Abstract
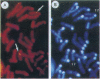
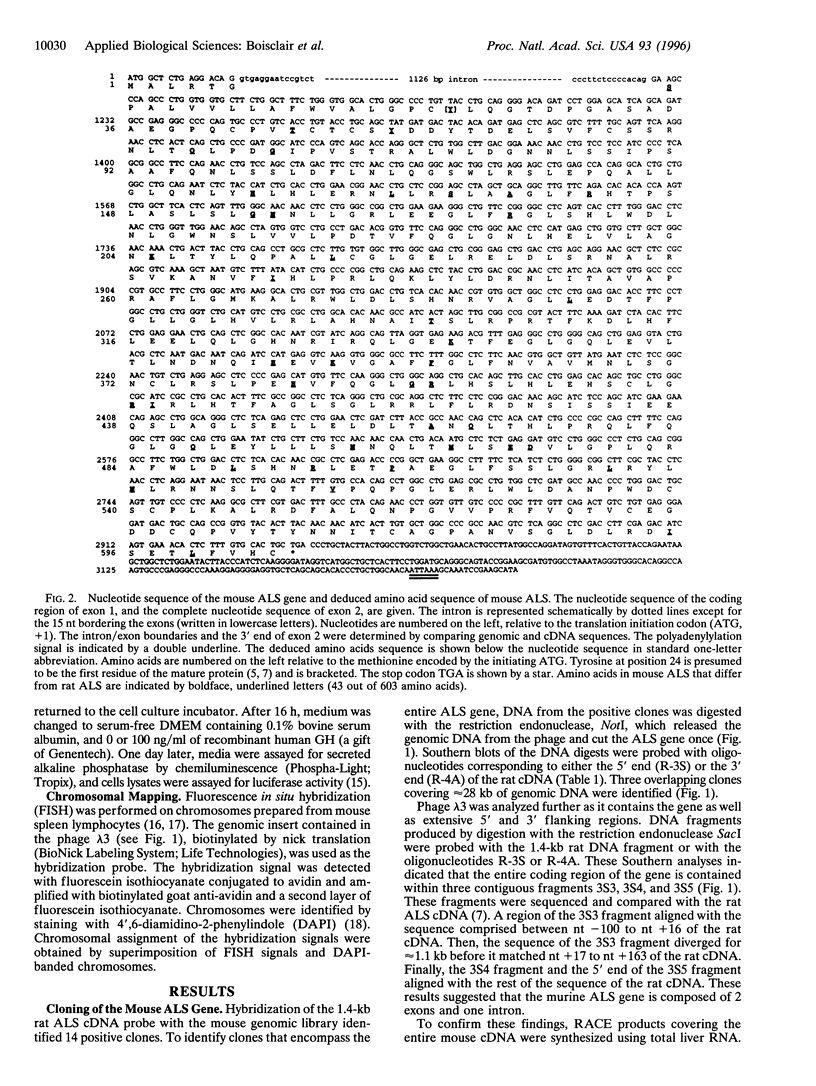
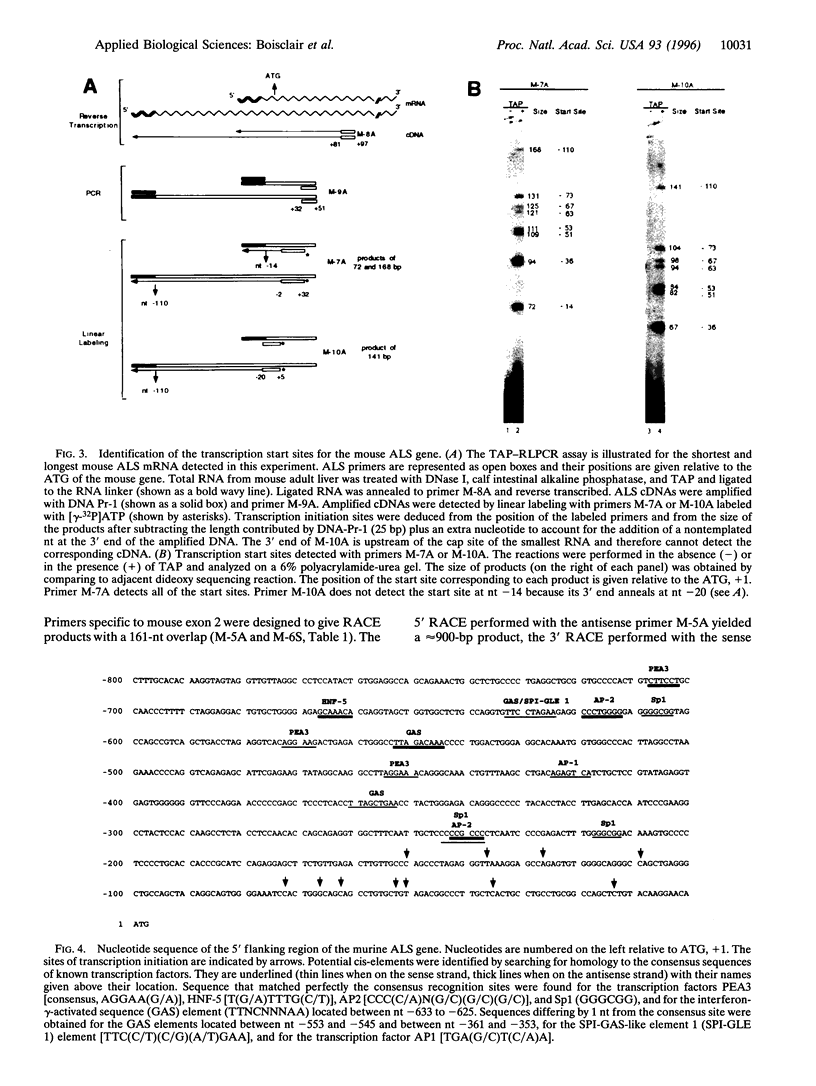
After birth, most of insulin-like growth factor I and II (IGFs) circulate as a ternary complex formed by the association of IGF binding protein 3-IGF complexes with a serum protein called acid-labile subunit (ALS). ALS retains the IGF binding protein-3-IGF complexes in the vascular compartment and extends the t1/2 of IGFs in the circulation. Synthesis of ALS occurs mainly in liver after birth and is stimulated by growth hormone. To study the basis for this regulation, we cloned and characterized the mouse ALS gene. Comparison of genomic and cDNA sequences indicated that the gene is composed of two exons separated by a 1126-bp intron. Exon 1 encodes the first 5 amino acids of the signal peptide and contributes the first nucleotide of codon 6. Exon 2 contributes the last 2 nt of codon 6 and encodes the remaining 17 amino acids of the signal peptide as well as the 580 amino acids of the mature protein. The polyadenylylation signal, ATTAAA, is located 241 bp from the termination codon. The cDNA and genomic DNA diverge 16 bp downstream from this signal. Transcription initiation was mapped to 11 sites over a 140-bp TATA-less region. The DNA fragment extending from nt -805 to -11 (ATG, +1) directed basal and growth hormone-regulated expression of a luciferase reporter plasmid in the rat liver cell line H4-II-E. Finally, the ALS gene was mapped to mouse chromosome 17 by fluorescence in situ hybridization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Hsu G. W., Myers M. G., Jr, Billestrup N., White M. F., Carter-Su C. Growth hormone, interferon-gamma, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995 Jun 16;270(24):14685–14692. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- Baxter R. C. Circulating levels and molecular distribution of the acid-labile (alpha) subunit of the high molecular weight insulin-like growth factor-binding protein complex. J Clin Endocrinol Metab. 1990 May;70(5):1347–1353. doi: 10.1210/jcem-70-5-1347. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Dai J. Purification and characterization of the acid-labile subunit of rat serum insulin-like growth factor binding protein complex. Endocrinology. 1994 Feb;134(2):848–852. doi: 10.1210/endo.134.2.7507839. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Martin J. L., Beniac V. A. High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. J Biol Chem. 1989 Jul 15;264(20):11843–11848. [PubMed] [Google Scholar]
