Abstract
Although the cure rate of newly diagnosed acute lymphoblastic leukemia (ALL) has improved over the past four decades, the outcome for patients who relapse remains poor. New therapies are needed for these patients. Our previous global gene expression analysis in a series of paired diagnosis-relapse pediatric patient samples revealed that the antiapoptotic gene survivin was consistently upregulated upon disease relapse. In this study, we demonstrate a link between survivin expression and drug resistance and test the efficacy of a novel antisense agent in promoting apoptosis when combined with chemotherapy. Gene-silencing experiments targeting survivin mRNA using either short-hairpin RNA (shRNA) or a locked antisense oligonucleotide (LNA-ON) specifically reduced gene expression and induced apoptosis in leukemia cell lines. When used in combination with chemotherapy, the survivin shRNA and LNA-ON potentiated the chemotherapeutic antileukemia effect. Moreover, in a mouse primary xenograft model of relapse ALL, the survivin LNA-ON decreased survivin expression in a subset of animals, and produced a statistically significant decrease in tumor progression. Taken together, these findings suggest that targeting endogenous levels of survivin mRNA by LNA-ON methods may augment the response to standard chemotherapy by sensitizing otherwise resistant tumor cells to chemotherapy.
Keywords: survivin, pediatric, acute lymphoblastic leukemia, EZN-3042
Introduction
Acute lymphoblastic leukemia (ALL) is the most common form of childhood cancer. Although long-term survival rates approach 80% for B-precursor ALL, outcomes for those who relapse are poor. We recently performed gene expression profiling on matched pairs of leukemic blasts from individual patients at diagnosis and relapse.1 This study identified a set of 117 genes that were differentially expressed between diagnosis and relapse in B-precursor ALL. We focused on survivin (BIRC5), a gene upregulated at leukemia relapse, given its involvement in many other cancers, as well as the availability of therapeutic agents to abrogate survivin expression and/or function.
Survivin, a dual regulator of cell-cycle progression and apoptosis (reviewed in Knauer et al.2 and Pennati et al.3) is overexpressed in practically all human cancers, but is low in most normal terminally differentiated tissues with the exception of liver, ovary, testes and hematopoietic progenitor cells.4 Several mechanisms of deregulation have been reported, including transcriptional and post-transcriptional modifications, chromosomal amplification and subcellular relocalization.5–11 Survivin has been shown to be upregulated in response to several chemotherapeutic agents, such as doxorubicin and paclitaxel.12–14 Also, expression of survivin is inversely correlated with a good therapeutic response and has been used as a prognostic marker for treatment resistance in some tumor types, such as breast cancer, bladder cancer and lymphoma.15–20 Thus, survivin is an attractive target in cancer therapy and currently, several clinical trials employing different approaches including antisense oligonucleotides, small molecule inhibitors and immunotherapy are in progress.3,21
Given our finding of upregulation of survivin at relapse,1 we hypothesized that targeting survivin would be an effective treatment strategy in relapsed ALL and that downregulation of survivin expression would improve the response of ALL cells to conventional cytotoxic chemotherapy. In this study, we used antisense technologies to downregulate the expression of survivin in leukemia cell lines to determine the impact of targeting survivin in vitro and to assess the effect on chemotherapy response. We also used a murine xenograft model of adult and pediatric relapsed ALL to assess the effect of the antisense in vivo. As controls, we modulated the expression of securin/pituitary tumor transforming gene1 (PTTG1), topoisomerase II alpha (TOP2A) and cyclin B1 (CCNB1), other genes shown to have increased expression at relapse.1 These studies demonstrated that downregulation of survivin or securin with shRNAs induced apoptosis in ALL cell lines and potentiated the antileukemic effect of chemotherapy, lowering the IC50’s to etoposide, doxorubicin and predisolone by as much as threefold. Furthermore, we demonstrated the antitumor effect of a novel third generation antisense drug EZN-3042, which specifically targets survivin, in treating pediatric ALL in a primary mouse xenograft model. These data suggest that the use of EZN-3042 in combination with chemotherapy may be beneficial for patients with relapsed ALL.
