Abstract
Background
Mechanisms of atrial fibrillation (AF) initiation are incompletely understood. We hypothesized that rate-dependent changes (restitution) in action potential duration (APD) and activation latency are central targets for clinical interventions that induce AF. We tested this hypothesis using clinical experiments and computer models.
Methods and Results
In 50 patients (20 persistent, 23 paroxysmal AF, 7 controls), we used monophasic action potential catheters to define left atrial APD restitution, activation latency and AF incidence from premature extrastimuli. Isoproterenol (n=14), adenosine (n=10), or rapid pacing (n=36) were then initiated to determine impact on these parameters. Compared with baseline in AF patients, isoproterenol and rapid pacing decreased activation latency (64±14vs 31±13 vs 24±14 ms, p<0.05), steepened maximum APD restitution slope (0.8±0.7 vs 1.7±0.5 vs 1.1±0.5, p<0.05), and increased AF incidence (12% vs 64% vs 84%, p<0.05). Conversely, adenosine shortened APD (p<0.05), yet increased activation latency (86±27 ms, p=0.002) so that maximum APD restitution slope did not steepen (1.0±0.5, p=NS) and AF incidence was unchanged (10%, p=NS). In controls, no intervention steepened APD restitution or initiated AF. Computational modeling revealed that isoproterenol steepened APD restitution by increased ICaL and decreased activation latency via enhanced IKr inactivation, while rapid pacing steepened APD restitution via increased IK1.
Conclusions
Steep APD restitution is a common pathway for AF initiation by isoproterenol and tachycardia, via reduced activation latency that enables engagement of steep APD restitution at rapid rates. Modeling suggests AF initiation from each intervention uses distinct ionic mechanisms. This insight may help design interventions to prevent AF.
Keywords: action potentials, atrial fibrillation, computers, electrophysiology, pacing
Introduction
Our present understanding of atrial fibrillation (AF) initiation involves the interaction of static (including fibrosis-related conduction slowing1 and tissue anisotropy2) and dynamic factors (particularly the rate-response of action potential duration [APD]3 and conduction velocity4), leading to functional wavefront block, reentry, and AF.
Recent work has shown steep APD restitution near human pulmonary veins5 may enable single premature atrial complexes (PACs) to initiate paroxysmal AF by amplifying APD oscillations, resulting in wavebreak.3 However, steep APD restitution was not observed in persistent AF patients, possibly due to activation latency which prevented early beats from engaging steep APD restitution,6 questioning the mechanistic role of APD restitution in patients with severe atrial remodeling.
Nevertheless, AF can be effectively induced in patients in the electrophysiology laboratory using a variety of techniques including isoproterenol,7 adenosine,8 or rapid pacing.7 However, the profibrillatory effects9 and ionic mechanisms10 of these interventions are incompletely understood.
We used clinical experiments to test the hypothesis that steep APD restitution is a common pathway for AF initiation from these interventions, mediated via reductions in activation latency that “unmask” steep APD restitution, particularly in patients with remodeled atria. We used computer models of human atrial tissue to explore differences in the ionic mechanisms from each intervention.
Methods
We enrolled patients referred for electrophysiology study at the University of California San Diego and Veterans Affairs San Diego Healthcare System. AF patients were studied prior to AF ablation, while controls had no history of AF but required left atrial access for clinical ablation. The study was approved by our joint Institutional Review Board, and all patients provided written informed consent.
Electrophysiology Study
Electrophysiology study was performed in the fasted state, >5 half-lives after discontinuing antiarrhythmic medications (>4 weeks after discontinuing amiodarone). Transseptal puncture was guided by intracardiac echocardiography and left atrial (LA) geometry was created using NavX (St. Jude Medical, Sylmar, CA) referenced to patient-specific computed tomography data. A deflectable 7F monophasic action potential (MAP) catheter (2.5 mm electrode spacing, Boston Scientific, Sunnyvale, CA) was advanced to record the NavX-verified antrum of the left or right superior pulmonary veins.
Electrophysiologic Recordings
MAP recordings were filtered at 0.05–500 Hz, other intracardiac signals between 30–250 Hz. Signals were digitized at 1 kHz to 16-bit resolution and exported from the physiologic recorder (Bard Pro, Billerica, MA) for analysis using custom PC software written by SMN in Labview (National Instruments, Austin, TX). Recordings showing excessive baseline wander, artifact, or noise were excluded.
Baseline Pacing Protocol
The protocol was performed before ablation. Patients presenting in AF were electrically cardioverted to sinus rhythm, and the protocol was commenced after 18±5 minutes. Pacing was performed at twice diastolic threshold using the MAP catheter, and action potentials (APs) were recorded in routine fashion.11 A drive train of 8 beats at a cycle length (CL) of 500 ms (S1) (or 450 ms if resting CL was <500 ms) was followed by single PACs (S2), coupled at 450, then 400, reduced in 20 ms steps to 300, then in 10 ms steps to effective refractory period (ERP) or AF initiation.
Measurement of PAC-Related APD Restitution
We measured APD using validated software.5 Briefly, AP onset was assigned as the time of maximal computed upstroke dV/dt (figure 1A). We identified phase II just after the peak of atrial AP, and phase IV voltage as mean voltage preceding and following the beat. APD at 90% repolarization (APD90) spans the interval from AP onset to 90% voltage recovery from phase II to baseline. Diastolic interval spans the interval from APD90 of the prior beat to next AP. When repolarization was contaminated by noise (e.g. pacing artifact), mean APD90 of 2 prior beats at a stable CL was used.
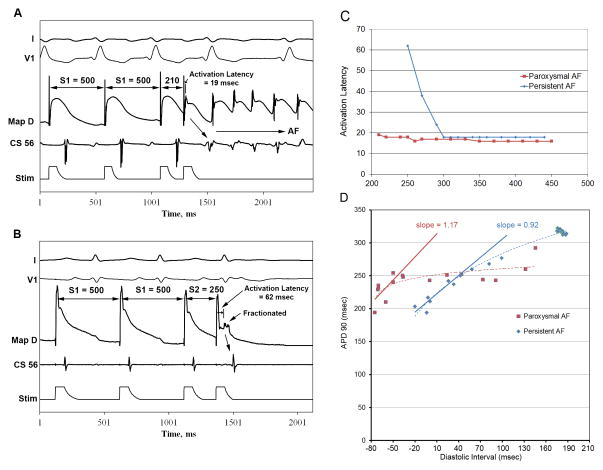
Figure 1.
