. CNY was supported by an American Physiological Society Fellowship.
Footnotes
CONFLICT OF INTEREST / DISCLOSURES
The authors have no conflict of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 4.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 8.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 9.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 10.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Lechan RM, Nestler JL, Jacobson S, Reichlin S. The hypothalamic ‘tuberoinfundibular’ system of the rat as demonstrated by horseradish peroxidase (HRP) microiontophoresis. Brain Res. 1980;195:13–27. doi: 10.1016/0006-8993(80)90862-8. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985;243:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 14.Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, Ferguson AV. The subfornical organ: A central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009;296:R512–20. doi: 10.1152/ajpregu.90858.2008. [DOI] [PubMed] [Google Scholar]
- 15.Fry M, Ferguson AV. The sensory circumventricular organs: Brain targets for circulating signals controlling ingestive behavior. Physiol Behav. 2007;91:413–423. doi: 10.1016/j.physbeh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Cottrell GT, Ferguson AV. Sensory circumventricular organs: Central roles in integrated autonomic regulation. Regul Pept. 2004;117:11–23. doi: 10.1016/j.regpep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: Critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 18.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25–32. doi: 10.1152/physiolgenomics.00048.2004. [DOI] [PubMed] [Google Scholar]
- 19.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 20.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinnayah P, Lazartigues E, Sakai K, Sharma RV, Sigmund CD, Davisson RL. Genetic ablation of angiotensinogen in the subfornical organ of the brain prevents the central angiotensinergic pressor response. Circ Res. 2006;99:1125–1131. doi: 10.1161/01.RES.0000250259.66683.f5. [DOI] [PubMed] [Google Scholar]
- 22.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: Comparison of two viral vector systems. Hypertension. 2002;39:603–608. doi: 10.1161/hy0202.103295. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 24.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press, Inc; San Diego: 1997. [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Huo L, Gamber KM, Grill HJ, Bjorbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology. 2008;149:492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res. 2012 doi: 10.1007/s10286-012-0168-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 31.Munzberg H. Differential leptin access into the brain--a hierarchical organization of hypothalamic leptin target sites? Physiol Behav. 2008;94:664–669. doi: 10.1016/j.physbeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 33.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 34.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG, Jr, Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol. 2010;298:R720–8. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 37.Gruber K, McRae-Degueurce A, Wilkin LD, Mitchell LD, Johnson AK. Forebrain and brainstem afferents to the arcuate nucleus in the rat: Potential pathways for the modulation of hypophyseal secretions. Neurosci Lett. 1987;75:1–5. doi: 10.1016/0304-3940(87)90065-6. [DOI] [PubMed] [Google Scholar]
- 38.Riediger T, Schmid HA, Young AA, Simon E. Pharmacological characterisation of amylin-related peptides activating subfornical organ neurones. Brain Res. 1999;837:161–168. doi: 10.1016/s0006-8993(99)01697-2. [DOI] [PubMed] [Google Scholar]
- 39.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 40.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39:486–490. doi: 10.1161/hy0202.102836. [DOI] [PubMed] [Google Scholar]
- 41.Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, Barzel B, Head GA. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55:862–868. doi: 10.1161/HYPERTENSIONAHA.109.141119. [DOI] [PubMed] [Google Scholar]
- 42.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: Evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 43.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.