- Boisclair Y. R., Brown A. L., Casola S., Rechler M. M. Three clustered Sp1 sites are required for efficient transcription of the TATA-less promoter of the gene for insulin-like growth factor-binding protein-2 from the rat. J Biol Chem. 1993 Nov 25;268(33):24892–24901. [PubMed] [Google Scholar]
- Boisclair Y. R., Brown A. L. Use of reverse ligation-PCR to identify transcriptional start sites in GC-rich TATA-less genes: application to the rat IGFBP-2 gene. DNA Cell Biol. 1995 Aug;14(8):731–739. doi: 10.1089/dna.1995.14.731. [DOI] [PubMed] [Google Scholar]
- Bronson S. K., Smithies O. Altering mice by homologous recombination using embryonic stem cells. J Biol Chem. 1994 Nov 4;269(44):27155–27158. [PubMed] [Google Scholar]
- Chin E., Zhou J., Dai J., Baxter R. C., Bondy C. A. Cellular localization and regulation of gene expression for components of the insulin-like growth factor ternary binding protein complex. Endocrinology. 1994 Jun;134(6):2498–2504. doi: 10.1210/endo.134.6.7515002. [DOI] [PubMed] [Google Scholar]
- Dai J., Baxter R. C. Molecular cloning of the acid-labile subunit of the rat insulin-like growth factor binding protein complex. Biochem Biophys Res Commun. 1992 Oct 15;188(1):304–309. doi: 10.1016/0006-291x(92)92385-b. [DOI] [PubMed] [Google Scholar]
- Dai J., Baxter R. C. Regulation in vivo of the acid-labile subunit of the rat serum insulin-like growth factor-binding protein complex. Endocrinology. 1994 Dec;135(6):2335–2341. doi: 10.1210/endo.135.6.7527331. [DOI] [PubMed] [Google Scholar]
- Dai J., Scott C. D., Baxter R. C. Regulation of the acid-labile subunit of the insulin-like growth factor complex in cultured rat hepatocytes. Endocrinology. 1994 Sep;135(3):1066–1072. doi: 10.1210/endo.135.3.8070348. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Faber P. W., van Rooij H. C., Schipper H. J., Brinkmann A. O., Trapman J. Two different, overlapping pathways of transcription initiation are active on the TATA-less human androgen receptor promoter. The role of Sp1. J Biol Chem. 1993 May 5;268(13):9296–9301. [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont-Racine M., Bertrand E., Pictet R., Grange T. A highly sensitive method for mapping the 5' termini of mRNAs. Nucleic Acids Res. 1993 Apr 11;21(7):1683–1684. doi: 10.1093/nar/21.