Abstract
The dismal outcome of blast crisis chronic myelogenous leukemia (CML-BC) patients underscores the need for a better understanding of the mechanisms responsible for the development of drug-resistance. Altered expression of the anti-apoptotic Bcl-xL has been correlated with BCR-ABL leukemogenesis; however, its involvement in the pathogenesis and evolution of CML has not been formally been demonstrated yet. Thus, we generated an inducible mouse model in which simultaneous expression of p210-BCR-ABL1 and deletion of bcl-x occurs within hematopoietic stem and progenitor cells. Absence of Bcl-xL did not affect development of the chronic phase-like myeloproliferative disease, but none of the deficient mice progressed to an advanced phenotype, suggesting the importance of Bcl-xL in survival of progressing early progenitor cells. Indeed, pharmacologic antagonism of Bcl-xL, with ABT-263, combined with PP242-induced activation of BAD markedly augmented apoptosis of CML-BC cell lines and primary CD34+ progenitors but not those from healthy donors, regardless of drug-resistance induced by bone marrow stromal cell-generated signals. Moreover, studies in which BAD or Bcl-xL expression was molecularly altered strongly support their involvement in ABT-263/PP242-induced apoptosis of CML-BC progenitors. Thus, suppression of the antiapoptotic potential of Bcl-xL together with BAD activation represents an effective pharmacologic approach for patients undergoing blastic transformation.
INTRODUCTION
Despite successful implementation of imatinib and second generation tyrosine kinase inhibitors (TKI) as first line therapies for chronic myelogenous leukemia (CML) in chronic phase (CML-CP), the majority of CML-BC and Philadelphia-positive (Ph+) B-cell acute lymphoblastic leukemia (B-ALL) patients do not show long-term responses to TKIs or any other therapeutic option1-6. The molecular mechanisms responsible for blastic transformation and drug-resistance in CML-BC are still unclear but likely involve both BCR-ABL1 kinase-dependent and –independent mechanisms4. Presence of BCR-ABL1 mutations can only in part explain the development of TKI-resistance7; in fact, both cell autonomous (e.g. enhanced Src and LYN kinase activity)8 and microenvironment-induced signals9, 10 contribute to development of drug-resistance and increased survival of CD34+ CML-BC progenitors4. The latter seems to depend, at least in part, on increased levels and/or activity of antiapoptotic Bcl-211, Bcl-xL9, 12, 13, and Mcl-19, 14, 15. While Mcl-1, but not Bcl-2, is essential for survival of normal and Ph+ leukemic stem cell (LSC) populations16-19, the role of Bcl-xL in their maintenance in vivo is still unknown. Although loss of Bcl-xL by itself or its pharmacologic antagonism in combination with that of Bcl-2 in B-ALL mouse models did not dramatically improve survival20-22, exposure of TKI-resistant CML-BC stem and progenitor cells to the Bcl-xL/Bcl-2 antagonist ABT-737 induced apoptosis by partially restoring sensitivity to imatinib23. However, therapeutic CML-BC strategies involving pharmacologic antagonism of Bcl-xL could be further refined and potentiated not only by associating a Bcl-xL/Bcl-2 antagonist with TKIs, as BCR-ABL1 kinase mutation-independent relapse is the common outcome for TKI-treated CML-BC patients24, but also by combining the orally bioavailable formulation of ABT-737 (i.e. ABT-263) that reportedly has a clinically-manageable toxicity profile25, with other non toxic drugs capable of further modulating apoptosis. Since the BCR-ABL1-regulated26-28 pro-apoptotic factor BAD is the primary binding partner of Bcl-xL25, and it undergoes phosphorylation (inhibition) upon cytokine- or oncogene-induced activation of Akt and mTORC1/2 signaling29, pharmacologic restoration of BAD activity combined with suppression of Bcl-xL activity might fully restore TKI sensitivity or, per se, strongly initiate apoptosis of CML-BC progenitors even when BCR-ABL kinase-independent signals (e.g. microenvironmental-induced9, 10) control survival of CML-BC progenitors. Thus, it is highly plausible that dual inhibitors of the BCR-ABL1-activated30, 31 and PI3K-Akt-dependent mTORC complexes1/229 (e.g. OSI-02732 and PP24233) that reportedly limit proliferation and colony forming ability of mononuclear cells (MNCs) from CML-BC patients34, 35, have the strong ability to activate BAD and likely potentiate the effects of Bcl-xL/Bcl-2 antagonism in CML-BC.
Here we show deletion of the bcl-x gene in the BCR-ABL1+ LSC-enriched cell compartment neither altered stem cell frequency nor improved mice survival albeit none of the bcl-x deficient mice underwent disease progression and developed a lymphoid CML-BC-like leukemia phenotype36; suggesting that Bcl-xL may be important for the survival of BCR-ABL1+ progenitors undergoing progression. Additionally, we found that PP242 has the ability to activate BAD and potentiate the effects of ABT-263-mediated antagonism of Bcl-xL. Combination of ABT-263 with PP242 efficiently and selectively induced apoptosis in BCR-ABL1+ cell lines and primary CML-BC progenitors, but not CD34+ progenitors from healthy donors, and overcame TKI-resistance induced by signals generated by stromal cells. Furthermore, shRNA studies confirmed efficacy of this strategy depends, at least in part, on PP242-induced BAD activation. Likewise, genetic manipulation of the BCR-ABL1/Bcl-xL/BAD interplay through shRNA-mediated impairment of the BCR-ABL1-regulated heterogeneous ribonuclear protein A1 (hnRNP A1)37 resulted in lower levels of Bcl-xL expression and BCR-ABL1 kinase activity, and increased sensitivity of CD34+ CML-BC progenitors to the pro-apoptotic activity of PP242, suggesting the efficacy of ABT-263 in these studies results from its ability to inhibit Bcl-xL, and not Bcl2. Furthermore, antagonism of Bcl-xL while activating BAD may represent an efficient pharmacologic approach to augment TKI-based therapeutic protocols for CML patients with advanced and drug-insensitive stages of the disease.
METHODS
Generation and analysis of the Bcl-xL-deficient BCR-ABL+ transgenic mice
Inducible SCLtTA-BCR-ABL1-cre-bcl-x fl/fl mice were generated through cross breeding of SCLtTA36, pTRE-BCR-ABL138, and tet-O-cre39 lines, with mice carrying loxP sites flanking exons 1 and 2 of the bcl-x gene40. Breeding was done while administering tetracycline in drinking water38; PCR-mediated genotyping was performed as described38 with gene specific primers (Table 1). Efficiency of recombination within bcl-x was assessed by 3-primer (A, B and C) PCR40 on DNA isolated from bone marrow (BM) and splenic MNCs. Following recombination, primers A and C generate the 280 base pair product (bp). In the presence of a non-recombined allele, primers A and C do not amplify and the 300 bp product from primers A and B is observed. Induction of BCR-ABL1 (p210) transgene and cre recombinase was achieved by tetracycline withdrawal. Mice were induced at 6 to 8 weeks of age and studies were performed with approval of the Medical College of Wisconsin’s IACUC.
Table 1.
