Abstract
Purpose
An association between birth order and reduced unaided vision (a surrogate for myopia) has been observed previously. We examined the association between birth order and myopia directly in 4 subject groups.
Methods
Subject groups were participants in 1) the Avon Longitudinal Study of Parents and Children (ALSPAC; UK; age 15 years; N=4,401), 2) the Singapore Cohort Study of Risk Factors for Myopia (SCORM; Singapore; age 13 years; N=1,959), 3) the Raine Eye Health Study (REHS; Australia; age 20 years; N=1,344), and 4) Israeli Defense Force recruitment candidates (IDFC; Israel; age 16-22 years; N=888,277). Main outcome: Odds ratio (OR) for myopia in first born versus non-first born individuals after adjusting for potential risk factors.
Results
The prevalence of myopia was numerically higher in first-born versus non-first-born individuals in all study groups, but the strength of evidence varied widely. The adjusted ORs (95% CI) were: ALSPAC, 1.31 (1.05-1.64); SCORM, 1.25 (0.89-1.77); REHS, 1.18 (0.90-1.55); IDFC, 1.04 (1.03-1.06). In the large IDFC sample, the effect size was greater (a) for the first born versus fourth or higher born comparison than for the first born versus second/third born comparison (P<0.001) and (b) with increasing myopia severity (P<0.001).
Conclusions
Across all studies, the increased risk of myopia in first born individuals was low (OR <1.3). Indeed, only the studies with >4000 participants provided strong statistical support for the association. The available evidence suggested the relationship was independent of established risk factors such as time outdoors/reading, and thus may arise through a different causal mechanism.
Keywords: Refractive error, myopia, birth order, ALSPAC, SCORM, RAINE
Introduction
Myopia occurs when the length of the unaccommodated eye is too long relative to its optical power. This leads to symptoms of blurred distance vision, requiring the use of spectacles, contact lenses or refractive surgery. Furthermore, the axial elongation typically seen in myopia puts the eye at an increased risk of sight-threatening conditions such as retinal detachment, glaucoma, cataract, and chorio-retinal degeneration1. Both genetic and environmental factors are implicated in causing the high and increasing prevalence of myopia observed in many populations2-5. Because myopia often develops during school-age, environmental risk factors present during this period have been studied intensively. A causal link between myopia and the amount of time children spend doing nearwork has been hypothesised for decades, with some support6. However, careful longitudinal studies have suggested the influence of nearwork is limited7. More recently, the time children spend outdoors has been shown to be negatively associated with myopia3, 8-12. Also, the discovery of associations between antenatal and early postnatal factors such as maternal age, parental smoking, birth weight, breastfeeding, and birth order has renewed interest in this period of early life and its role in refractive development13-17.
A number of studies have assessed the relationship between birth order and myopia. However, all of these prior studies suffered from one of following limitations: employing an indirect method of detecting myopia (namely, unaided distance vision of 6/12 or worse)14, 18, 19, categorising myopes and hyperopes together20, or investigating a highly selected group of subjects (closely inbred pedigrees)21. Here we report an investigation of the relationship between birth order and myopia in 4 groups that are largely representative of the general population in 4 different countries.
Methods
Subjects
ALSPAC (Avon Longitudinal Study of Parents and Children)
ALSPAC recruited 14,541 pregnant women resident in Avon, UK with expected dates of delivery 1st April 1991 to 31st December 1992. Of the initial 14,541 pregnancies, 13,988 children were alive at 1 year. The original cohort was largely representative of the UK 1991 Census; however, families of low socioeconomic status, families in which the mother was teenaged at the birth of her child, and families of non-White ethnic origin were underrepresented22. For the present study, subjects from multiple births (e.g. twins) or of non-Caucasian ethnicity (~2% of the total) were omitted from the analysis. During pregnancy, mothers were asked for details of all previous pregnancies resulting in either a live birth or stillbirth, from which the ALSPAC child’s birth order was derived23.
SCORM (Singapore Cohort study Of Risk factors for Myopia)
Children aged 7–9 years at baseline attending 3 schools in Singapore were invited to participate. Individuals with syndromic myopia, congenital cataract, serious systemic diseases or who refused instillation of eyedrops were excluded. Of the 2819 eligible children, 1979 (70.2%) participated at baseline. Follow-up data were collected at yearly visits using the same clinical procedures as at baseline. For each SCORM participant, information on the number of siblings, and their ages, was obtained from the baseline questionnaire. Birth order was calculated from the sibling information.
REHS (Raine Eye Health Study)
The Western Australian Pregnancy Cohort (Raine) Study recruited 2868 individuals from Perth, Australia between 1989 and 1991. The original cohort was a population-based sample of Western Australians. Mothers were recruited into the study during the 18th week of pregnancy. Prenatal and birth data including birth order information were collected prospectively from participants’ mothers. Subjects from multiple births were omitted from the analysis.