Materials and methods
Plasmids, cell lines and transfections
Molt4 (T-ALL), Reh (B-ALL), RS4;11 (B-ALL) and MV4;11 (B-ALL) cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum, 10mm 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1%Pen/Strep under 5% CO2 at 37°C. All cell lines were purchased from American Type Culture Collection and are authenticated according to their protocols (http://www.atcc.org). Short-hairpin RNA (shRNA) vectors were created by annealing single-stranded oligonucleotides and ligating them into the pSiren-ZsGreen expression vector (Clontech, Mountain View, CA, USA). The targeted sequences were as follows: survivin (5′-TGACGACCCCA TAGAGGAA-3′), securin (5′-CCTGCAATAATCCAGAATG-3′), cyclin B1 (5′-TGCAGAAGATGGAGCTGAT-3′) and TOP2A (5′-CTGCTAGTCCACGATACAT-3′). A control shRNA with scrambled sequence was provided by Clontech. Control (EZN-3046)- and survivin (EZN-3042)-targeted locked antisense oligonucleotide (LNA-ON) were provided by Enzon (Piscataway, NJ, USA).22 Transfections of Molt4 cells were performed using the Nucleofector Kit V from Lonza (Walkersville, MD, USA) with the following optimized parameters: 2 × 106 cells were resuspended in 100 (µl solution V, mixed with 5 µg of plasmid or 9.4 µm LNA-ON and transfected by nucleofection using program A-30. Transfections of B-precursor ALL cells were performed using the 96-well Lonza shuttle with the following optimized parameters: 0.4 × 106 cells were resuspended in 20 µl solution SF (Reh & MV4;11) or SE (RS4;11), mixed with 3.3 µm of LNA-ON and transfected with program DS137 (Reh), DS150 (MV4;11) or DC100 (RS4;11). For gymnotic delivery experiments,23 Reh cells were plated at a concentration of 1 × 106 cells/ml and 10 µM LNA-ON was added directly to the media. Stock solutions of dexamethasone (Sigma-Aldrich, St Louis, MO, USA), doxorubicin (Sigma-Aldrich), vincristine (Sigma-Aldrich) and L-asparaginase (Sigma-Aldrich) were prepared in H20, etoposide (Sigma-Aldrich) was prepared in dimethyl sulfoxide (stock 40 mm) and prednisolone (Pharmacia, St Paul, MN, USA) was prepared in 0.9% NaCl. Drugs were serially diluted in RPMI and added to the culture media at indicated concentrations. Cells were incubated with chemotherapy for 24 h except vincristine and L-asparaginase, which were incubated for 48 h.
Quantitative reverse transcription PCR analysis
To determine the relative expression of each gene of interest, total RNA was extracted using the RNEasy Mini Kit (Qiagen, Valencia, CA, USA), and reverse transcription (RT)-PCR was performed using I-Script II complementary DNA Synthesis kit (Biorad, Hercules, CA, USA) and QuantiTect SYBR Green PCR kit (Qiagen). Synthesis of PCR products was monitored by the DNA Engine Opticon System (MJ Research, Waltham, MA, USA) and normalized to B2microglobulin amplification. Data were plotted relative to mRNA levels in control samples using the ΔΔCt method. The PCR primers used were as follows: B2microglobulin (5′-atgtgtctgggtttcatccatcc-3′and 5′-AGTCAC ATGGTTCACACGGCA-3′), survivin common primers (5′-CAT CTCTACATTCAAGAACTGG-3 and 5′-GGTTAATTCTTCAAACT GCTTC-3′), survivin main isoform (5′-CCACCGCATCTCTACA TTCA-3′ and 5′-TATGTTCCTCTATGGGGTCG-3′), survivin 2B isoform (main forward and 5′-AGTGCTGGTATTACAGGCGT-3′), survivin ΔEX3 isoform (main forward and 5′-TTTCCTTTGCA TGGGGTC-3′), survivin 2α isoform (5′- CAGTGTTTCTTCTGC TTCAAGG 3′ and 5′- GCAACCCTCCCATACTAAGTGTC 3′), survivin 3B isoform (5′- ACAGAGAAAGAGCCAAGAAC 3′ and 5′- TTTTGCTTCCAGTCCCTCCCTG 3′), securin (5′-TTTCTGCC AAAAAGATGACT-3 and 5′-GAGACTGCAACAGATTGGAT-3), TOP2A (5′-CTGATTCAGAGGGGATATGA-3′ and 5′-CCACAAA TCTGATGGACTCT-3′), and cyclin B1 (5′-TGACTTTGCTTTTGT GACTG −3′ and 5′-GTGTCCATTCACCATTATCC-3′).