Sequences of primers used for genotyping, real-time, and 3-primer PCR
| Primers used for genotyping |
| Bcl-x f forward 5’-GTCCTGGCCCTGTCACTTA-3’ |
| Bcl-x f reverse 5’-CCCTTCCCACCTCACTTCCT-3’ |
| BCR/ABL forward 5’-GAGCGTGCAGAGTGGAGGGAGAACA-3’ |
| BCR/ABL reverse 5’-GGTACCAGGAGTGTTTCTCCAGACTG-3’ |
| Cre forward 5’-ACCTGAAGATGTTCGCGATTATCT-3’ |
| Cre reverse 5’-ACCGTCAGTACGTGAGATATCT-3’ |
| tTA forward 5’-GCTAGGTGTAGAGCAGCCTAC-3’ |
| tTA reverse 5’-GGCGGCATACTATCAGTAGTA-3’ |
|
|
| Primers used for real-time PCR |
| Bcl-x forward 5’-ACTTTTGTGGATCTCTACGGGAAC-3’ |
| Bcl-x reverse 5’-CTGAAGAGTGAGCCCAGCAG-3’ |
| Bcl-2 forward 5’-CTTCGCAGAGATGTCCAGTCAG-3’ |
| Bcl-2 reverse 5’-GAACTCAAAGAAGGCCACAATCC-3’ |
| Mcl-1 forward 5’-TTGTAAGGACGAAACGGGACTG-3’ |
| Mcl-1 reverse 5’-TATGCCAGACCAGCCCCTAC-3’ |
| BCR/ABL forward 5’-CGTCCACTCAGCCACTGGAT-3’ |
| BCR/ABL reverse 5’-GGCTTCACTCAGACCCTGAGG-3’ |
| L19 forward 5’-TCTGGTTGGATCCCAATGAGA-3’ |
| L19 reverse 5’-GTCACAGGCTTGCGGATGAT-3’ |
|
|
| 3-primer PCR (recombination) |
| Primer A 5’-CGGTTGCCTAGCAACGGGGC-3’ |
| Primer B 5’-CTCCCACAGTGGAGGACCTCG-3’ |
| Primer C 5’-TCAGAAGCCGCAATATCCCC-3’ |
Culture of cell lines and primary cells, colony forming, and long term culture-initiating cell (LTC-IC) assays
The CML-BC cell lines 32D-BCR-ABL1 (6.15 clone), LAMA84 (kindly provided by Dr. A. Reid, Imperial College, London UK) and K562 were maintained in culture in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% FBS and 2 mM L-glutamine. For maintenance, cellular fractionation, and drug treatments, 32Dcl3 and derived lines were cultured in the presence of 10% (v/v) WEHI conditioned medium as source of IL-3. For experiments requiring the use of conditioned medium (CM) from the telomerase-immortalized (TERT+) human mesenchymal stem cell lines (hTERT+ stromal line41; kindly provided by Dr. D. Campana, NUS, Singapore), LAMA84 cells were maintained in 100% CM 18 hours preceding and during drug treatments (24 hr.).
Frozen CD34+ Normal Bone Marrow (NBM) cells from different healthy donors were obtained from Cincinnati Children’s Hospital and The Ohio State University (OSU). Studies with human CML specimens included those obtained from The Ohio State University Leukemia Tissue Bank; the Division of Hematology, Maisonneuve-Rosemont Hospital, Montréal QC and from the Department of Hematology, Aarhus University, Denmark, and were carried out with approval from The OSU Institutional Review Board. The percentage of Ph+ cells analyzed by FISH ranged from 91% to 100%. The CD34+ fraction was isolated by magnetic cell sorting (MACS, Miltenyi Biotec, Auburn, CA) and cultured in IMDM containing 30% FBS, 2 mM L-glutamine and supplemented with recombinant human cytokines (StemSpan CC100; Stem Cell Technologies, Vancouver, BC). Mouse lineage-negative/Sca-1+/c-Kit+ (LSK) cells were isolated from femur and/or spleen of induced and non-induced (WT) animals as described36. All in vitro studies using primary mouse cells were done with the OSU IACUC’s approval. Colony forming (CFC) and replating assays, determination of LTC-IC frequency, and lentiviral production and transduction were performed as described in Supplemental Methods.
Isolation of stem/progenitor cell-enriched fractions and flow cytometry-based assays
Total and lineage-depleted mouse BM cells were isolated as described36. FACS-mediated analysis of hematopoietic markers was performed with combinations of the following antibodies: anti-Gr-1 PE, anti-Mac-1 FITC, anti-B220 APC, anti-CD19 PeCy7, anti-Ter119 PeCy7, and anti-c-kit APC AF750 (eBioscience, San Diego, CA), anti-CD71 Biotin and, anti-Sca-1 PeCy7 (BD Biosciences). CML specimens were subjected to CD34 positive-selection, and the hematopoietic stem cell-enriched fraction (CD34+/CD38−) along with common myeloid progenitors (CMP, CD34+/CD38+/CD123+/CD45Rα−) and granulocyte monocyte progenitors (GMPs, CD34+/CD38+/CD123+/CD45Rα+) were separated following staining with anti-CD34 AF647 (4H11) and anti-CD38 PeCy7 (HIT2) (eBioscience), anti-CD123 PE (9F5) (BD Pharmingen), and anti-CD45Rα PE Texas Red (Invitrogen) antibodies and cell sorting (Aria, Becton Dickinson, Franklin Lakes, NJ). Determination of the percentage of apoptotic cells in untreated and after 3 (cell lines) and 6 (primary cells) days of drug treatment were assessed by Annexin V PE staining (BD Biosciences) and Sytox Blue Live/Dead Stain (Invitrogen). All analyses were performed on a tri-laser fluorescent-activated cell sorter (FACS) (LSRII, Becton Dickinson). Cells were thereafter used for RNA isolation, Real Time PCR and Western blot analyses as described in detail in Supplemental Methods.
Reagents (Chemical Inhibitors and Plasmids)
Culture medium containing cell lines and primary cells seeded at a density of 105 and 106 per milliliter, respectively, were exposed to inhibitors at the doses indicated in the results section. Cell lines were treated for 72 hours, except for LAMA84 cells which were treated for 24 hours due to sensitivity to all treatments. The drugs used include Imatinib (Novartis), LY294002 (Cayman Chemical, Ann Arbor, MI), Rapamycin (Sigma, St Louis, MO), ABT-263 (ChemieTek, Indianapolis, IN), PP242 (Chemdea, Ridgewood, NJ), and U0126 (Promega).
The pLL3.7-hnRNPA1(shRNA) construct was obtained by cloning the annealed oligonucleotides 5’-tAGCAAGAGATGGCTAGTGCttcaagagaGCACTAGCCATCTCTTGCTtttttggaac-3’ into the HpaI and NotI sites of the pLL3.7 lentiviral plasmid. Bases specific for hnRNP A1 shRNA are capitalized. The BAD shRNA-containing lentiviral construct was generated as described42.
Statistical analysis
Data are expressed as means ± SEM and were compared using the Student t and/or Fisher exact tests. P values ≤ 0.05 are considered significant.