IDFC (Israeli Pre-recruitment Candidates)
Military service is compulsory in Israel except for specific minority groups. The IDFC sample comprised N=888,277 subjects aged 16–22 years examined in pre-recruitment offices and who had refractive evaluation. All data were obtained from the database of the Israel Defense Forces Induction Center, without any details of personal identity. “Region of family origin” was categorized as Israeli, Western (European, American, or Oceanic), or Eastern (Asian or African) according to the father’s country of birth or, for subjects whose father was born in Israel, by the grandfather’s country of birth. The analysis was restricted to non-orthodox recruits, since refractive development has been reported to differ markedly in this group24. Birth order was reported by the conscript as part of the pre-recruitment evaluation.
Ethical approval
Each study adhered to the tenets of the Declaration of Helsinki for research involving human subjects and received ethical approval from its local ethics committee: ALSPAC, the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees; SCORM, the Singapore Eye Research Institute Ethics Committee; REHS, University of Western Australia Ethics Committee; IDFC, The IDF Institutional Review Board.
Refractive errors and potential risk factors
ALSPAC
Noncycloplegic autorefraction measurements (Canon R50 instrument) were obtained during a visit to a research clinic when the children were approximately 15 years old, as described25. The spherical equivalent (SE) for each eye was calculated as the sphere power plus half the cylinder power, and then averaged for the two eyes of each subject (SEAV). Subjects were classed as “likely myopes” if they had an SEAV ≤−1.00 D and as “likely non-myopes” otherwise. A validation study suggested the −1.00 D cut-point provided 96% specificity and 89% sensitivity (area under ROC curve 92%) in diagnosing myopia corresponding to a subjective refraction of ≤−0.50 D. Subjects were classified, additionally, as “likely emmetropes/hyperopes” if they had an SEAV ≥−0.25 D, using the threshold previously adopted by Jones-Jordan et al.26 Socio-economic status, intrauterine growth retardation (small for gestational age), maternal tobacco smoking during the first 3 months of pregnancy, maternal age, time spent outdoors, time spent reading for pleasure, and parental myopia were assessed as described previously23, 25, 27, 28. For first-born subjects, “only child” status was inferred if the mother reported that the study child had no siblings (related or foster children) living at home, in a questionnaire completed when the study child was aged 11 years.
SCORM (Singapore Cohort study Of Risk factor for Myopia)
Details of the assessment of refractive error have been reported previously29, 30. Briefly, refractive error was measured by cycloplegic autorefraction using a Canon RK-F1 autorefractor, and subjects were classified as myopic if the spherical equivalent refractive error in the right eye was ≤−0.50 D.
REHS (Raine Eye Health Study)
At age 20 years, 1344 participants underwent a comprehensive eye examination that included cycloplegic autorefraction (Nidek 510A instrument). Complete ocular and general phenotypic data were available on 1266 (94.2%) participants. Subjects were diagnosed as myopic if the spherical equivalent in their right eye was ≤−0.50 D. At the examination, participants completed a questionnaire containing the question, “In summer, when not at work, how much time do you spend in the sun?” The questionnaire also asked how many parents were myopic. Other phenotypic information was available from the previous cohort follow-ups.
IDFC (Israeli Pre-recruitment Candidates)
Details of the assessment of refractive error have been reported previously31, 32. Briefly, subjects with unaided vision of 6/6 on a Snellen chart with not more than one mistake were assumed to be non-myopic. For subjects with a corrected visual acuity of 6/6 (with not more than one mistake) the prescription of their optical correction was taken as their refractive error or the subject underwent a subjective refraction examination by an optometrist. All other subjects underwent subjective non-cycloplegic refraction. Subjects were classified as myopic if the spherical equivalent refractive error in the right eye was ≤−0.50 D.
Statistical analyses
Fisher’s Exact test or the Chi squared test were used to compare subjects classified as myopic versus non-myopic by first-born status. Multivariate binary logistic regression was used to study the influence of multiple risk factors. To be included in the multiple regression model, a variable had to be associated with myopia status (P<0.05) in univariate analysis and be associated with first-born status (P<0.05) in univariate analysis. Note that we restricted attention to variables associated with both myopia status and first born status in univariate analyses, since a variable not meeting our criterion for association with being first born was deemed unlikely to act as a confounder. The availability of information on potential risk factors for myopia in each study group is listed in the Results section, along with details of which variables were tested in univariate analyses, and those variables that were included in the final logistic regression model. Adjusted odds ratios (OR) and 95% confidence intervals (CI) are presented. Analyses were carried out using SPSS version 18 (SPSS Inc., Chicago, USA).
For the IDFC and SCORM studies, where recruitment occurred over several years, some of the subjects included in the analysis were siblings. We developed a simulation model to assess whether the presence of siblings biased estimates of the risk of myopia associated with birth order (Appendix 1, Online Supplementary Material). The simulation suggested that there would be minimal or no observable bias when analyses were conducted ignoring the relatedness between subjects. Theoretical reasoning suggested that, because of the study designs employed, the age of the subject was not a potentially confounding factor (Appendix 2, Online Supplementary Material). This was important given that, within families, first-born children are always older than their second-born siblings, and so on, and since myopia tends to increase with age.
Results
The demographics of the 4 samples studied are shown in Tables 1 and 2. Consistent with theoretical expectations (Appendix 2, Online Supplementary Material), the age of subjects was found to be similar across birth orders (Table 2). The numbers of subjects classified as myopic in each cohort are shown in Table 3. Information on potential risk factors for myopia included in the analysis of each cohort are presented in Table 4.