(A) Premature extrastimuli initiate AF at CL 500/210 in a paroxysmal AF patient, with an activation latency of 19 msec. (B) Patient with Persistent AF with activation latency of 62 msec at 500/250, and AF is not initiated. Maximum activation latency (C) is greater in persistent (blue) versus paroxysmal (red) AF, illustrated by these representative patients. Baseline action potential duration (APD) restitution slope (D) was <1 in persistent AF (blue) but >1 in paroxysmal (red), as shown for these individuals. Key: ABL-D=distal electrode of MAP catheter.
We constructed APD restitution curves (figure 1D) using (diastolic interval, APD90) pairs for each extrastimulus. Maximum slope was obtained from a linear fit of 30-ms diastolic interval segments.9 When alternans was observed,12 APD/diastolic interval pairs for even or odd beats were analyzed separately to compute tissue restitution. Patients were assigned to isoproterenol, adenosine, or rapid pacing interventions and APD restitution and AF incidence were remeasured.
Analysis of Activation Latency – Rate Relationship
Activation latency was defined as the time between pacing stimulus to maximum dV/dt during AP upstroke of each paced beat (figure 1B). We used (S1–S2 interval, activation latency) pairs to plot activation latency restitution (figure 1C).13
Pharmacologic Interventions
The isoproterenol group (n=14: 6 persistent, 5 paroxysmal, 3 control) received an infusion of 1 mcg/min, titrating upward until the heart rate increased by 20%. The adenosine group (n=10: 6 persistent, 4 paroxysmal) received an infusion of 140 mcg/kg/min, as utilized for stress testing.14 APD restitution was remeasured after single extrastimulus pacing and AF incidence recorded.
Rapid Pacing
In the rapid pacing group (n=36: 12 persistent, 19 paroxysmal, 5 control), pacing for 30 seconds was delivered at CLs of 500, 450, then 400 msec, decrementing by 20 msec until 300 msec, then in 10 msec steps until AF onset or loss of atrial capture. APD restitution was constructed from the last paced beats at each CL, and AF incidence was recorded.
Computer Modeling
We constructed a computer model of human atrial tissue, based upon the Courtemanche-Ramirez-Nattel (CRN) model,15 to investigate the mechanisms by which APD and activation latency restitution are altered by isoproterenol, adenosine, and rapid pacing in the setting of persistent AF. Methods detail and can be found in the online supplement. Briefly, models were initially paced at CL 750ms (600ms for isoproterenol) until steady state. The clinical pacing protocols were then performed to determine the contribution of altered ion channel densities and kinetics resulting from isoproterenol, adenosine, and rapid pacing to APD and activation latency restitution.
Statistical Analysis
Continuous data are represented as mean±standard deviation. One-way ANOVA with post-hoc Bonferroni testing was used to compare continuous variables in table 1. The chi-square test with Bonferroni correction was used to compare categorical data, while the Fisher exact test was employed for categorical comparisons when expected frequency values were <5. The Mann-Whitney U test was used to compare differences in AF duration. For individual comparisons between baseline and each intervention, the McNemar test was used to compare categorical variables and the paired t test was used for continuous data; subgroup means and standard deviations are reported for each comparison. All tests are two-tailed; a probability of <5% was considered statistically significant. Statistical analyses were performed using SPSS 19 (IBM, Somers, NY, USA).
Table 1.
Study Demographics
| Characteristics | Paroxysmal AF | Persistent AF | Control | p Value |
|---|---|---|---|---|
| n (male) | 23 (22) | 20 (20) | 7 (5) | |
| Age (years) | 61±9a | 63±9b | 47±18a,b | 0.01 |
| Duration of AF, months (25–75%) | 40 (13–56) | 55 (16–96) | 0.33 | |
| Left atrial diameter, mm | 41±5 | 47±5b | 35±4b | <0.001 |
| Left ventricular EF, % | 58±8 | 56±12 | 61±12 | 0.58 |
| CHF | 3 (13) | 5 (25) | 0 | 0.35 |
| Hypertension | 14 (61) | 18 (90)b | 1 (14)b | 0.001 |
| Diabetes mellitus | 8 (35) | 5 (25) | 1 (14) | 0.53 |
| Hyperlipidemia | 15 (65)a | 13 (65)b | 0a,b | 0.006 |
| Coronary disease | 8 (35) | 6 (30) | 0 | 0.1 |
| RCA disease | 5 (22) | 2 (10) | 0 | 0.36 |
| Prior Myocardial Infarction | 4 (17) | 3 (15) | 0 | 0.50 |
| Prior PCI | 7 (30) | 2 (10) | 0 | 0.09 |
| CABG | 2 (9) | 2 (10) | 0 | 0.69 |
| COPD | 1 (4) | 3 (15) | 0 | 0.23 |
| Medications | ||||
| Beta-Blocker | 13 (57) | 14 (70) | 2 (29) | 0.16 |
| ACEI/ARB | 13 (57) | 12 (60) | 2 (29) | 0.34 |
| Digoxin | 4 (17) | 4 (20) | 0 | 0.45 |
| Calcium Channel Blockers | 7 (33) | 6 (33) | 1 (33) | 0.93 |
| Flecainide/propafenone | 2 (9) | 1 (5) | 0 | 0.68 |
| Amiodarone | 1 (4) | 2 (10) | 0 | 0.57 |
| Sotalol | 6 (26) | 4 (20) | 0 | 0.32 |
| Dofetilide | 4 (18) | 1 (5) | 0 | 0.23 |
| Coumadin | 17 (74)a | 20 (100)b | 0a,b | <0.001 |
| Aspirin | 6 (26) | 1 (5) | 1 (14) | 0.17 |
| Statin | 15 (65)a | 9 (45) | 0a | 0.01 |
denotes paroxysmal AF versus control, p<0.05;
denotes persistent AF versus control, p<0.05. Bonferroni correction was made for comparisons within each characteristic.