RESULTS
The survival factor Bcl-xL is dispensable for development of CML in vivo
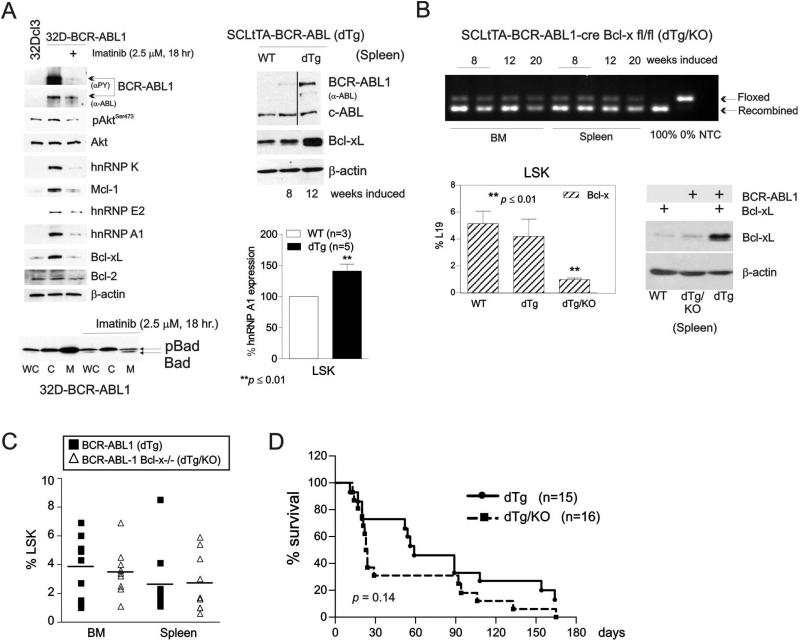
BCR-ABL1-dependent induction of Bcl-xL expression, albeit not required for the emergence of Ph+-ALL in animals22, seems to be important, at least in vitro, for survival of CML-BC cell lines12, 13. High levels of BCR-ABL1 expression similar to those found in CML-BC blasts43 resulted in the imatinib-sensitive induction of survival factors Mcl-1 and Bcl-xL, but not Bcl-2, and in increased expression and activity of their post-transcriptional modulators37, 43, 44 (e.g. hnRNP A1) and upstream regulators of cell survival (e.g. Akt ) (Fig. 1A, top left). Accordingly, Akt-regulated activity of pro-apoptotic BAD was restored upon kinase inhibition of BCR-ABL1, as indicated by the appearance of the non-phosphorylated (active45) BAD in the mitochondrial (M) fraction of imatinib-treated 32D-BCR-ABL1 cells (Fig. 1A, bottom left). To assess whether expression of Bcl-xL has a role in CML-development, maintenance and/or progression in vivo, we crossed SCLtTA-BCR-ABL1 (dTg) mice, which upon induction of BCR-ABL1 develop a CML-like myeloproliferative disorder (MPD) that progresses into a lymphoid blast crisis (L-BC)-like disease in 30% of mice36, with inducible bcl-x-deficient animals22 to generate the SCLtTA-BCR-ABL1-cre-Bcl-x fl/fl (dTg/KO) mouse line (Fig. 1B, top). SCL-driven expression of BCR-ABL1 increased protein levels of Bcl-xL and that of its post-transcriptional modulator hnRNP A137 in MNC and stem cell-enriched (LSK) cell fractions, respectively, isolated from spleens of 8 and/or 12 week-induced dTg mice, (Fig. 1A, top and bottom right). Note that MNCs and LSKs from non-induced littermates (wild type; WT) were used as controls. However, the almost complete loss of Bcl-xL mRNA (~75% reduction) and protein (≥90% reduction) expression in BM and/or splenic LSKs (Fig. 1B, bottom left) and MNCs (Fig.1B, bottom right), respectively, neither altered the frequency of BCR-ABL1+ LSK cells (Fig. 1C) nor prevented the development of a CML-like MPD as indicated by increased presence of Gr-1+/Mac-1+ myeloid cells36 in PB of 8, 12 and 16 week-induced dTg/KO animals (Fig. 2A, left and Suppl. Fig 1A). dTg/KO mice developed splenomegaly (Suppl. Fig 1B, left) and did not demonstrate significantly different overall survival (p=0.14) (Figure 1D), suggesting that the anti-apoptotic potential of Bcl-xL might be dispensable for both the maintenance of human Ph+ stem cell compartment and development of CML. In fact, succumbed dTg/KO mice had a phenotype mostly superimposable with that of the original SCLtTA-BCR-ABL1 mouse model36. In addition to splenomegaly and high percentages of Gr-1+/Mac-1+ cells in PB, BM and spleen (Suppl. Fig. 1A), they also presented pale brittle bones (not shown), and massive infiltration of myeloid cells into spleen, liver and kidney (Suppl. Fig 1B, right). Likewise, deletion of Bcl-x did not alter the frequency of erythroid (Ter119+/CD71+) and lymphoid B- (B220+/CD19+) cells (Suppl. Fig. 1A). Consistent with the existence of a BCR-ABL1-induced and hnRNP A1-mediated posttranscriptional control of Bcl-xL expression37, we found almost identical levels of bcl-x mRNA in WT and dTG LSK cells (Fig. 1B bottom lef) whereas higher Bcl-xL protein (Fig. 1A and 1B bottom right) and hnRNP A1 levels (Fig. 1A bottom right) were detected in MNC and/or LSK cells from dTg animals.
Figure 1. Expression of Bcl-xL is dispensable for BCR-ABL1-driven myeloproliferative disease (MPD) in vivo.
(A) Top left: Western blots show levels and/or activity of Mcl-1, Bcl-xL, Bcl-2, hnRNP K, hnRNP E2, hnRNP A1, total (α-ABL) and active (αPY) BCR-ABL1, and active Akt (pAktSer473) in parental, untreated and imatinib-treated BCR-ABL1-expressing myeloid progenitor 32Dcl3 cells. Top right: Bcl-xL and BCR-ABL1 protein expression in mononuclear cells (MNCs) from spleens of wild type (non-induced: WT) (lane 1) and leukemic SCLtTA-BCR-ABL1 (dTg) (lanes 2 and 3) mice. Bottom left: BAD expression in whole cell lysates (WC), cytoplasmic (C), and mitochondrial (M) subcellular fractions of untreated and imatinib-treated 32D-BCR-ABL1 cells. Bottom right: Flow cytometry analysis of hnRNP A1 expression in lineage-negative/Sca-1+/c-Kit+ (LSK) cells from the bone marrow of WT and 8-week induced dTg mice. (B) Top: PCR shows levels of recombination of Bcl-x floxed alleles in the bone marrow (BM) and spleen of 8, 12, and 20 week-induced SCLtTA-BCR-ABL1- cre Bcl-x fl/fl (dTg/KO) mice. Controls demonstrating complete recombination, absence of recombination, and the absence of a template (NTC) are shown in the last three lanes. Bottom left: bcl-x mRNA levels determined by Real-Time PCR in LSK cells from BM of WT and leukemic dTg and dTg/KO mice is reported as percentages of L19 expression. Results are given as mean ± Standard Error of the Mean (SEM); n = 3. Bottom right: Levels of Bcl-xL protein in MNCs from spleen lysates of WT, leukemic dTg/KO, and dTg mice. (C) Left: Frequency by flow cytometry of lineage-negative/Sca-1+/c-Kit+ (LSK) cells in BM and spleen of dTg and dTg/KO mice. (D) Kaplan-Meier plot shows survival times of dTg and dTg/KO leukemic mice. Asterisks (*) indicate statistical significance.
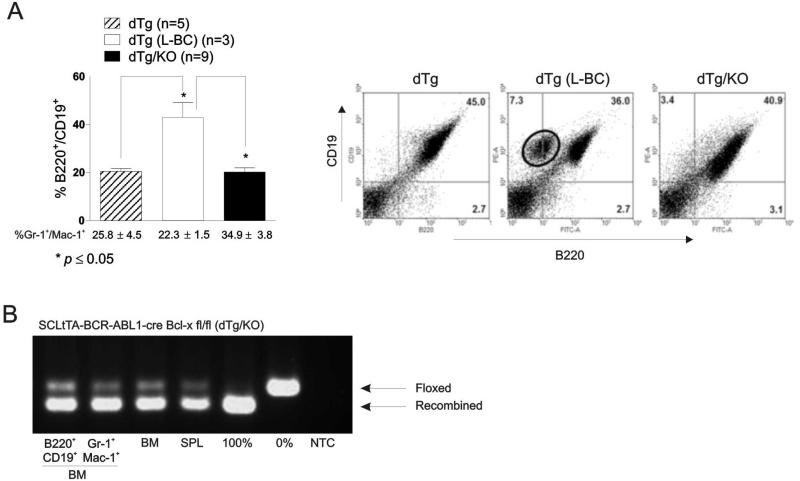
Figure 2. Bcl-x deficient BCR-ABL1 expressing mice do not progress to lymphoid blast crisis.
(A) Left: Flow cytometric analysis shows percentage (mean ± SEM) of B220+/CD19+ and Gr-1+/Mac-1+ cells in the peripheral blood of leukemic dTg, dTg which progressed to lymphoid blast crisis (L-BC), and dTg/KO mice. Right: representative FACS panels of B220/CD19 populations in the spleen of 12 week-induced: dTg (n=5) (left panel), dTg (L-BC; n=3) (middle panel), and dTg/KO (n=12) (far right panel) mice. (B) PCR demonstrates levels of recombination in B220+/CD19+ (lane 1) and Gr-1+/Mac-1+ (lane 2) cells from the marrow, as well as whole bone marrow (BM) (lane 3) and spleen (SPL) (lane 4) of dTg mice 12 weeks after induction. Lane 5 and 6 are controls demonstrating complete and absence of recombination. Results shown are representative of three different mice.