Table 1. General demographic characteristics of the study groups.
| Study group | ALSPAC | SCORM | REHS | IDFC |
|---|---|---|---|---|
| Recruitment site | UK | Singapore | Australia | Israel |
| Cohort type | Birth cohort | School-based cohort | Birth cohort | Military recruitment candidates |
| Sample size | 4,401 | 1,959 | 1,266 | 888,277 |
| Ethnicity§ | White European 100% |
Chinese (75%) Malay (17%) Indian (7%) Eurasian (<1%) Other (1%) |
White European = 85% Chinese=5% Indian = 4% Other = 6% |
Israeli family origins, within 2 generations: Israeli 10% Western 36% Eastern 54% |
| Age (years) Mean±SD and range |
15.4 ± 0.3 (range 14-17) |
13.05 ± 2.33 (range 7-20) |
20.05 ± 0.44 (range 20-22) |
17.3 ± 0.5 (range 16–22) |
| Sex (%male) | 47.2 | 50.6% | 52% | NA* |
| Method of refractive estimation |
Non-cycloplegic autorefraction |
Cycloplegic autorefraction |
Cycloplegic autorefraction |
Spectacle focimetry or subjective refraction |
| Refraction (D) Mean±SD |
−0.40 ± 1.27 | −2.15 ± 2.48 | −0.13 ± 1.60 | NA* |
For the IDFC, the “region of family origin” was used to categorize subjects’ ethnicity: Western = European, American, or Oceanic, Eastern = Asian or African.
information not available for release.
Table 2. Demographic characteristics of the study groups by birth order.
| Number of subjects (%) | ||||
|---|---|---|---|---|
|
|
||||
| Birth order | ALSPAC | SCORM | REHS | IDFC |
| 1 | 2168 (49.3%) | 1800 (91.9%) | 597 (47.2%) | NA |
| 2 | 1525 (34.7%) | 155 (7.9%) | 382 (30.2%) | NA |
| 3 | 532 (12.1%) | 4 (0.2%) | 186 (14.7%) | NA |
| ≥4 | 176 (4.0%) | - | 101 (8.0%) | NA |
|
| ||||
| Age of subjects (mean ± S.D.) in years | ||||
|
|
||||
| Birth order | ALSPAC | SCORM | REHS | IDFC |
| 1 | 15.4 ± 0.3 | 13.1 ± 2.3 | 20.0 ± 0.4 | NA |
| 2 | 15.4 ± 0.3 | 12.6 ± 2.2 | 20.1 ± 0.5 | NA |
| 3 | 15.4 ± 0.3 | 13.0 ± 0.0 | 20.1 ± 0.5 | NA |
| ≥4 | 15.4 ± 0.3 | - | 20.0 ± 0.4 | NA |
NA: Information not available for release.
Table 3. Number of subjects classified as myopic or emmetropic/hyperopic.
| Study group | Myopic (%) | Emmetropic/ hyperopic |
Non-myopic | Total |
|---|---|---|---|---|
| ALSPAC | 755 (17.2%) | 2184 | 3646 | 4401 |
| SCORM | 1337 (68.2%) | - | 622 | 1959 |
| REHS | 301 (23.8%) | - | 965 | 1266 |
| IDFC* | NA (30%) | - | NA | NA |
For the IDFC, we used the myopia prevalence from a 2008 study of this cohort32, since the figure for the set of subjects explicitly analysed here was not available (NA).
Table 4. Potential confounding variables for each study group.
| Variable | Study group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALSPAC | SCORM | REHS | IDFC | ||||||||
| Avail. | Uni. | Incl. | Avail. | Uni. | Incl. | Avail. | Uni. | Incl. | Avail. | Incl*. | |
| Sex | ✓ | ✓ | - | ✓ | ✓ | - | ✓ | ✓ | - | ✓ | ✓ |
| Ethnicity | NA | NA | - | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | ✓ |
| Parental myopia | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | ✓ | - | - | - |
| Time reading for pleasure | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | - | - | - | - |
| Time spent outdoors | ✓ | ✓ | - | ✓ | - | - | ✓ | ✓ | - | - | - |
| Socioeconomic status | ✓ | ✓ | ✓ | ✓ | ✓ | - | ✓ | - | - | ✓ | ✓ |
| Maternal age | ✓ | ✓ | ✓ | - | - | - | ✓ | ✓ | ✓ | - | - |
| Smoking during pregnancy | ✓ | ✓ | - | ✓ | - | - | ✓ | ✓ | - | - | - |
| Prematurity | ✓ | ✓ | - | - | - | - | ✓ | ✓ | - | - | - |
| Small for gestational age | ✓ | ✓ | - | - | - | - | ✓ | ✓ | - | - | - |
| Breast feeding | ✓ | - | - | ✓ | - | - | ✓ | - | - | - | - |
| IQ | ✓ | - | - | ✓ | - | - | ✓ | - | - | ✓ | ✓ |
| BMI | ✓ | - | - | ✓ | - | - | ✓ | - | - | ✓ | ✓ |
| Height | ✓ | - | - | ✓ | ✓ | - | ✓ | - | - | ✓ | ✓ |
| Season of birth | ✓ | - | - | ✓ | - | - | ✓ | - | - | ✓ | ✓ |
| Place of birth | NA | NA | - | ✓ | - | - | NA | NA | - | ✓ | ✓ |
Avail: tick indicates information on variable was available; Uni: tick indicates variable was tested in univariate analysis for association with birth order and myopia; Incl: tick indicates risk factor was associated with both myopia and birth order, and was included in the final logistic regression model. (*Note that for the IDFC sample, all available potential confounders were included in the final model).