Results
We studied 50 patients (20 persistent, 23 paroxysmal AF, and 7 controls) whose characteristics are summarized in table 1. Patients with persistent AF had greater left atrial diameters than those with paroxysmal AF or controls (p<0.001) and a higher incidence of hypertension. Electrophysiologic data for AF patients are summarized in online supplement table S1.
AF Initiation, APD Restitution, and Activation Latency
Single PACs rarely initiated AF in AF patients at baseline (5/43 patients, 12%). Administration of isoproterenol (7/11 patients, p=0.031) and rapid pacing (26/31 patients, p<0.001) increased AF incidence, while adenosine did not (1/10, p=1.0).
At baseline, AF initiation from a single PAC was more likely in patients with steep (>1) than flat (< 1) APD restitution (5/24 versus 0/23, p=0.0496). Figure 1A shows initiation of AF by a PAC in a patient with paroxysmal AF and steep APD restitution. In comparison, figure 1B shows failure of a PAC to initiate AF in a patient with persistent AF and flat restitution. In these patients, note that maximum activation latency was lower when AF was induced (figures 1A). Activation latency restitution for these representative patients is shown in figure 1C. Notably, maximum APD restitution slope was >1 for a paroxysmal AF patient in whom a PAC initiated AF (figure 1D, arrow).
For the population, APD restitution from single PACs had maximum slope >1 in 20/23 patients with paroxysmal AF versus 3/20 patients with persistent AF (p<0.001). Maximum activation latency was longer for patients with persistent AF (80±6 msec) than paroxysmal AF (64±9 msec, p=0.035). Of control patients, none had maximum slope >1 (0.6±0.4) at baseline, and none showed AF initiation from PACs.
Effects of Isoproterenol on APD and Activation Latency Restitution
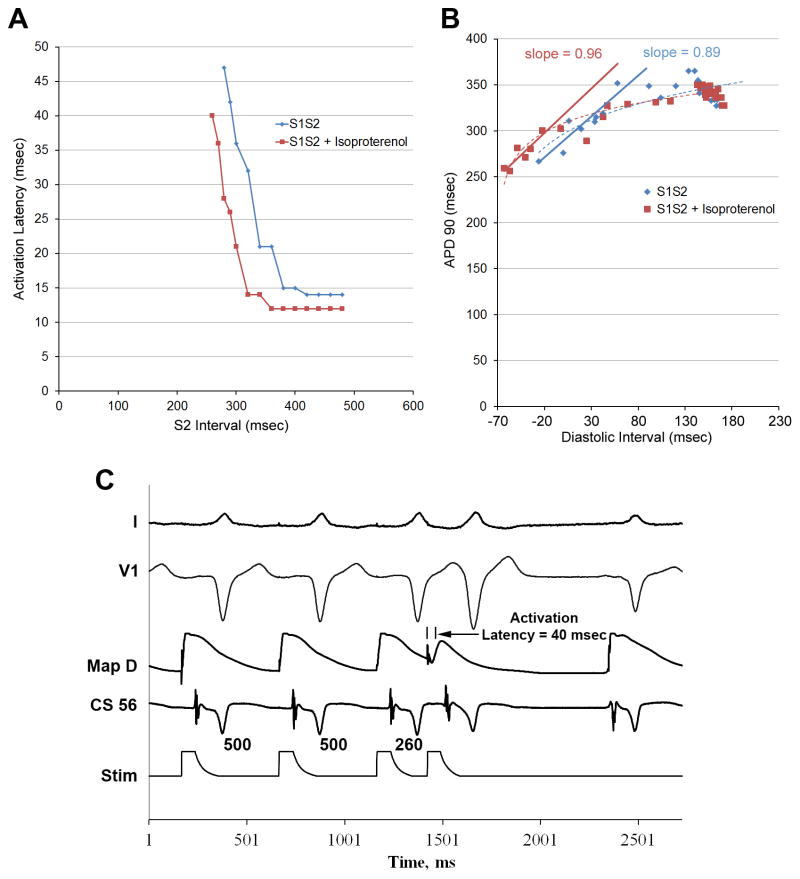
In AF patients, isoproterenol decreased maximum activation latency versus baseline (31±13 vs 64±14 ms, p=0.007). In a persistent AF patient, figure 2A shows a baseline PAC (left) just above atrial ERP (500/270) capturing atrial tissue with an activation latency of 64 msec. During isoproterenol (figure 2A, right), maximum activation latency shortens to 50 msec at 450/210, just above atrial ERP. Figure 2B shows the effects of isoproterenol on the activation latency curve for this patient during PAC delivery at baseline (blue) and during isoproterenol infusion (red).
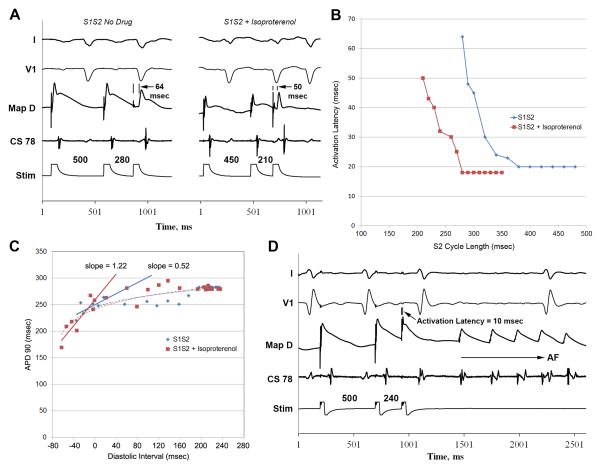
Figure 2.
(A) Extrastimulus pacing at baseline (left) and during isoproterenol infusion (right) in a patient with persistent AF. (B) Activation latency curves in the same patient, showing isoproterenol infusion (red) decreases maximum activation latency versus baseline (blue) and permits shorter extrastimuli to conduct. (C) APD restitution, for this patient, illustrate how isoproterenol (red) extends the curve to shorter diastolic intervals (leftward) versus baseline (blue), permitting shorter APDs and steepening APD restitution. (D) Premature extrastimulus induces AF in the same patient during isoproterenol, in whom no AF was observed at baseline.