Bcl-xL expression is required for CML disease progression in vivo
To determine whether Bcl-xL plays a role in CML blastic transformation, a cohort of 8-12 week-induced dTg (n=8) and dTg/KO (n=12) animals presenting with marked neutrophilia, as evidenced by the percentage of Gr-1+/Mac-1+ cells almost twice that of non-induced littermates [%Gr-1+/Mac-1+: 24.05±3.0 (dTg); 34.9±3.8 (dTg/KO); and 13.6±1.7 (non-induced control mice; n=3)], were monitored for signs of disease progression36. A significantly increased number of B220+/CD19+ cells in PB (Fig. 2A, left) and the appearance of a B220dim/CD19+ (Fig. 2A, right) population of lymphoblasts in the spleen was observed in 3 out of 8 dTg but not in the dTg/KO mice (n=12) between 8 and 12 weeks post BCR-ABL1 induction, indicating that loss of Bcl-xL impairs the transformation of a CML-CP-like disorder into a L-BC-like acute leukemia36 (p≤0.05). Consequently, dTg mice with the transformed L-BC-like disease but not dTg/KO animals presented B220+/BP-1+ lymphoblasts in PB, lymph nodes, and BM as well (not shown). BM examination of dTg/KO animals demonstrated nearly complete gene recombination in purified populations of both myeloid (Gr-1+/Mac-1+) and lymphoid (B220+/CD19+) cells (Fig. 2B).
Inhibition of Bcl-xL triggers apoptosis of BCR-ABL1+ myeloid progenitors and is potentiated by reactivation of BAD
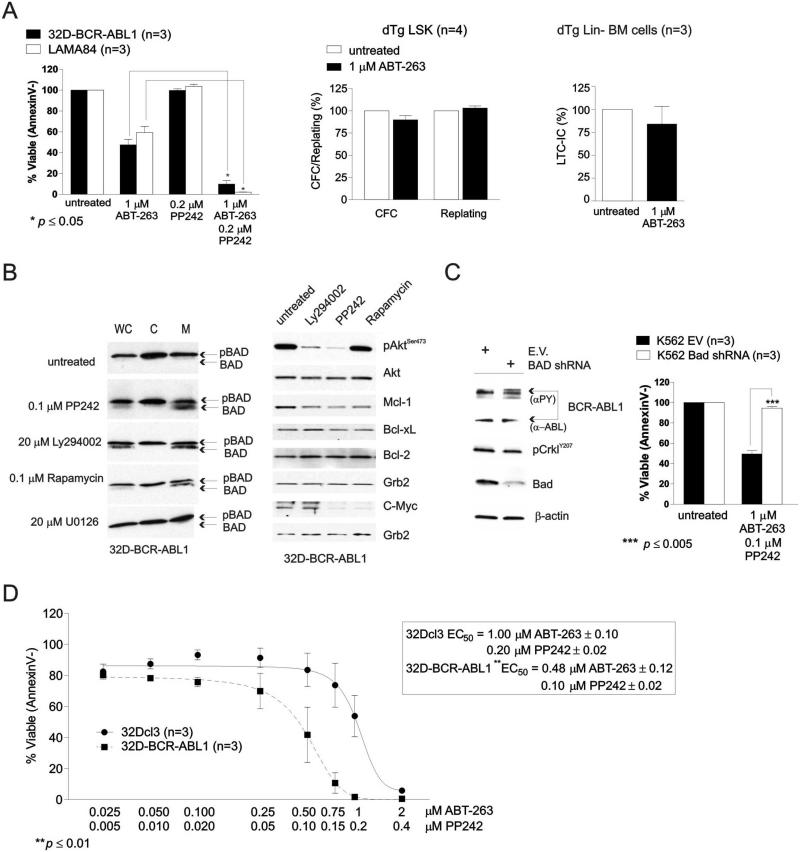
Previous studies report that it is the anti-apoptotic activity of Bcl-xL, but not Bcl-2, which reconstitutes most, albeit not entirely, the leukemogenic potential4, 12, 46 of BCR-ABL1 in CML-BC-progenitors. To assess whether Bcl-xL can be used as a therapeutic target in CML-BC, 32D-BCR-ABL1 and LAMA84, which are models of blast crisis, were used to assess sensitivity of these cells to the Bcl-xL/Bcl-2 antagonist ABT-263. In three independent experiments, flow cytometric analysis of Annexin V- and Sytox Blue-stained cells revealed that treatment with a single dose of ABT-263 (1 μM) induced a ~50% decrease in cell survival compared to vehicle-treated cells (Fig. 3A, left). Furthermore, ABT-263 (1 μM) did not alter the percentage of dTg (n=4) LSK-derived colony forming cells (~10% inhibition) and their replating efficiency (Fig. 3A, middle). Similarly, the LTC-IC frequency of Lin− BM cells from 8 week-induced dTg mice (n=3) remained almost identical in untreated and ABT-263-treated cells (~15% reduction) (Fig. 3A, right), suggesting that loss of Bcl-xL does not influence the self-renewal and survival of BCR-ABL1-transformed hematopoietic stem cells.
Figure 3. Inhibition of Bcl-xL triggers apoptosis of BCR-ABL1+ myeloid progenitors and is potentiated by reactivation of BAD.
(A) Left: Plot shows effect of ABT-263 and PP242 used alone or in combination on survival (expressed as the mean ± SEM of the percentage of AnnexinV− cells; n=3) of untreated and drug-treated 32D-BCR-ABL1 (black bars) and LAMA84 (white bars) cells; middle: Methylcellulose-based clonogenic potential and replating efficiency of untreated and ABT-263-treated LSK cells from spleens of dTg mice; right: effect of ABT-263 treatment on the long-term culture-initiating cell frequency in lineage-negative (Lin−) cells from BM of 8 week-induced dTg mice. (B) Levels of the active (BAD) and inactive (pBAD) BAD (left panels) and of Akt, Mcl-1, Bcl-xL, Bcl-2, and c-Myc in whole cell (WC) and cytoplasmic (C) and mitochondrial (M) subcellular fractions (left panels) and total lysates (right panels) of 32D-BCR-ABL1 cells untreated or treated with the indicated chemical inhibitors of known survival/proliferation pathways. (C) Right: Survival analysis shows that shRNA-mediated BAD downregulation suppresses the potentiating killing activity of PP242 in ABT-263-treated K562 cells. Left: Western blot shows downregulation of BAD by shRNA. Levels of total (α-ABL) and active (αPY) BCR-ABL1, pCrklY207, and in empty vector (EV) and BAD shRNA-expressing K562 cells are shown as controls. (D): Annexin V-staining shows effect of increasing doses of ABT-263/PP242 combination on survival of parental (solid line) and BCR-ABL1-expressing 32Dcl3 cells (dashed line). Inset shows EC50 values for 32Dcl3 and 32D-BCR-ABL1 cells. All data are representative of at least three independent experiments.
Thus, because of the important role played by BAD in BCR-ABL1-driven leukemogenesis26-29 and in the regulation of Bcl-xL activity25, we evaluated whether pharmacologic activation of BAD achieved through interference with the PI3K/Akt/mTORC1/229 or MEK1/MAPK47 signaling enhances ABT-263-induced apoptosis of BCR-ABL1+ cells. 32D-BCR-ABL1 cells were treated for 18 hours with the archetypical PI3-Kinase inhibitor LY294002 (20 μM), mTORC1 inhibitor Rapamycin (0.1 μM), mTORC1/2 inhibitor PP242 (0.1 μM), or the MAP-Kinase inhibitor U0126 (25 μM) and levels of phosphorylated (pBAD) and non-phosphorylated BAD as well as that of other survival signaling molecules (e.g. Akt, Mcl-1, Bcl-xL, Bcl-2 and c-Myc) were determined. Western blot analysis performed on subcellular fractionated (Fig. 3B, left) and total protein lysates (Fig. 3B, right) show that PP242, LY294002 and Rapamycin induced BAD activation as indicated by the readily detectable non-phosphorylated BAD in whole cell (WC) and mitochondrial (M) lysates (Fig. 3B, left). By contrast, inhibition of MEK1 by U0126 did not induce BAD activation (Fig. 3B), consistent with persistence of Akt- and p70 S6 kinase-dependent BAD phosphorylation on serine 13629. As expected, BAD was heavily phosphorylated/inactive in vehicle treated (untreated) (Fig. 3B, left) 32D-BCR-ABL1 cells. Likewise, levels of Mcl-1 and that of c-Myc were significantly reduced by treatment with LY294002, PP242 or Rapamycin, and PP242 or Rapamycin, respectively (Fig. 3B, right), while expression of Bcl-xL and Bcl-2 were not influenced by suppression of PI-3K/Akt/mTORC1/2-mediated signals (Fig. 3B, right).