ALSPAC
Prior to adjustment for potential risk factors, being first born was associated with an increased risk of “likely myopia” in children from the ALSPAC birth cohort (OR=1.18, 95% CI: 1.01-1.38). However, there was no indication of a dose-response relationship (that is, children with a birth order of two were classified as “likely myopic” no more often than those with a birth order of three, and so on). Several variables showed evidence of association with both likely myopia and with first-born status in univariate trials. Specifically, positive associations were observed for: an increased number of myopic parents, a higher social class of parents, more time spent reading for pleasure and older maternal age. Adjusting for these covariates increased the estimated effect size for the association between first-born status and likely myopia (Table 5; adjusted OR=1.31, 95% CI: 1.05-1.64). When the analysis described above was repeated using the comparison “likely myopia” versus “likely emmetropia/hyperopia” (as opposed to “likely myopia” versus “likely not myopia”) the effect size and statistical significance level were qualitatively the same (Table 5).
Table 5. Risk of likely myopia in first born versus non-first born subjects in the ALSPAC cohort.
Note that sample size differs between the adjusted and unadjusted analyses due to missing information for some children.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N | OR (95% CI) |
P-value | N | OR (95% CI) |
P-value | |
|
|
||||||
| Including only children | ||||||
| Likely myopic vs. likely not myopic |
4401 | 1.18 (1.01-1.38) |
0.038 | 2499 | 1.31 (1.05-1.64) |
0.016 |
| Likely myopic vs. likely emmetropic/hyperopic |
2939 | 1.23 (1.05-1.46) |
0.014 | 1679 | 1.41 (1.11-1.78) |
0.004 |
| Excluding only children | ||||||
| Likely myopic vs. likely not myopic |
3527 | 1.16 (0.97-1.38) |
0.102 | 2194 | 1.37 (1.07-1.74) |
0.012 |
Adjusted analyses included the variables: The number of myopic parents, the social class of parents, time spent reading for pleasure and maternal age.
After excluding “only children” (children with no related/fostered brothers or sisters), the increased risk of likely myopia in first-born subjects remained essentially unchanged (Table 5). This finding implied that the risk associated with being first born did not arise because of a peculiarly high prevalence of myopia in “only children”, but rather that all first-born children were at an increased risk of myopia regardless of whether or not they had younger siblings. (Specifically, the prevalence of “likely myopia” was 17.9% in “only children”, 18.0% in first-born children who had younger siblings, and 16.0% in children who were not first born).
Note that data for at least one risk factor variable was missing for 1882 out of the 4401 ALSPAC participants. Subjects with full information were an average of 14 days younger (P<0.001) and −0.08 D more myopic (P=0.042) than those with missing information. The proportion of boys and girls was the same in those with and without missing information, however subjects with full information were much more likely to be first-born (54% vs. 43%, P<0.001).
SCORM (Singapore Cohort study Of Risk factor for Myopia)
Prior to adjustment for other potential risk factors, being first born was associated with an increased risk of myopia in children in the SCORM study (OR=1.46, 95% CI: 1.03-2.06). However, after adjusting for ethnicity, which was the only risk factor that was associated both with myopia and first-born status in univariate analyses, the magnitude and statistical evidence for the association between being first born and myopia was markedly reduced towards the null (OR=1.25, 95% CI: 0.89-1.77, P=0.20).
REHS (Raine Eye Health Study)
In unadjusted analyses, the prevalence of myopia was higher in first-born than in non-first-born subjects, but the degree of association was not significant (OR=1.04, 95% CI: 0.80-1.34, P=0.79). After adjusting for maternal age, which was the only variable associated both with myopia and first-born status in this cohort, the risk associated with being first born was shifted away from the null, but remained non-significant (OR=1.18, 95% CI: 0.90-1.55, P=0.24).
IDFC (Israeli Defense Force recruitment Candidates)
After adjusting for “region of family origin”, socioeconomic status, intelligence, body mass index (BMI), height, birth place, season of birth, and sex, birth order was associated with all degrees of myopia in the IDFC sample (all P<0.001; Table 6). Furthermore, for mild and moderate levels of myopia, the risk (OR) associated with being first born was greater in comparison to individuals with a birth order of four or more than in comparison to those who were second or third born in this study group. For high myopia, however, the risk in comparison to those who were second or third born (OR=1.18, 95% CI: 1.13 1.23, P<0.001) was similar to that seen in comparison to those with a birth order of four or more (OR=1.19, 95% CI:1.11 1.27, P<0.001).