Notably, isoproterenol increased maximum APD restitution slope (0.8±0.7 to 1.7±0.5, p=0.035) in AF patients, and increased AF initiation (as discussed above). In persistent AF patients, APD restitution slope increased from 0.4±0.3 to 1.5±0.9 (p=0.04). In paroxysmal AF patients, maximum APD restitution slope was >1 at baseline (1.6±0.5), increasing non-significantly to 1.8±0.3 with isoproterenol (p=0.7). As expected, isoproterenol shortened minimum atrial APD (154±32 versus 196±39 msec, p=0.015) for study patients.
Figure 2C shows APD restitution for a patient with persistent AF at baseline (blue) and during isoproterenol (red). By decreasing activation latency and shortening APD, isoproterenol shifted APD restitution leftward and increasing maximum slope from 0.52 to 1.22, and AF was induced (figure 2D). Maximum activation latency was shorter with isoproterenol.
Effects of Rapid Pacing on APD and Activation Latency Restitution
Like isoproterenol, rapid pacing decreased maximum activation latency versus baseline (24±14 vs 64±15 ms, p=0.029). Figure 3A shows maximum activation latency reduction in a persistent AF patient. In another persistent AF example, figure 3B shows that activation latency was significantly attenuated during rapid pacing (red) versus baseline S1S2 pacing (blue).
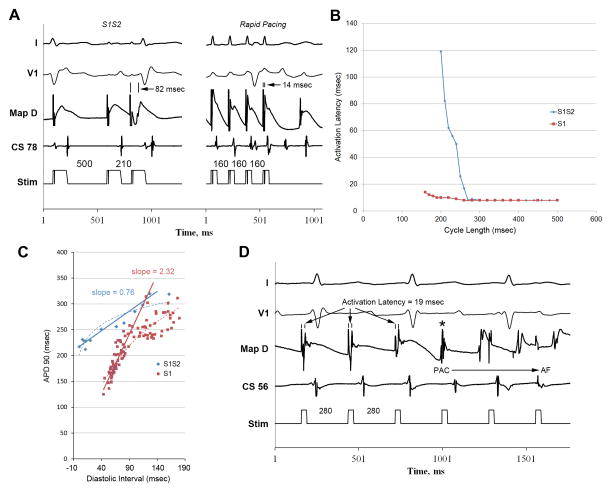
Figure 3.
(A) Baseline single extrastimulus pacing (left) and rapid pacing (right) in a persistent AF patient. Maximum activation latency is less during rapid pacing (14 msec) than baseline (82 msec). Activation latency restitution for the same patient (B) show rapid pacing suppresses maximum activation latency (red) versus baseline S1S2 pacing (blue). Rapid pacing steepens action potential duration (APD) restitution versus S1S2 pacing, as illustrated in the APD restitution plot (C) from this patient. Atrial fibrillation was inducible with rapid pacing in this patient (D), but not at baseline.
In persistent AF patients, rapid pacing also increased APD restitution slope (example patient illustrated in figure 3C) compared to single PACs (1.1±0.5 vs 0.4±0.3, p=0.04). APD restitution slope was >1 for 8/12 persistent AF patients during rapid pacing versus 2/12 with single PACs (p=0.03). Like isoproterenol, rapid pacing decreased minimum atrial APD vs baseline (115±11 vs 185±39, p=0.02). Figure 3D shows AF initiation during rapid pacing in a persistent AF patient in whom AF was not inducible with single extrastimulus pacing.
Effects of Adenosine on APD and Activation Latency Restitution
Like isoproterenol and rapid pacing, adenosine infusion shortened minimum APD versus baseline (183±19 vs 207±19 msec, p=0.02). Unlike isoproterenol and rapid pacing, however, adenosine increased maximum activation latency (86±27 vs 74±22 ms, p=0.004) and increased minimum diastolic interval versus baseline (20±19 vs −1±17 msec, p=0.04). Figures 4A shows prolongation of activation latency during adenosine (red) versus baseline (blue) in a persistent AF patient.
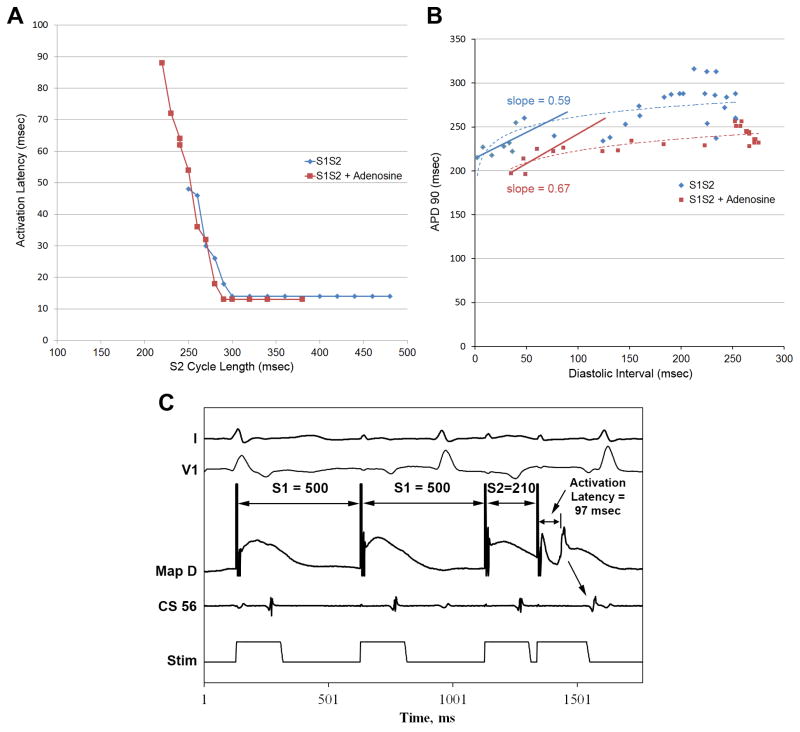
Figure 4.