Activation of BAD in PP42-treated 32D-BCR-ABL1 and LAMA84 cells did not alter survival (Fig 3A); however, 90-95% were apoptotic (Annexin V+) after exposure of both BCR-ABL1+ lines to single treatment with a combination of 1 μM ABT-263 and 0.2 μM PP242 (n=3) (Fig. 3A, left). Although previous work reported a modest decrease (MTT-based assay) in proliferation/survival in PP242-treated BCR-ABL1+ cell lines35, PP242 failed to induce apoptosis of both LAMA84 and 32D-BCR-ABL cells when used at lower concentrations (0.2 μM) (Fig. 3A, top), most likely due to high Bcl-xL levels. The potentiating effect of this TORC1/2 inhibitor on the pro-apoptotic activity of ABT-263 in cell line models of blast crisis may depend on its ability to activate BAD which in turn, antagonizes the anti-apoptotic function of Bcl-xL25. We tested this hypothesis by genetic manipulation of BAD expression with shRNA which showed that ~50% BAD knock-down in K562 cells (Fig. 3C, left) is sufficient to prevent PP42 from augmenting the pro-apoptotic effects of ABT-263 (Fig. 3C, right). Furthermore, Annexin V-based apoptosis assays revealed that 32D-BCR-ABL1 cells are two times more sensitive than 32Dcl3 cells to combined pharmacologic inhibition of Bcl-xL with ABT-263 (0.025-2 μM) and activation of BAD by PP242 (0.005-0.4 μM) with EC50 values of 0.48 μM ABT-263/0.1μM PP242 for 32D-BCR-ABL1, and 1.0 μM ABT-263/0.2 μM PP242 for 32Dcl3 cells (Fig. 3D).
The combination of ABT-263 with PP242 triggers apoptosis of CML-BC but not CML-CP or normal CD34+ progenitor cells, and overcomes microenvironment-induced TKI resistance
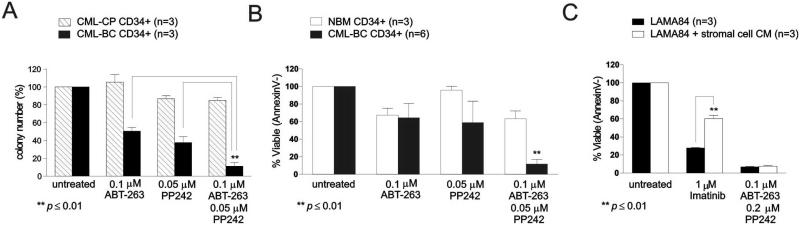
Methylcellulose-based clonogenic assays revealed that the combination of ABT-263 (0.1 μM) and PP242 (0.05 μM), used at one-tenth and one-fourth of the concentrations given to cell lines, dramatically decreases size (not shown) and number (~90% inhibition) of cytokine-driven myeloid colony forming cells from CD34+ BM CML-BC, but not CML-CP (~15% reduction) progenitors (n=3) (Fig. 4A). Marked (~85-95%) apoptosis (Annexin V+) was induced by the same drug combination in CD34+ progenitors isolated from BM of CML-BC (n=6) (Fig. 4B, black bars) but not healthy (n=3) donors in which a 6-day exposure to both drugs resulted in a 40% reduction in viability (Fig. 4B, white bars). A significant but modest (~50% reduction) impairment of CD34+ CML-BC (n=3) colony formation was observed when these drugs were used separately (Fig. 4A). This correlated with a 30% decrease in viability of ABT-263- but not PP242-treated CD34+ BM cells from CML-BC (n=6) and healthy (n=3) individuals (Fig. 4B).
Figure 4. The combination ABT-263 and PP242 triggers apoptosis of leukemic but not normal CD34+ progenitor cells, and overcomes microenvironment-induced TKI resistance.
(A) Clonogenic and/or (B) survival analyses (mean ± SEM, p ≤ 0.01) of untreated (DMSO) and drug (ABT-263 and/or PP242)-treated CD34+ BM progenitors from CML-CP, CML-BC patients and/or healthy individuals (NBM: normal bone marrow). (C) Survival analysis (mean ± SEM; n=3) shows effect of extrinsic drug-resistance signals provided by hTERT+ stromal cell-conditioned medium (CM) on the pro-apoptotic activity of Imatinib and ABT263/PP242 in Ph+ LAMA84 cells.
Previous studies show that the BM stroma protects CML-BC (cell lines9 and primary cells10) from TKI-induced cell death. To determine whether PP242 and ABT-263 treatment overcomes microeviromentally-induced drug resistance, LAMA84 cells were cultured for 42 hrs. in conditioned medium from the TERT+ human mesenchymal stem cell line, while exposed for 24 hr. to either 1μM imatinib or the combination 0.1 μM ABT-236 and 0.2 μM PP242. Flow cytometric analysis of Annexin V/Sytox Blue-stained cells showed near complete cell death in LAMA84 cells treated for 24 hr. with ABT-263 and PP242 regardless of the presence of BM stroma CM (Fig. 4C). As expected, TKI (e.g. imatinib)-induced apoptosis was strongly inhibited by culturing LAMA84 cells in hTERT+ stromal cell CM, suggesting that suppression of Bcl-xL/Bcl-2 and TORC1/2 pathways efficiently overcomes extrinsic BM stromal signals conferring resistance of Ph+ leukemic progenitors to TKI-induced apoptosis.
Impaired Bcl-xL expression by hnRNP A1 shRNAs mimics the pro-apoptotic effects of ABT-263 and potentiates efficacy of PP242
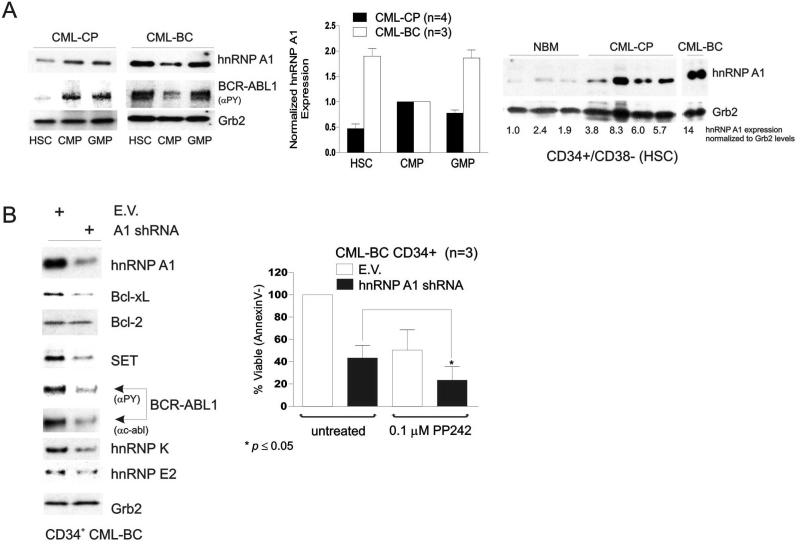
HnRNP A1 expression was found to gradually increase, in a BCR-ABL kinase-dependent manner, in paired CML-CP and –BC BM MNC samples37. We found that levels of hnRNP A1 are specifically elevated in the CML-BC cell populations which, as reported, show innate or acquired TKI-resistance48, have the ability to self-renew49 and, most likely, represent the origin of the progressing cell clone4. In fact, hnRNP A1 expression was increased in CD34+/CD38− (HSC) and GMP cell fractions of CML-BC (n=3) when compared to the CML-BC CMP (Fig. 5A, left and middle) and to HSC and GMP cells factions from BM of healthy (n=3) and CML-CP (n=4) individuals (Fig. 5A). Expression of hnRNP A1 was ~3 times higher in chronic phase (n=4) and almost 10 times higher in a blast crisis patient (Fig. 5A, right).