Table 6. Risk of myopia in first born versus non-first born subjects in the IDFC cohort.
| First born vs. second or third born |
First born vs. fourth of higher born |
|||
|---|---|---|---|---|
|
|
||||
| OR (95% CI) |
P-value | OR (95% CI) |
P-value | |
|
|
||||
| Degree of myopia | ||||
| Mild (>−3.00 and ≤−0.50 D) |
1.04 (1.03-1.06) |
<0.001 | 1.12 (1.09-1.14) |
<0.001 |
| Moderate (>−6.00 and ≤−3.00 D) |
1.12 (1.09-1.14) |
<0.001 | 1.14 (1.10-1.18) |
<0.001 |
| Severe (≤−6.00) |
1.18 (1.13-1.23) |
<0.001 | 1.19 (1.11-1.27) |
<0.001 |
Analyses were adjusting for “region of family origin”, socioeconomic status, intelligence, body mass index (BMI), height, birth place, season of birth, and sex.
Discussion
Overall, our findings from the 4 subject groups suggest that there is a small, increased risk of myopia in first-born vs. non-first-born subjects, as implied previously from studies employing indirect refraction assessment methods14, 18, 19. The association we observed in the largest study group (IDFC) is unlikely to be have arisen by chance, whilst those seen in the other studies provided weaker evidence consistent with their smaller sample sizes, and thus need to be interpreted with caution. Nevertheless, all of the study groups showed a similar direction and (low) level of risk (Figure 1). In the largest study group, the IDFC, there was strong evidence of a greater difference in myopia prevalence in first born versus fourth-or-higher born individuals compared to first born versus second or third born individuals, as has already been reported for a large UK-based cohort, the NCDS14. However, this relationship was not seen in the other groups we studied.
Figure 1. Adjusted odds ratios (with 95% CI) for each study sample.
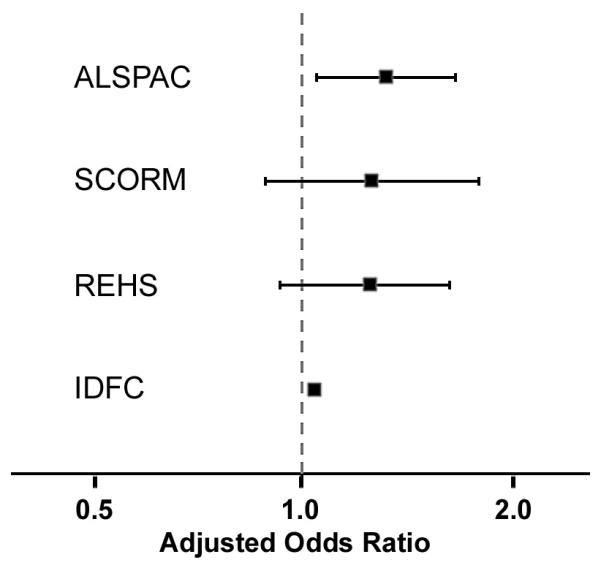
The lack of uniformity of the adjusted ORs across the 4 study groups (Figure 1) is likely to have been influenced by sample size-related variation in statistical power, as mentioned above. However, in addition, subject-related differences in the population groups that were investigated may also have had an impact; for instance, the participants in the REHS and IDFC were generally older than those in SCORM and ALSPAC, the age range of the IDFC was wider than those of the other 3 cohorts, and a larger proportion of the SCORM participants were first born than was the case in the other cohorts. Furthermore, the level of exposure to environmental risk factors for myopia is thought to vary between Singapore, Israel, Australia and the UK33. Differences in the statistical analysis of the groups may also have contributed to the variability in the adjusted ORs; the availability and inclusion of potential confounding variables such as socioeconomic status and parental myopia differed markedly across the study groups (Table 4). These between-site differences can be viewed as an important limitation of our attempt to draw together results from different cohorts. Conversely, epidemiology studies that deliberately explore findings from disparate study populations also offer the attraction of providing some insurance against confounding34, since a causal association, for instance, should produce a consistent magnitude and direction of effect across diverse study sites.
The low risk associated with being first born implies that birth order has less influence on refractive development compared to known risk factors for myopia such as parental refractive error and time spent engaged in sports/outdoor activity2, 25, 26. In the ALSPAC study, adjustment for behaviours implicated in causing variations in myopia risk within a family actually increased the estimated effect size (unadjusted OR = 1.18, adjusted OR = 1.31). Thus, the data do not support the idea that the association between birth order and myopia is confounded by the behavioural differences we adjusted for, although other behavioural differences may be involved. There were small differences in the age and refractive error of ALSPAC subjects with and without missing data, along with a surprisingly large difference in the proportion of first-born individuals (43% vs. 54%, respectively), i.e. the subjects in the adjusted analysis were not a random sample of all those attending the research clinic at this age, and in particular, parents of first-born children were more likely to have fully completed questionnaires about their child. (Moreover, attendance of ALSPAC children at the research clinic is also known to vary depending on the age, sex and socioeconomic status of the participant25). Potentially, the risk factor-myopia relationship profile of subjects with full vs. missing information could also have varied. These demographic and potential risk profile differences may have contributed to the observed difference between the unadjusted and adjusted OR estimates, meaning that caution is needed in extrapolating these values to the entire population.