(A) Adenosine allows more premature extrastimuli to conduct, but with greater activation latency in a persistent AF patient. (B) In the same patient, action potential duration (APD) restitution slope is <1 at baseline (blue) and during adenosine infusion (red). In a separate persistent AF patient, an extrastimulus (210 msec) fails to induce AF during adenosine infusion. Note substantial activation latency (97 msec).
Adenosine infusion did not alter APD restitution slope for patients with persistent AF (0.4±0.2 vs 0.3±0.3, p=0.3), and did not facilitate AF. Figure 4B illustrates atrial APD restitution at baseline (blue) and during adenosine (red) in a persistent AF patient. Restitution is shifted rightward due to greater activation latency at short coupling intervals with no significant change in slope. Figure 4C shows significant activation latency during adenosine in a persistent AF patient and no AF initiation.
Isoproterenol and Rapid Pacing in Control Patients
In control patients, isoproterenol did not change minimum APD, minimum diastolic interval, or maximum activation latency versus baseline (see online supplement, table S2). Figure 5A shows activation latency curves at baseline and during isoproterenol in a control. Importantly, isoproterenol did not significantly increase APD restitution slope, and no controls had APD restitution slope >1 with isoproterenol. Figure 5B shows flat APD restitution curves at baseline and during isoproterenol in a control patient. Figure 5C shows failure of single extrastimulus pacing to initiate AF in a control patient just above atrial ERP during isoproterenol.
Figure 5.
(A) Activation latency curves in a representative control patient at baseline (blue) and during isoproterenol (red) show a similar reduction in minimum conducted S2 and maximum activation latency to AF patients. However, action potential duration restitution slope (B) remains <1 during isoproterenol, as shown in this representative patient. (C) shows failure of shortest conducted extrastimulus to induce AF in a control patient during isoproterenol.
Rapid pacing decreased maximum activation latency and increased minimum diastolic interval compared to baseline and isoproterenol (online supplement, table S2). Like isoproterenol, rapid pacing did not steepen APD restitution slope > 1 in controls, and AF was not induced.
Mechanisms for AF Initiation: Insights from Computational Modeling
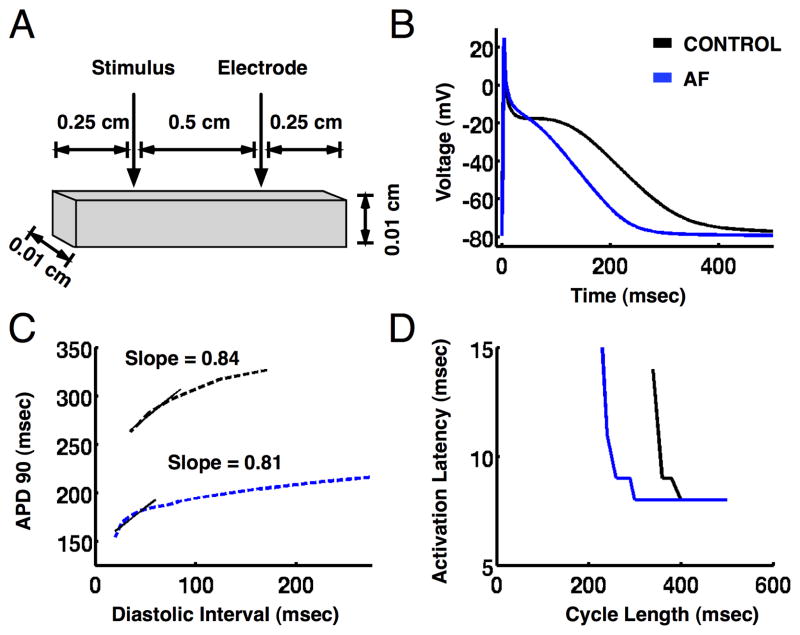
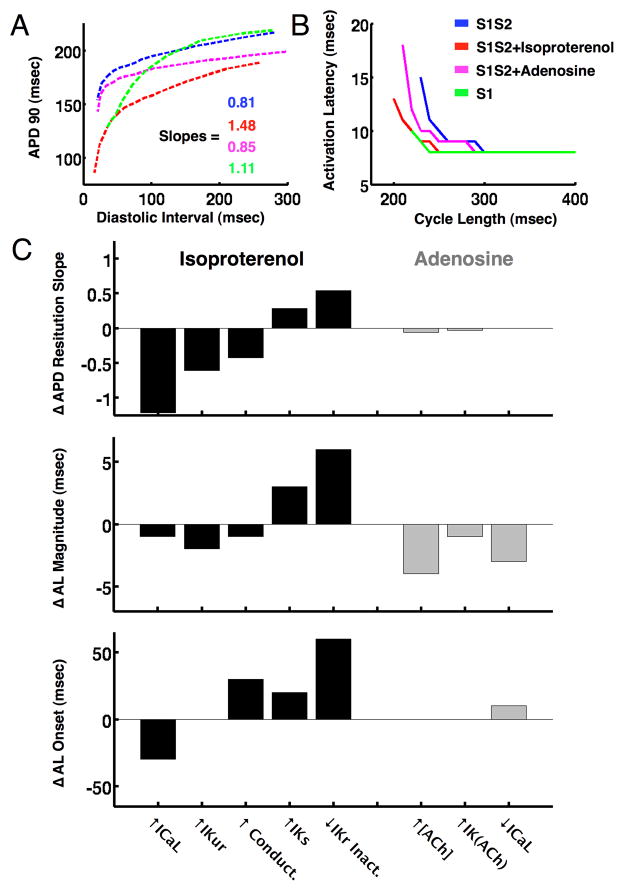
In the atrial tissue model (figure 6A), reducing ICaL, Ito and IKur to simulate AF electrical remodeling16 reproduced the changes in APD (figure 6B), APD restitution slopes (figure 6C), and activation latency dynamics (figure 6D) observed clinically. Simulating the effects of rapid pacing, isoproterenol, and adenosine via changes in ion channel densities and kinetics reproduced alterations in APD restitution slope (Figure 7A) and activation latency dynamics (Figure 7B) consistent with the clinical data (online supplement figures S1–3, and Table S1).