Figure 5. Impaired Bcl-xL expression by hnRNP A1 shRNAs mimics the pro-apoptotic effects of ABT-263 and potentiates those of PP242.
(A) Western blots show expression levels of hnRNP A1 and BCR-ABL1 kinase activity (αPY) in CD34+/CD38- stem cell (HSC)-enriched, common myeloid (CMP) and granulocyte-macrophage (GMP) progenitors from BM of NBM (n=3), CML-CP (ntot=7), and CML-BC (ntot=4). Grb2 was detected as an internal control for equal loading. Plot (middle) shows normalized (mean ± SEM) hnRNP A1 levels in the indicated BM subpopulations from CML-CP (n=4) and –BC (n=3) patient samples. (B) Right: Survival analysis (mean ± SEM) in untreated (DMSO) and PP242-treated empty vector (EV) and hnRNP A1 shRNA lentivirally-transduced CD34+ CML-BC progenitors (n=3) shows that hnRNP A1-induced downregulation of Bcl-xL but not Bcl-2 mimics the effects of ABT-263. Left: Western blots on lysates of empty vector (EV) and hnRNP A1 shRNA1 lentivirally-transduced CD34+ CML-BC BM cells (n=3) shows the effect of hnRNP A1 shRNA expression on levels of hnRNP A1, BCR-ABL1 and BCR-ABL1 downstream effectors (Bcl-xL, Bcl-2, SET, hnRNP K and hnRNP E2).
Since hnRNP A1 is a positive post-transcriptional modulator of Bcl-xL expression37, we modulated levels of hnRNP A1 with shRNA to better understand the molecular mechanisms by which the Bcl-xL/Bcl-2 antagonist ABT-263 exerts its pro-apoptotic activity in BCR-ABL+ CML-BC progenitors. Knock-down of hnRNP A1 in primary CD34+ CML-BC BM cells (n=3) resulted in downregulation of Bcl-xL but not Bcl-2 (Fig. 5B, left), and mimicked the effect of ABT-263 when hnRNP A1 shRNA-expressing CD34+ CML-BC BM cells (n=3) were exposed to 0.1 μM PP242 (Fig. 5B, right). Annexin V staining revealed shRNA-mediated decreased levels of hnRNP A1 impaired survival of CD34+ CML-BC progenitors by ~60% 6 days after GFP-selection compared to vector-transduced progenitors (Fig. 5B). Viability of shRNA infected cells was further reduced (p≤0.05) upon addition of 0.1 μM PP242 (~20% survival), suggesting that expression of Bcl-xL rather than Bcl-2 is important for survival of CML-BC progenitors, and that ABT-263 exerts its proapoptotic activity in CML-BC cells through inhibition of Bcl-xL. As expected, shRNA-mediated suppression of hnRNP A1 expression (~65% inhibition) resulted in downregulation of the hnRNP A1-target SET, thereby leading to PP2A reactivation4, 50, and, consequently, downregulation of BCR-ABL1 and its downstream effectors (e.g. hnRNP E2 and K)43, 44 in CD34+ CML-BC progenitors (Fig. 5B).
DISCUSSION
The dismal outcome of patients with CML-BC treated with either TKIs or other experimental drugs reflects our lack of a clear understanding of which BCR-ABL kinase-dependent and/or –independent pathways are significantly contributing to disease progression2, 4. Among these, several regulators of apoptosis (e.g. Bcl-xL) have been proposed to be important for survival of CML-BC progenitors51; however, whether their contribution is critical for disease progression in vivo is still unclear. By using a mouse model of CML blastic transformation36, we showed that the anti-apoptotic factor Bcl-xL is dispensable for development and maintenance of a CML-CP-like disease in mice but required for transformation into an L-BC-like disorder (Fig. 1, 2 and S1). Development of leukemia in the absence of bcl-x expression in vivo was unexpected because of both the dependence of Bcl-xL expression on BCR-ABL1 kinase activity, and the numerous in vitro studies suggesting a role for Bcl-xL in BCR-ABL1 kinase-dependent and -independent survival of CML-BC cells and their resistance to pro-apoptotic stimuli9, 12, 13. We also showed that genetic and pharmacologic (ABT-263) loss of Bcl-xL expression and/or activity did not alter BCR-ABL1+ stem cell (LSK) number, survival and self-renewal activities while preventing in vivo expansion of more committed progenitors which, like the CML-BC GMPs4, 49, represent a secondary CML cell population demonstrating increased BCR-ABL1 expression, survival/proliferation advantage, increased genomic instability and, likely, self-renewal. However, while the L-BC-like disease maintains BCR-ABL1 kinase-dependence in dTg mice, relapse and BCR-ABL kinase-independence are two phenomena commonly observed in TKI-treated CML-BC patients36, 38. Furthermore, despite the proposed role for Bcl-2 in disease progression46, 52, expression studies done in CML patients indicate that disease progression does not directly correlate with Bcl-2 levels53, suggesting that Bcl-xL, and possibly its negative regulator BAD, may play an important role in both CML-BC development and BCR-ABL1-independent TKI resistance, which is likely induced by microenvironment-generated signals rather than depending on the presence of leukemic cell clone(s) harboring BCR-ABL1 mutations9, 10.
In support of a significant biological role played by both Bcl-xL and BAD in CML-BC and not CML-CP, we showed that low concentrations of the orally-available Bcl-2/Bcl-xL inhibitor ABT-263 (100 nM) exerts a strong and selective cytotoxicity towards CD34+ CML-BC but not CP or normal progenitors (Fig. 3 and 4) when used in combination with suboptimal concentrations of drugs (e.g. 50 nM PP242) which lead to BAD activation (Fig. 3). Indeed, treatment of both BCR-ABL1+ cell lines and CD34+ CML-BC progenitors with combined low doses of ABT-263 and PP242 reduced viability by ~90% without having any significant effect on CD34+ hematopoietic cells from healthy individuals. The anti-leukemic effect of a combined Bcl-xL/Bcl-2 antagonist (i.e., ABT-737 or ABT-263) and PP242 treatment has been previously investigated in cell line models of Burkitt’s lymphoma (0.5 μM ABT-737/1.25 μM PP242) and acute T-cell leukemia (T-ALL) (0.01-1 μM ABT-263/ 0.01-1 μM PP242)54, 55. However, while the ABT-263/PP242 combination strongly resulted in apoptosis of primary CML-BC cells and cell lines, these drugs had only a modest killing (30% induction of apoptosis) in Burkitt’s lymphoma and a very limited synergistic effect in T-ALL cell lines54, 55 , suggesting that the Bcl-xL/BAD interplay specifically plays a crucial role in survival of CML-BC but not all leukemic progenitors. Note that alone, neither ABT-263 nor PP242 had a significant effect on survival of CML-BC progenitors when used at 0.1 μM and 0.050 μM concentrations, respectively (Fig. 4), although it has been shown that higher doses of PP242 decreased clonogenic potential of CML-BC cells35, most likely through its inhibitory effect on mTORC1/2-Akt1-regulated Mcl-1 expression (Fig. 3). Consistent with our data obtained with 100 nM ABT-263 in both leukemic and normal CD34+ progenitors, it has been reported23 that suppression of Bcl-xL/Bcl-2 activities by 100 nM ABT-737 accounts only for 20-30% of apoptosis. Furthermore, low or no sensitivity to the ABT-737/ABT-263 compounds, even if used at concentrations as high as 10 μM, has been reported for Ph+ cell lines and primary CML stem/progenitor cells23, 25, 56. The limitation of this drug as a single therapeutic agent in CML-BC is supported by evidence indicating resistance to its pro-apoptotic activity is induced in malignancies (e.g., CML-BC9, 12, 13) where Bcl-xL and/or Mcl-1 are overexpressed23, 57.