Our novel finding that “only children” appeared to be at a similarly elevated risk of myopia as first-born children with younger siblings adds weight to the hypothesis that birth order confers an increased risk of myopia independent of known risk factors, since it argues against a causal mechanism involving first-born children modifying the myopia-related environmental risk factor exposure of their younger siblings.
Birth order could conceivably be linked causally to myopia via a direct physiological mechanism, for example relating to the in utero environment. An association between birth order and refraction could also arise if maternal age (or paternal age) is a risk factor for myopia in offspring. Supporting potential confounding between birth order and maternal age, Rudnicka et al.14 reported an elevated risk of reduced uncorrected vision (i.e. likely myopia) with increasing maternal age (OR=1.10 per 5-year increase, 95% CI: 1.04-1.17) in a meta-analysis of 3 UK-based cohorts. In a more detailed analysis of one of the Rudnicka et al. study samples, Rahi et al.13 found evidence pointing towards a complex relationship in which both low and high maternal age increased the risk of myopia compared with a maternal age between 21-30 years, along with a further rise in risk in those born to mothers aged ≥35 years (OR=1.5, 95% CI: 1.1-2.0). However, in regression models that adjusted for maternal age, birth order appeared to confer an independent risk for myopia in the 3 groups of subjects studied by Rudnicka et al.14, and this was also the case for the ALSPAC participants studied here.
Although there are reports of birth order-disease associations with a physiological basis unconnected to maternal age, for instance in conditions such as metabolic syndrome, schizophrenia, asthma and cancer35-38, the causal pathways involved are not generally known. First-born babies tend to have a lower birth weight for their gestational age than non-first born-babies (sometimes termed “small for dates”)39 and are over-represented in those showing “catch-up growth” during the first 2 years of infancy, followed by obesity and insulin resistance later in childhood40-42. Given the evidence from animal models for a role of glucagon and insulin in emmetropization43, 44, this raises the interesting possibility that altered signalling through the insulin/glucagon axis relating to birth order may impact not only on bodily growth but also on ocular growth.
A higher prevalence of myopia has been observed repeatedly in communities living in urban rather than rural locations45-47. An interesting implication of an association between birth order and myopia is that it has the potential to contribute to this difference in prevalence, because the birth rate (or, strictly, the total fertility rate) of women living in urban regions is typically lower than that of women living in rural areas, after accounting for any difference in child mortality rates (see USAID-funded MEASURE Demographic and Health Survey project (http://www.measuredhs.com/). We constructed a simulation model (details available on request) to test the likely magnitude of birth order in contributing to the urban vs. rural myopia prevalence rates (assuming the following causal path: Low birth rate → High proportion of subjects who are first born → Increased prevalence of myopia ← Other causes). The results suggested that the birth order-myopia association would account for differences in prevalence of <5%, i.e. much less than the often marked difference in myopia prevalence between urban and rural locations45-47.
To summarize, the strength of our presentation of these studies is that it facilitates the comparison of multiple groups of subjects of differing age, ethnicity and myopia prevalence, enabling us to examine the generality of the myopia-birth order association, and the very large sample size of the IDFC cohort. Weaknesses affecting cross-study comparison are that non-uniform assessment methods were employed across study groups, that cycloplegia was not used to assess refractive error in all study groups, that the range of potential myopia risk factors used in the regression analyses was not uniform across study groups, and that none of the groups of subjects was fully representative of the general population. Indeed, for the above reasons we decided that it would be inappropriate to perform a meta-analysis of the results in the 4 subjects groups. Furthermore, we made no attempt to search for interactions between birth order and other potential myopia risk factors, due to a perceived lack of statistical power in the 3 smaller study groups and lack of information about key risk factors in the IDFC. Such analyses may be an interesting avenue for further research.
In conclusion, we found robust evidence in the two largest studies that being a first-born child increased the risk of myopia to a small extent, substantiating the conclusions of existing studies that relied on more indirect methods (see Introduction). Being first born was also a risk factor for high-degree myopia. Moreover, adjusting for known myopia risk factors and excluding “only children” did little to modify the risk associated with being first born, suggesting the possibility of an independent mechanism of action (perhaps of physiological and maternal origin). In the Israeli subjects, there was also support for a relationship of decreasing risk of myopia with increasing birth order. There is uncertainty regarding whether these associations hold true for the smaller studies and/or whether they might vary in different ethnicities, but the data are suggestive that the association between birth order and myopia is a widespread phenomenon. Future epidemiology studies could help confirm or refute this by routinely collecting and reporting information on participants’ birth orders. If there are other (non-behavioural) causal mechanisms involved it may be informative to explore their contribution to myopia development.
Supplementary Material
Acknowledgements
The ALSPAC researchers are extremely grateful to all the families who took part in this study, the midwives for their help with recruitment, and the other members of the ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The Raine researchers thank all participants and their families and the whole Raine Study team. This publication is the work of the authors and Jeremy Guggenheim will serve as guarantor for the contents of this paper.