Figure 6.
Action potential duration (APD) and activation latency restitution in a computer model of human atrial tissue. (A) Atrial tissue dimensions. (B) Action potentials for pacing cycle length of 500 ms. (C) APD restitution and (D) activation latency restitution for control (black) and persistent atrial fibrillation (blue) models during extrastimulus pacing.
Figure 7.
Action potential duration (APD) and activation latency (AL) restitution for rapid pacing, isoproterenol and adenosine in the atrial fibrillation tissue model. (A) Isoproterenol and rapid pacing (S1) increased APD restitution slope >1, while adenosine did not. (B) Isoproterenol and rapid pacing (S1) decreased maximum activation latency, while adenosine increased maximum activation latency (C). Differences in APD restitution slope (top), activation latency magnitude (middle) and activation latency onset (bottom) between the isoproterenol and adenosine AF models (see online supplement for details, note that differences are the inverse of the contribution of each ionic current modification shown in panels A and B).
Modeling revealed that APD restitution in AF was steepened by isoproterenol via 3 mechanisms: increased ICaL, IKur and tissue conductivity (Figure 7C, top left), with the ICaL effect predominating. The simulated activation latency was shifted to shorter pacing coupling intervals by primarily by reduced IKr inactivation and to a lesser extent by increased IKs (Figure 7C, middle left, additional data in online supplement figure S2).
Once increased extracellular potassium ([K+]o) was incorporated into the model (see online supplement), we found that rapid pacing increased the slope of APD restitution by a rate-dependent elevation in IK1 (online supplement figure S1).
Our model of adenosine intervention in AF found that increased IKACh (via enhanced conductance) and elevated extracellular acetylcholine concentration tended to increase APD restitution slope. However, IKACh is a relatively small current when compared to the other potassium currents involved in repolarization, and overall there was no significant change in APD restitution slope with adenosine. All three parameter modifications increased activation latency (Figure 7C, middle right, additional data in online supplement figure S3), with [ACh] elevation predominating.
Discussion
In studying why clinical interventions initiate AF in patients with varying extents of atrial remodeling, we report 3 major findings. First, our data support the concept that steep APD restitution was a final ‘pathway’ for clinical interventions that initiated human AF. Second, we show that activation latency truncates the leftmost portion of APD restitution in patients with atrial remodeling (persistent AF). Adrenergic stimulation and tachycardia decreased activation latency to engage steep APD restitution and initiate AF. Third, these observations and computational modeling revealed mechanistic differences in the effects of isoproterenol and rapid pacing on AF initiation. These results provide fundamental insights into the mechanisms of AF initiation in patients with and without atrial remodeling that may assist in designing preventive interventions for AF.
Steep APD Restitution as a Common Pathway Preceding AF Initiation
Steep APD restitution is known to amplify APD fluctuations, ultimately leading to local conduction block, unidirectional wavebreak, and reentry.17 Previously, steep APD restitution has been shown to facilitate arrhythmia initiation during single extrastimulus pacing with no other pro-arrhythmic interventions in paroxysmal AF patients.5 However, the mechanisms for AF initiation in patients with remodeled atria and persistent AF, and how isoproterenol and sustained rapid pacing are clinically profibrillatory, had been unclear.
Isoproterenol is commonly used to initiate AF.7 However, prior reports have not identified whether it operates via APD shortening,18 APD restitution steepening9 or other mechanisms. Rapid pacing is also commonly used to initiate AF in the EP laboratory.7 Tachycardia has been shown to steepen APD restitution in an open chest dog model,19 but the applicability of this finding, particularly in patients with persistent AF and remodeled atria, was uncertain.
In this work, we found that clinical interventions which steepen APD restitution >1, including isoproterenol and rapid pacing, increase AF incidence regardless of other properties such as minimum APD. We also show that adenosine, which did not steepen APD restitution at studied doses, did not increase AF incidence, despite decreasing minimum APD akin to isoproterenol and rapid pacing. In control patients without AF, no intervention steepened APD restitution >1, and none initiated AF.
Prior work has reported that AF is not dependent on APD restitution in a canine model, and that AF had variable effects on APD restitution during arrhythmia.20 Our findings may differ for several reasons: (a) we examined AF in patients with clinical atrial remodeling versus a canine acetylcholine-AF model, and (b) we examined the mechanisms of AF initiation, rather than those that perpetuate AF. Interestingly, recent animal studies also support the role of steep APD restitution in AF initiation from rapid pacing.19
Isoproterenol and Tachycardia Decrease Activation Latency to Engage Steep APD Restitution
Prior work has suggested that greater activation latency in persistent AF patients prevented sufficiently premature extrastimuli from activating atrial tissue, thus obscuring the leftmost portions of APD restitution that were steep (>1) in patients with paroxysmal AF.5
The impact of tissue activation latency upon the maximum rate of cardiac depolarization has been explored by Koller et al.6 Using MAP catheters in human atria, they concluded that local activation latency (a) was predominantly due to activation delay, but also conduction slowing, and (b) curbed the targeted atrial response interval. Although these findings should truncate the leftmost portion of APD restitution, no studies to our knowledge have examined the relationship between activation latency and APD restitution, or its relationship to human AF initiation.
In this study, we found that activation latency is greater in persistent AF patients (with maximum APD restitution slope <1) than paroxysmal AF (with maximum APD restitution slope >1). Interventions that decreased activation latency (isoproterenol and rapid pacing) steepened APD restitution and increased AF incidence. Thus, in patients with persistent AF in whom AF could not be initiated by PACs at baseline, these interventions reduced activation latency to increase maximum APD restitution slope to > 1, a proarrhythmic condition that favors alternans, conduction block, and wavebreak.17
Quantifying a Pro-fibrillatory Spectrum of Electrical Remodeling
This study suggests that APD restitution and activation latency may characterize phenotypes of atrial remodeling relevant for human AF initiation. Compared to patients with paroxysmal AF with APD restitution slope >1, patients with persistent AF had substantial activation latency and baseline APD restitution slope <1. Control patients had neither steep APD restitution nor substantial activation latency. This mechanistic spectrum is consistent with clinical presentations. In persistent AF, conduction slowing (activation latency) facilitates reentrant AF but also reduces the dependence upon AF triggers. The onset and perpetuation of persistent AF are facilitated by both isoproterenol and rapid pacing, which steepen APD restitution in this study, but not by continuous-infusion adenosine, which did not steepen APD restitution. Paroxysmal AF is triggered by PACs, consistent with steep APD restitution, as we have shown. Finally, control patients exhibit neither steep APD restitution nor substantial activation latency.