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargosky S. E., Tapanainen P., Rosenfeld R. G. Administration of growth hormone (GH), but not insulin-like growth factor-I (IGF-I), by continuous infusion can induce the formation of the 150-kilodalton IGF-binding protein-3 complex in GH-deficient rats. Endocrinology. 1994 May;134(5):2267–2276. doi: 10.1210/endo.134.5.7512499. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Schmid C., Froesch E. R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989 Dec;121(6):753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Heng H. H., Squire J., Tsui L. C. High-resolution mapping of mammalian genes by in situ hybridization to free chromatin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9509–9513. doi: 10.1073/pnas.89.20.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng H. H., Tsui L. C. Modes of DAPI banding and simultaneous in situ hybridization. Chromosoma. 1993 May;102(5):325–332. doi: 10.1007/BF00661275. [DOI] [PubMed] [Google Scholar]
- Ing Y. L., Leung I. W., Heng H. H., Tsui L. C., Lassam N. J. MLK-3: identification of a widely-expressed protein kinase bearing an SH3 domain and a leucine zipper-basic region domain. Oncogene. 1994 Jun;9(6):1745–1750. [PubMed] [Google Scholar]
- Javahery R., Khachi A., Lo K., Zenzie-Gregory B., Smale S. T. DNA sequence requirements for transcriptional initiator activity in mammalian cells. Mol Cell Biol. 1994 Jan;14(1):116–127. doi: 10.1128/mcb.14.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. I., Clemmons D. R. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995 Feb;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kollmar R., Farnham P. J. Site-specific initiation of transcription by RNA polymerase II. Proc Soc Exp Biol Med. 1993 Jun;203(2):127–139. doi: 10.3181/00379727-203-43583. [DOI] [PubMed] [Google Scholar]
- Kollmar R., Sukow K. A., Sponagle S. K., Farnham P. J. Start site selection at the TATA-less carbamoyl-phosphate synthase (glutamine-hydrolyzing)/aspartate carbamoyltransferase/dihydroorotase promoter. J Biol Chem. 1994 Jan 21;269(3):2252–2257. [PubMed] [Google Scholar]
- Leong S. R., Baxter R. C., Camerato T., Dai J., Wood W. I. Structure and functional expression of the acid-labile subunit of the insulin-like growth factor-binding protein complex. Mol Endocrinol. 1992 Jun;6(6):870–876. doi: 10.1210/mend.6.6.1379671. [DOI] [PubMed] [Google Scholar]