Funding/Support: The UK Medical Research Council (Grant ref: 74882) the Wellcome Trust (Grant ref: 076467) and the University of Bristol provide core support for ALSPAC. The Raine Eye Health Study was funded by the Australian Foundation for the Prevention of Blindness, The Ophthalmic Research Institute of Australia (ORIA) and the National Health and Medical Research Council (NHMRC Grant ref: 1021105). The Western Australian Pregnancy Cohort (Raine) Study core funding is provided by The University of Western Australia (UWA), The Telethon Institute for Child Health Research, Raine Medical Research Foundation, UWA Faculty of Medicine, Dentistry and Health Science, Women’s and Infant’s Research Foundation, Curtin University, NHMRC. This work was specifically funded by grant SCIAD 053 from the National Eye Research Centre, Bristol (JAG, CW), a NIHR career development fellowship (CW), NIH grant R01-EY018838 (RAS), the Paul and Evanina Bell Mackall Foundation Trust (RAS), Research to Prevent Blindness (RAS), Singapore National Medical Research Council grant NMRC/0695/2003 (SMS).
Footnotes
Conflicts of interest: None of the authors have any proprietary interests or conflicts of interest related to this submission.
Originality of submission: This submission has not been published anywhere previously and that it is not simultaneously being considered for any other publication.
References
- 1.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic and Physiological Optics. 2005;25(5):381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clinical Genetics. 2011;79(4):301–20. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524–32. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip JM, Huynh SC, Robaei D, et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye. 2007;22(5):649–56. doi: 10.1038/sj.eye.6702701. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-P, Hocking PM, Wang L, et al. Selective breeding for susceptibility to myopia reveals a gene-environment interaction. Invest Ophthalmol Vis Sci. 2011;52:4003–11. doi: 10.1167/iovs.10-7044. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfield M, Gilmartin B. Myopia and nearwork: Causation or merely association? In: Rosenfield M, Gilmartin B, editors. Myopia and nearwork. Butterworth-Heinemann; Oxford, UK: 1998. [Google Scholar]
- 7.Mutti DO, Zadnik K. Has Near Work’s Star Fallen? Optometry and Vision Science. 2009;86(2):76–8. doi: 10.1097/OPX.0b013e31819974ae. [DOI] [PubMed] [Google Scholar]
- 8.Mutti DO, Mitchell GL, Moeschberger ML, et al. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43(12):3633–40. [PubMed] [Google Scholar]
- 9.Ip JM, Saw SM, Rose KA, et al. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49(7):2903–10. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]
- 10.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Saw S-M, Hong C-Y, Chia K-S, et al. Nearwork and myopia in young children. Lancet. 2001 Feb 3;357:390. doi: 10.1016/S0140-6736(05)71520-8. [DOI] [PubMed] [Google Scholar]
- 12.Dirani M, Tong L, Gazzard G, et al. Outdoor Activity and Myopia in Singapore Teenage Children. British Journal of Ophthalmology. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- 13.Rahi JS, Cumberland PM, Peckham CS. Myopia over the lifecourse: Prevalence and early life influences in the 1958 British Birth Cohort. Ophthalmology. 2011;118(5):797–804. doi: 10.1016/j.ophtha.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Rudnicka AR, Owen CG, Richards M, et al. Effect of breastfeeding and sociodemographic factors on visual outcome in childhood and adolescence. American Journal of Clinical Nutrition. 2008;87(5):1392–9. doi: 10.1093/ajcn/87.5.1392. [DOI] [PubMed] [Google Scholar]
- 15.Stone RA, Wilson LB, Ying GS, et al. Associations between childhood refraction and parental smoking. Investigative Ophthalmology & Visual Science. 2006;47(10):4277–4287. doi: 10.1167/iovs.05-1625. [DOI] [PubMed] [Google Scholar]
- 16.Saw SM, Chia KS, Lindstrom JM, et al. Childhood myopia and parental smoking. British Journal of Ophthalmology. 2004;88(7):934–7. doi: 10.1136/bjo.2003.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip JM, Robaei D, Kifley A, et al. Prevalence of hyperopia and associations with eye findings in 6- and 12-year-olds. Ophthalmology. 2007;115(4):678–85. doi: 10.1016/j.ophtha.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 18.Douglas JWB, Ross JM, Simpson HR. The ability and attainment of short-sighted pupils. Journal of the Royal Statistical Society Series A (General) 1967;130(4):479–504. [Google Scholar]
- 19.Peckham CS, Gardiner PA, Goldstein H. Acquired Myopia in 11-Year-Old Children. British Medical Journal. 1977;1(6060):542–5. doi: 10.1136/bmj.1.6060.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker G. Visual-acuity, birth-order, achievement versus affiliation, and other Edwards personal preference schedule scores. Journal of Psychosomatic Research. 1965;9(3):277–83. doi: 10.1016/0022-3999(65)90052-8. [DOI] [PubMed] [Google Scholar]
- 21.Basu SK, Jindal A. Genetic aspects of myopia among the Shia Muslin Dawoodi Bohras of Udaipur, Rajsthan. Human Heredity. 1983;33:163–9. doi: 10.1159/000153369. [DOI] [PubMed] [Google Scholar]