Conversely, other measurable indices of electrical remodeling were less clearly linked to AF onset. Adenosine significantly shortened APD, yet it increased activation latency at these doses and did not increase AF incidence. Thus, short minimum APD alone did not predict steep APD restitution or clinical AF initiation.
Different Ionic Mechanisms Underlie the Proarrhythmic Effects of Isoproterenol and Tachycardia
Detailed computer modeling provides novel insight into the pro-fibrillatory ionic mechanisms of these clinical interventions. Specifically, we found that isoproterenol and rapid pacing in persistent AF increased the slope of APD restitution above 1 via distinct mechanisms. First, the alteration of calcium dynamics from increased ICaL by isoproterenol significantly steepened APD restitution slope. This finding is consistent with the results by Gong et al,21 demonstrating that when ICaL is increased in the setting of shortened atrial APD, APD restitution slope steepens, promoting AF initiation. Second, potassium accumulation during rapid pacing increased the slope of APD restitution to >1 by a rate-dependent elevation in IK1, thereby promoting AF initiation. This result is consistent with prior work demonstrating that elevated IK1 plays a significant role in rate-dependent APD shortening.22
Discrepancies with Prior Work
In our study, we did not see an increase in atrial arrhythmias with adenosine unlike prior work.23 This may be explained by differences in the mode of administration: we administered adenosine as a constant infusion while prior work delivered adenosine as a bolus. Bolus infusion, however, is impractical for evaluating APD restitution due to (a) the short half-life of adenosine and the time required for ADPR studies (several minutes), and (b) heart block caused by higher doses of adenosine. Infused adenosine doses were likely sufficient since this approach is validated as an effective strategy for cardiac stress imaging.14
Clinical Implications
These results suggest that APD restitution and activation latency may characterize phenotypes of atrial remodeling relevant for human AF initiation. Our results may explain why drugs with specific targets (e.g. class III agents prolonging atrial ERP) fail to control AF in isolation, since AF is initiated and sustained by multiple mechanisms. Moreover, these results may help to inform future strategies to prevent AF initiation, such as interventions that inhibit multiple pro-fibrillatory ionic mechanisms.
Limitations
First, we focused on APD restitution near the superior PVs. Therefore, further studies should define regional differences24 and their relationship to AF initiation. Second, AF did not induce with PACs in all patients with steep restitution. Future studies should determine what other factors are required for AF initiation with a single premature beat. Third, some patients presented in AF and others in sinus rhythm, questioning the contribution of recent AF to our results. Although paroxysmal AF patients typically presented in sinus rhythm and persistent AF patients in AF, two paroxysmal AF patients presented in AF, and one persistent AF patient presented in sinus rhythm. Their EP properties followed their respective groups, mitigating this concern. Fourth, patients were sedated during the study, which may alter autonomic balance. Fifth, activation latency is the sum of tissue latency and conduction velocity slowing. Our atrial tissue monodomain model does not include tissue latency, only conduction velocity changes. This may explain some of the discrepancies between modeling and clinical data, particularly the shorter activation latency magnitude in the model compared to MAPs recorded in patients. More detailed bidomain computer models are required for more accurate simulation of activation latency, and are in preparation in our laboratory. Nevertheless, modeled trends matched observed data, providing insight into underlying mechanisms. Sixth, in our model we quantify APD and activation latency restitution based on membrane voltage and action potential propagation, for which MAP recordings are only a surrogate. Seventh, the CRN model15 cannot fully reproduce the effects of all the pharmacological interventions used in this study, such as PKA-dependent phosphorylation by isoproterenol. However, it is unique in that it has been validated for use in tissue and whole organ level simulations at fast rates.25 Once newer human atrial cell models are formulated for use in tissue and organ level simulation studies, such as those by Grandi et al,26 which include a robust B-adrenergic framework, they could be used to provide additional insight into these effects. Eighth, rate-dependent elevation of [K+]o during rapid pacing is not an intrinsic feature of the CRN model; our modifications to the model may introduce inaccuracies in the calculation of the potassium dependent sarcolemmal currents. Ninth, our AF model does not include atrial structural remodeling such as fibrosis, which could potentially alter conduction. Tenth, we were unable to perform all measures in all patients, increasing the potential impact of confounders. Finally, studies with a more balanced gender mix are required.
Conclusions
Clinical induction methods promote AF initiation by various mechanisms. Steepening of APD restitution slope to >1 promoted AF initiation, while AF did not initiate when APD restitution slope was < 1. Isoproterenol and rapid pacing steepened maximum APD restitution slope by decreasing activation latency thus enabling AF initiation. Computational modeling revealed that these changes were due to increased ICaL and decreased IKr inactivation with isoproterenol, and to increased IK1 during rapid pacing. Accordingly, these data may help design strategies to prevent AF.
Supplementary Material
Clinically, atrial fibrillation (AF) is often refractory to antiarrhythmic medical management. A possible explanation is that narrowly-targeted drug therapy cannot completely prevent the development of AF if there are multiple ionic pathways to the pro-arrhythmic substrate underlying the arrhythmia. To test this hypothesis, we examined the electrophysiologic changes which occurred during different interventions used to initiate AF in the electrophysiology laboratory. We studied patients with a spectrum of AF ranging from persistent and paroxysmal AF to controls without AF. We evaluated three electrophysiologic interventions: isoproterenol infusion, rapid pacing, and adenosine infusion. Isoproterenol infusion and rapid pacing increased AF incidence in this population. Importantly, the final common pathway of AF initiation for both interventions was the pro-arrhythmic state of steep action potential duration (APD) restitution. In steep APD restitution, fluctuations in the diastolic interval or action potential duration are amplified over time, resulting in conduction block and reentry. We also found that both of these interventions decreased activation latency in patients with severely remodeled atria (e.g. persistent AF patients) to allow steep APD restitution. Detailed computer modeling revealed that isoproterenol and rapid pacing achieved steep APD restitution via distinct ionic pathways. These data support the idea that steep APD restitution is central to human AF initiation, and that multiple ionic alterations may lead to this pro-arrhythmic state. Future studies should evaluate the success of antiarrhythmic strategies which block several of the predicted mechanisms of steep APD restitution simultaneously.