- Lobie P. E., Wood T. J., Sliva D., Billestrup N., Waters M. J., Enberg B., Norstedt G. The cellular mechanism of growth hormone signal transduction. Acta Paediatr Suppl. 1994 Dec;406:39–47. doi: 10.1111/j.1651-2227.1994.tb13420.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Stephenson E. W., Johnson L., Cochran B. H., Schwartz J. The serum response element can mediate induction of c-fos by growth hormone. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6721–6725. doi: 10.1073/pnas.90.14.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M. Insulin-like growth factor binding proteins. Vitam Horm. 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- Rotwein P., Gronowski A. M., Thomas M. J. Rapid nuclear actions of growth hormone. Horm Res. 1994;42(4-5):170–175. doi: 10.1159/000184189. [DOI] [PubMed] [Google Scholar]
- Sliva D., Wood T. J., Schindler C., Lobie P. E., Norstedt G. Growth hormone specifically regulates serine protease inhibitor gene transcription via gamma-activated sequence-like DNA elements. J Biol Chem. 1994 Oct 21;269(42):26208–26214. [PubMed] [Google Scholar]
- Subramanian A., Teixeira J., Wang J., Gil G. A STAT factor mediates the sexually dimorphic regulation of hepatic cytochrome P450 3A10/lithocholic acid 6 beta-hydroxylase gene expression by growth hormone. Mol Cell Biol. 1995 Sep;15(9):4672–4682. doi: 10.1128/mcb.15.9.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderKuur J., Allevato G., Billestrup N., Norstedt G., Carter-Su C. Growth hormone-promoted tyrosyl phosphorylation of SHC proteins and SHC association with Grb2. J Biol Chem. 1995 Mar 31;270(13):7587–7593. doi: 10.1074/jbc.270.13.7587. [DOI] [PubMed] [Google Scholar]
- Wahle E., Keller W. The biochemistry of 3'-end cleavage and polyadenylation of messenger RNA precursors. Annu Rev Biochem. 1992;61:419–440. doi: 10.1146/annurev.bi.61.070192.002223. [DOI] [PubMed] [Google Scholar]
- Wood T. J., Sliva D., Lobie P. E., Pircher T. J., Gouilleux F., Wakao H., Gustafsson J. A., Groner B., Norstedt G., Haldosén L. A. Mediation of growth hormone-dependent transcriptional activation by mammary gland factor/Stat 5. J Biol Chem. 1995 Apr 21;270(16):9448–9453. doi: 10.1074/jbc.270.16.9448. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Futo E., Hussain M., Rutishauser J., Maack C. A., Froesch E. R. Intravenously injected insulin-like growth factor (IGF) I/IGF binding protein-3 complex exerts insulin-like effects in hypophysectomized, but not in normal rats. J Clin Invest. 1995 Jan;95(1):179–186. doi: 10.1172/JCI117636. [DOI] [PMC free article] [PubMed] [Google Scholar]