- 22.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–85. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 23.Leary S, Smith GD, Ness A. Smoking during pregnancy and components of stature in offspring. American Journal of Human Biology. 2006;18(4):502–512. doi: 10.1002/ajhb.20518. Alspac Study T. [DOI] [PubMed] [Google Scholar]
- 24.Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in jewish teenagers. Journal of Pediatric Ophthalmology and Strabismus. 1993;30:319–22. doi: 10.3928/0191-3913-19930901-12. [DOI] [PubMed] [Google Scholar]
- 25.Guggenheim JA, Northstone K, McMahon G, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: A prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53:2856–65. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones-Jordan LA, Mitchell GL, Cotter SA, et al. Visual activity prior to and following the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011;52:1841–50. doi: 10.1167/iovs.09-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams C, Miller LL, Gazzard G, Saw SM. A comparison of measures of reading and intelligence as risk factors for the development of myopia in a UK cohort of children. British Journal of Ophthalmology. 2008;92(8):1117–21. doi: 10.1136/bjo.2007.128256. [DOI] [PubMed] [Google Scholar]
- 28.Golding J, Pembrey M, Jones R, ALSPAC Study Team ALSPAC-The Avon Longitudinal Study of Parents and Children - I. Study methodology. Paediatric and Perinatal Epidemiology. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 29.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51–7. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 30.Saw SM, Tan SB, Fung D, et al. IQ and the association with myopia in children. Invest Ophthalmol Vis Sci. 2004;45(9):2943–8. doi: 10.1167/iovs.03-1296. [DOI] [PubMed] [Google Scholar]
- 31.Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology. 2008;115(4):686–92. doi: 10.1016/j.ophtha.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 32.Bar Dayan Y, Levin A, Morad Y, et al. The changing prevalence of myopia in young adults: a 13-year series of population-based prevalence surveys. Invest Ophthalmol Vis Sci. 2005;46(8):2760–5. doi: 10.1167/iovs.04-0260. [DOI] [PubMed] [Google Scholar]
- 33.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic and Physiological Optics. 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 34.Davey Smith G, Leary S, Ness A, Lawlor DA. Challenges and novel approaches in the epidemiological study of early life influences on later disease. Adv Exp Med Biol. 2009;646:1–14. doi: 10.1007/978-1-4020-9173-5_1. [DOI] [PubMed] [Google Scholar]
- 35.Cardwell CR, Stene LC, Joner G, et al. Birth order and childhood type 1 diabetes risk: a pooled analysis of 31 observational studies. International Journal of Epidemiology. 2011;40(2):363–74. doi: 10.1093/ije/dyq207. [DOI] [PubMed] [Google Scholar]
- 36.Kemppainen L, Veijola J, Jokelainen J, et al. Birth order and risk for schizophrenia: a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Acta Psychiatrica Scandinavica. 2001;104(2):148–52. doi: 10.1034/j.1600-0447.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohfuji S, Miyake Y, Arakawa M, et al. Sibship size and prevalence of allergic disorders in Japan: The Ryukyus Child Health Study. Pediatric Allergy and Immunology. 2009;20(4):377–84. doi: 10.1111/j.1399-3038.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 38.Von Behren J, Spector LG, Mueller BA, et al. Birth order and risk of childhood cancer: a pooled analysis from five US States. International Journal of Cancer. 2011;128(11):2709–2716. doi: 10.1002/ijc.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magnus P, Berg K, Bjerkedal T. The association of parity and birth-weight - Testing the sensitization hypothesis. Early Human Development. 1985;12(1):49–54. doi: 10.1016/0378-3782(85)90136-7. [DOI] [PubMed] [Google Scholar]
- 40.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. European Journal of Endocrinology. 2004;151(Suppl. 3):U131–U9. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 41.Ong KKL, Ahmed ML, Emmett PM, et al. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. British Medical Journal. 2000;320(7240):967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong KKL, Preece MA, Emmett PM, et al. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: Longitudinal birth cohort study and analysis. Pediatric Research. 2002;52(6):863–7. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Wallman J. Glucagon and insulin have opposite effects on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:13–23. doi: 10.1167/iovs.08-1702. [DOI] [PubMed] [Google Scholar]
- 45.He M, Zheng Y, Xiang F. Prevalence of myopia in urban and rural children in mainland China. Optometry and Vision Science. 2009;86(1):40–4. doi: 10.1097/OPX.0b013e3181940719. [DOI] [PubMed] [Google Scholar]
- 46.Ip JM, Rose K, Morgan I, et al. Myopia and the urban environment: Findings for a sample of 12-year old Australian school children. Invest Ophthalmol Vis Sci. 2008;49(9):3858–63. doi: 10.1167/iovs.07-1451. [DOI] [PubMed] [Google Scholar]
- 47.Shih YF, Chiang TH, Hsiao CK, et al. Comparing myopic progression of urban and rural Taiwanese schoolchildren. Japanese Journal of Ophthalmology. 2010;54(5):446–51. doi: 10.1007/s10384-010-0860-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