Given that microenvironment-induced TKI resistance has also been in part associated with the ability of extracellular BM soluble factors to increase Mcl-1, Bcl-xL, survivin, and mTORC1/2 levels in leukemic progenitors9, 58, and that downregulation of Mcl-1 restores sensitivity of leukemic cells to ABT-73759, 60, it is likely that a combined ABT-263/PP242 would be more effective than the single agent approaches. Indeed, we not only provided evidence indicating that PP242 is capable of reducing Mcl-1 levels but we also showed that ABT-263/PP242 treatment efficiently (90% induction) promoted apoptosis of CML-BC cells even in the presence of external factors (hTERT+ stromal cell CM) capable of inducing TKI resistance (Fig. 3 and 4). Mechanistically, shRNA-mediated suppression of BAD or hnRNP A1 that, in turn, leads to Bcl-xL but not Bcl-2 downregulation, allowed us to determine that inhibition of Bcl-xL and restoration of BAD activity mostly accounts for the apoptosis induced in CD34+ CML-BC progenitors by the Bcl-xL/Bcl2 antagonist ABT-263 and mTORC1/2 inhibitor PP242, respectively (Fig. 5). However, it is likely that PP242-induced inhibition of the mTORC1/2- and Akt-mediated survival signals also plays a key role in the apoptotic response of leukemic progenitors to the ABT-263/PP242 combination (Fig. 6).. Additionally, the strong apoptotic effect of the ABT-263/PP242 combination might also depend on interference with other BCR-ABL1 kinase-dependent and –independent survival signals. In fact, co-treatment of ABT-737 with imatinib induced not only a ~50% and ~25% apoptosis in CML-BC23, 56 and normal progenitors23, respectively, but also restored TKI sensitivity of CD34+/CFSEMAX CML-BC and CD34+/CD38− CML-CP stem cell-enriched populations23, 56, suggesting that BCR-ABL1-dependent and -independent survival pathways are simultaneously affected.
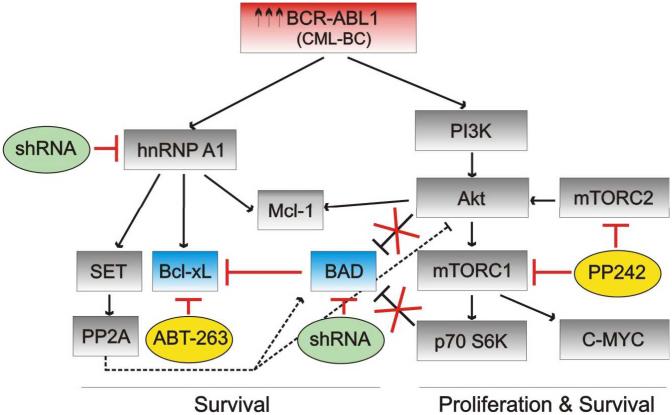
Figure 6. Effect of altered Bcl-xL and BAD expression and activity on BCR-ABL1 signaling in CML-BC.
Schematic representation of the survival/proliferation pathways induced by high levels of BCR/ABL-kinase activity and likely affected by altered Bcl-xL and BAD expression/activity (blue) upon exposure of CML-BC cells to ABT-263 or PP242 (yellow), or after shRNA-mediated downregulation (green) of BAD or hnRNP A1 in CML-BC. Dashed lines indicate pathways normally inactive in CML-BC. Red lines indicate pathways influenced by drug-treatment or shRNA expression.
In conclusion, although we cannot determine whether the combination of ABT-263 with PP242 would be more effective than TKIs in CML-BC therapy, our in vitro data strongly suggest that pharmacologic inhibition of Bcl-xL together with activation of its negative regulator BAD has a greater and more profound deleterious effect (90% induction of apoptosis in the presence of external cytokines) on survival of CML-BC progenitors regardless of the drug-resistance induced in these cells by BM-generated signals9, 10 .
Finally, it remains unknown whether TKIs with ABT-737/ABT-263 treatment would be sufficient to induce a sustained molecular remission, or the ~50% surviving drug-resistant CML-BC progenitors would account for relapse. Of clinical interest, our data support the application of an alternative therapeutic protocol in which patients would be initially de-bulked with TKIs to reduce leukemic blast burden and, thereafter, treated with ABT-263 and PP242 to eliminate residual TKI-resistant CML-BC clones.
Supplementary Material
ACKNOWLEDGEMENTS
D.P. is a Scholar of The Leukemia and Lymphoma Society. This work was supported in part by NIH grants CA095512 and CA163800 (D.P.), CA16058 (OSU-CCC); Fonds de Recherche Quebec Sante– TheCell (D.C.R.); Lauri Strauss Leukemia and BloodCenter Research Foundations (C.S.H.); the Danish Medical Research Council, the Danish Cancer Society and the Karen Elise Jensen Foundation (P.H) grants. J.G.H. was supported in part by NIH training grant HL-07209. We thank L. Hennighausen (NIH, Bethesda, MD) for providing Bcl-x f/f mice; H. Albertz and C. Reinbold (FACS Core Facility, Blood Research Institute, Milwaukee, WI) for technical assistance; J. Perrin (OSU Medical Center, Columbus OH) for helping in procuring CML specimens, and S. Lee (OSU Medical Center, Columbus OH) for editorial assistance.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests related to this work.