Acknowledgments
We are indebted to Kathleen Mills, BA for coordinating this study.
Funding Sources: American Heart Association 10BGIA3500045 and NIH (DEK), American Heart Association (JDB), National Science Foundation and NIH (NAT), and NIH (HL 70529, HL 83359) and Doris Duke Foundation (SMN).
Footnotes
Conflict of Interest Disclosures: Dr. Krummen has served as a consultant to InsilicoMed, and has received fellowship program support from Medtronic, Boston Scientific, St. Jude, Biotronik, and Biosense-Webster. Mr. Bayer, Dr. J. Ho, Dr. G. Ho, Ms. Smetak, and Mr. Clopton have no disclosures. Dr. Trayanova has served as a consultant to CardioSolv. Dr. Narayan has intellectual property and ownership interest in Topera Medical, and has served as a consultant to InsilicoMed. He has received fellowship program support from Medtronic, Boston Scientific, St. Jude, Biotronik, and Biosense-Webster.
References
- 1.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry EM, Ehrlich JR, Nattel S, Fenton FH. Pulmonary vein reentry--properties and size matter: Insights from a computational analysis. Heart rhythm. 2007;4:1553–1562. doi: 10.1016/j.hrthm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: The saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 4.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 5.Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: A mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koller BS, Karasik PE, Solomon AJ, Franz MR. Prolongation of conduction time during premature stimulation in the human atrium is primarily caused by local stimulus response latency. European heart journal. 1995;16:1920–1924. doi: 10.1093/oxfordjournals.eurheartj.a060848. [DOI] [PubMed] [Google Scholar]
- 7.Crawford T, Chugh A, Good E, Yoshida K, Jongnarangsin K, Ebinger M, Pelosi F, Jr, Bogun F, Morady F, Oral H. Clinical value of noninducibility by high-dose isoproterenol versus rapid atrial pacing after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:13–20. doi: 10.1111/j.1540-8167.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 8.Turley AJ, Murray S, Thambyrajah J. Pre-excited atrial fibrillation triggered by intravenous adenosine: A commonly used drug with potentially life-threatening adverse effects. Emerg Med J. 2008;25:46–48. doi: 10.1136/emj.2007.051227. [DOI] [PubMed] [Google Scholar]
- 9.Taggart P, Sutton P, Chalabi Z, Boyett MR, Simon R, Elliott D, Gill JS. Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation. 2003;107:285–289. doi: 10.1161/01.cir.0000044941.13346.74. [DOI] [PubMed] [Google Scholar]
- 10.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 11.Franz MR, Karasik PL, Li C, Moubarak J, Chavez M. Electrical remodeling of the human atrium: Similar effects in patients with chronic atrial fibrillation and atrial flutter. J Am Coll Cardiol. 1997;30:1785–1792. doi: 10.1016/s0735-1097(97)00385-9. [DOI] [PubMed] [Google Scholar]
- 12.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res. 2007;73:750–760. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Mohiuddin SM, Esterbrooks DJ, Gupta NC, Hilleman DE. Safety of different dosages of intravenous adenosine used in conjunction with diagnostic myocardial imaging techniques. Pharmacotherapy. 1993;13:476–480. [PubMed] [Google Scholar]
- 15.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am J Physiol. 1998;275:H301–321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 16.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: Insights from a mathematical model. Cardiovascular research. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 17.Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, Karagueuzian HS, Weiss JN, Chen PS. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stambler BS, Wood MA, Ellenbogen KA. Pharmacologic alterations in human type i atrial flutter cycle length and monophasic action potential duration. Evidence of a fully excitable gap in the reentrant circuit. J Am Coll Cardiol. 1996;27:453–461. doi: 10.1016/0735-1097(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Cui B, He B, Hu X, Wu W, Wu L, Huang C, Po SS, Jiang H. Distinct restitution properties in vagally mediated atrial fibrillation and six-hour rapid pacing-induced atrial fibrillation. Cardiovascular research. 2011;89:834–842. doi: 10.1093/cvr/cvq334. [DOI] [PubMed] [Google Scholar]
- 20.Burashnikov A, Antzelevitch C. Role of repolarization restitution in the development of coarse and fine atrial fibrillation in the isolated canine right atria. J Cardiovasc Electrophysiol. 2005;16:639–645. doi: 10.1046/j.1540-8167.2005.40689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, Christini DJ. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: A simulation study. Circulation. 2007;115:2094–2102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 22.Williams BA, Dickenson DR, Beatch GN. Kinetics of rate-dependent shortening of action potential duration in guinea-pig ventricle; effects of ik1 and ikr blockade. Br J Pharmacol. 1999;126:1426–1436. doi: 10.1038/sj.bjp.0702443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbisiero R, Kabell G, Cook JR, Fitzgerald TF, Kirchhoffer JB. Effects of adenosine on local stimulus-response latency and induction of atrial fibrillation by premature stimuli. Pacing and clinical electrophysiology : PACE. 1999;22:1378–1385. doi: 10.1111/j.1540-8159.1999.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 24.Fareh S, Villemaire C, Nattel S. Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98:2202–2209. doi: 10.1161/01.cir.98.20.2202. [DOI] [PubMed] [Google Scholar]
- 25.Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV, Zhang H. 3d virtual human atria: A computational platform for studying clinical atrial fibrillation. Progress in biophysics and molecular biology. 2011;107:156–168. doi: 10.1016/j.pbiomolbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and ca2+ model: Sinus rhythm and chronic atrial fibrillation. Circulation research. 2011;109:1055–1066. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.