REFERENCES
- 1.Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120(7):1390–7. doi: 10.1182/blood-2012-03-378919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hehlmann R. How I treat CML blast crisis. Blood. 2012;120(4):737–47. doi: 10.1182/blood-2012-03-380147. [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia (vol 23, pg 1054, 2009) Leukemia. 2010;24(5):1102–1102. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 4.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. Journal of Clinical Investigation. 2010;120(7):2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619–30. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milojkovic D, Apperley JF, Gerrard G, Ibrahim AR, Szydlo R, Bua M, et al. Responses to second-line tyrosine kinase inhibitors are durable: an intention-to-treat analysis in chronic myeloid leukemia patients. Blood. 2012;119(8):1838–43. doi: 10.1182/blood-2011-10-383000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karvela M, Helgason GV, Holyoake TL. Mechanisms and novel approaches in overriding tyrosine kinase inhibitor resistance in chronic myeloid leukemia. Expert Review of Anticancer Therapy. 2012;12(3):381–392. doi: 10.1586/era.12.10. [DOI] [PubMed] [Google Scholar]
- 8.Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leukemia & Lymphoma. 2008;49(4):615–619. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- 9.Bewry NN, Nair RR, Emmons MF, Boulware D, Pinilla-Ibarz J, Hazlehurst LA. Stat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistance. Molecular Cancer Therapeutics. 2008;7(10):3169–3175. doi: 10.1158/1535-7163.MCT-08-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O'Hare T, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2012;26(5):1140–1143. doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez-Garcia I, Grutz G. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5287–5291. doi: 10.1073/pnas.92.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DEH, Witte ON, Green DR. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-x(L) Oncogene. 1998;16(11):1383–1390. doi: 10.1038/sj.onc.1201664. [DOI] [PubMed] [Google Scholar]
- 13.Horita M, Andreu EJ, Benito A, Arbona C, Sanz C, Benet I, et al. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-(XL) Journal of Experimental Medicine. 2000;191(6):977–984. doi: 10.1084/jem.191.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105(8):3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 15.Soliera AR, Mariani SA, Audia A, Lidonnici MR, Addya S, Ferrari-Amorotti G, et al. Gfi-1 inhibits proliferation and colony formation of p210BCR/ABL-expressing cells via transcriptional repression of STAT 5 and Mcl-1. Leukemia. 2012;26(7):1555–63. doi: 10.1038/leu.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan EK, Holyoake TL, Craig AR, Jorgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25(6):985–994. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Hu Y, Michaels S, Segal D, Brown D, Li S. Inhibitory effects of omacetaxine on leukemic stem cells and BCR-ABL-induced chronic myeloid leukemia and acute lymphoblastic leukemia in mice. Leukemia. 2009;23(8):1446–54. doi: 10.1038/leu.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Herrero I, Vicente-Duenas C, Orfao A, Flores T, Jimenez R, Cobaleda C, et al. Bcl2 is not required for the development and maintenance of leukemia stem cells in mice. Carcinogenesis. 2010;31(7):1292–1297. doi: 10.1093/carcin/bgq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307(5712):1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda J, Kimura S, Andreeff M, Ashihara E, Kamitsuji Y, Yokota A, et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. British Journal of Haematology. 2008;140(2):181–190. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- 21.High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O'Brien R, Khaw SL, et al. The Bcl-2 Homology Domain 3 Mimetic ABT-737 Targets the Apoptotic Machinery in Acute Lymphoblastic Leukemia Resulting in Synergistic in Vitro and in Vivo Interactions with Established Drugs. Molecular Pharmacology. 2010;77(3):483–494. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
- 22.Harb JG, Chyla BI, Huettner CS. Loss of Bcl-x in Ph+ B-ALL increases cellular proliferation and does not inhibit leukemogenesis. Blood. 2008;111(7):3760–3769. doi: 10.1182/blood-2007-08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak DH, Wang RY, Schober WD, Konopleva M, Cortes J, Kantarjian H, et al. Activation of apoptosis signaling eliminates CD34(+) progenitor cells in blast crisis CML independent of response to tyrosine kinase inhibitors. Leukemia. 2012;26(4):788–794. doi: 10.1038/leu.2011.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milojkovic D, Apperley J. Mechanisms of Resistance to Imatinib and Second-Generation Tyrosine Inhibitors in Chronic Myeloid Leukemia. Clinical Cancer Research. 2009;15(24):7519–7527. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- 25.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Research. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DCS, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl(+) leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(40):14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salomoni P, Condorelli F, Sweeney SM, Calabretta B. Versatility of BCR/ABL-expressing leukemic cells in circumventing proapoptotic BAD effects. Blood. 2000;96(2):676–684. [PubMed] [Google Scholar]
- 28.Neshat MS, Raitano AB, Wang HG, Reed JC, Sawyers CL. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: Roles for phosphatidylinositol 3-kinase and Raf. Molecular and Cellular Biology. 2000;20(4):1179–1186. doi: 10.1128/mcb.20.4.1179-1186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27:S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 30.Ly C, Arechiga AF, Melo JV, Walsh CM, Ong ST. Bcr-Abl kinase modulates the translation regulators ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia cells via the mammalian target of rapamycin. Cancer Research. 2003;63(18):5716–5722. [PubMed] [Google Scholar]
- 31.Skorski T, Bellacosa A, NieborowskaSkorska M, Majewski M, Martinez R, Choi JK, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. Embo Journal. 1997;16(20):6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhagwat SV, Crew AP. Novel inhibitors of mTORC1 and mTORC2. Current Opinion in Investigational Drugs. 2010;11(6):638–645. [PubMed] [Google Scholar]
- 33.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. Plos Biology. 2009;7(2):371–383. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2-and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nature Medicine. 2010;16(2):205–U115. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koschmieder S, Gottgens B, Zhang P, Iwasaki-Arai J, Akashi K, Kutok JL, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105(1):324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- 37.Iervolino A, Santilli G, Trotta R, Guerzoni C, Cesi V, Bergamaschi A, et al. hnRNP A1 nucleocytoplasmic shuttling activity is required for normal myelopoiesis and BCR/ABL leukemogenesis. Molecular and Cellular Biology. 2002;22(7):2255–2266. doi: 10.1128/MCB.22.7.2255-2266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huettner CS, Zhang P, Van Etten RA, Tenen DG. Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nature Genetics. 2000;24(1):57–60. doi: 10.1038/71691. [DOI] [PubMed] [Google Scholar]
- 39.Radomska HS, Gonzalez DA, Okuno Y, Iwasaki H, Nagy A, Akashi K, et al. Transgenic targeting with regulatory elements of the human CD34 gene. Blood. 2002;100(13):4410–4419. doi: 10.1182/blood-2002-02-0355. [DOI] [PubMed] [Google Scholar]
- 40.Wagner KU, Claudio E, Rucker EB, Riedlinger G, Broussard C, Schwartzberg PL, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127(22):4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 41.Mihara K, Imai C, Coustan-Smith E, Dome JS, Dominici M, Vanin E, et al. Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. British Journal of Haematology. 2003;120(5):846–849. doi: 10.1046/j.1365-2141.2003.04217.x. [DOI] [PubMed] [Google Scholar]
- 42.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, et al. Modulation of prostate cancer genetic risk by ornega-3 and ornega-6 fatty acids. Journal of Clinical Investigation. 2007;117(7):1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrotti D, Cesi V, Trotta R, Guerzoni C, Santilli G, Campbell K, et al. BCR-ABL suppresses C/EBP alpha expression through inhibitory action of hnRNP E2. Nature Genetics. 2002;30(1):48–58. doi: 10.1038/ng791. [DOI] [PubMed] [Google Scholar]
- 44.Notari M, Neviani P, Santhanam R, Blaser BW, Chang JS, Galietta A, et al. A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential by regulating MYC mRNA translation. Blood. 2006;107(6):2507–2516. doi: 10.1182/blood-2005-09-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiang CW, Harris G, Ellig C, Masters SC, Subramanian R, Shenolikar S, et al. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin-3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97(5):1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- 46.Cirinna M, Trotta R, Salomoni P, Kossev P, Wasik M, Perrotti D, et al. Bcl-2 expression restores the leukemogenic potential of a BCR/ABL mutant defective in transformation. Blood. 2000;96(12):3915–21. [PubMed] [Google Scholar]
- 47.Fang XJ, Yu SX, Eder A, Mao ML, Bast RC, Boyd D, et al. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene. 1999;18(48):6635–6640. doi: 10.1038/sj.onc.1203076. [DOI] [PubMed] [Google Scholar]
- 48.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 49.Jamieson CHM, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. New England Journal of Medicine. 2004;351(7):657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 50.Eiring AM, Neviani P, Santhanam R, Oaks JJ, Chang JS, Notari M, et al. Identification of novel posttranscriptional targets of the BCR/ABL oncoprotein by ribonomics: requirement of E2F3 for BCR/ABL leukemogenesis. Blood. 2008;111(2):816–828. doi: 10.1182/blood-2007-05-090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103(11):4010–22. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 52.Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(17):10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravandi FM, Kantarjian HM, Talpaz M, O'Brien S, Faderl S, Giles FJ, et al. Expression of apoptosis proteins in chronic myelogenous leukemia. Cancer. 2001;91(11):9. doi: 10.1002/1097-0142(20010601)91:11<1964::aid-cncr1221>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 54.Evangelisti C, Ricci F, Tazzari P, Tabellini G, Battistelli M, Falcieri E, et al. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia. 2011;25(5):781–791. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 55.Spender LC, Inman GJ. Phosphoinositide 3-kinase/AKT/mTORC1/2 Signaling Determines Sensitivity of Burkitt's Lymphoma Cells to BH3 mimetics. Molecular Cancer Research. 2012;10(3):347–359. doi: 10.1158/1541-7786.MCR-11-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Airiau K, Mahon F-X, Josselin M, Jeanneteau M, Turcq B, Belloc F. ABT-737 increases tyrosine kinase inhibitor-induced apoptosis in chronic myeloid leukemia cells through XIAP downregulation and sensitizes CD34(+) CD38(-) population to imatinib. Experimental Hematology. 2012;40(5):367–378. doi: 10.1016/j.exphem.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119(24):5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng Z, Shi YX, Tsao T, Qiu Y, Kornblau SM, Baggerly KA, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120(13):2679–89. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvalo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 60.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.