Immunoblotting
For survivin, securin and cyclin B1 immunoblots, cell lysates were prepared as previously described,24 with the following primary antibodies and dilutions: antisurvivin, 1:100 (sc-17779, Santa Cruz Biotechnology, Santa Cruz, CA, USA) antisecurin, 1:200 (DCS-280 Abcam, Cambridge, MA, USA) and anticyclin B1 1:2000 (4135, Cell Signaling Technology, Danvers, MA, USA). Horseradish peroxidase-conjugated antimouse IgG, 1:10,000 (NA931V, GE Healthcare, Piscataway, NJ, USA) was used as the secondary antibody. TOP2A immunoblots were prepared according to the manufacturer’s instructions with the primary antibody antiTopo-II p170, 1:1000 (#2011-3, TopoGEN, Port Orange, FL, USA) and secondary antibody horseradish peroxidase-conjugated antirabbit IgG, 1:10 000 (NA934V, GE Healthcare). Signals were visualized using ECLplus (GE Healthcare).
Apoptosis assay
Apoptosis was determined at 48–72 h after transfection by Annexin V-PE and 7AAD staining (Annexin V-PE Apoptosis Detection Kit, BD Pharmingen, San Diego, CA, USA) followed by flow cytometry using the FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA). The percentage of Annexin V/7AAD positive and negative cells were analyzed by FlowJo software (version 7.2.5, Tree Star Inc., Ashland, OR, USA). For shRNA experiments, the population was gated on green fluorescent protein-positive cells to limit the analysis to transfected cells. Data were plotted relative to control transfection with no chemotherapy treatment. The IC50 values were determined by the Hill equation using KaleidaGraph software version 4.0 (Synergy Software, Reading, PA, USA), and the potentiation factor was calculated as the ratio of IC50 value of the drug plus EZN3046 (scrambled control)/IC50 value of drug plus EZN3042 (antisurvivin).
Cell-cycle analysis
Cell-cycle analysis was performed on Molt4 cells 48 h after transfection with control or survivin LNA-ON. Cells were stained with propidium iodide and analyzed by flow cytometry. Fluorescence intensity was measured and plotted relative to cell count.
In vivo drug testing using nonobese diabetic/severe combined immunodeficiencyxenografts
Under Institutional Animal Care and Use Committee and Institutional Review Board- approved protocols, nonobese diabetic/severe combined immunodeficiency primary xenografts were generated from four B-precursor ALL patient samples (ID 2365, 240, 4092 and 200) as previously described.25 Mice were maintained in sterile micro-isolator cages, housed in ventilated racks and given antibiotic prophylaxis with trimethoprim/sulfamethoxazole in drinking water. Mice were injected with 2–5 × 106 lymphoblasts from patient samples that had been previously xenografted into mice for expansion. Starting at 3–5 weeks post-injection, mice were screened for engraftment weekly by retro-orbital sampling of peripheral blood. Engraftment was detected by flow cytometry using antibodies recognizing CD19 and CD45 (Beckman Coulter, Brea, CA, USA). All antibodies are non-crossreactive to mouse proteins. After ALL engraftment, defined as greater than 5% peripheral blasts, mice were randomized to treatment with 25 mg/kg of ENZ-3042 (survivin LNA-ON) intraperitoneally (I.P) 3 days a week (Monday, Wednesday and Friday) vs scrambled control (ENZ-3046). After seven treatments, the dose was reduced to 12.5 mg/kg due to weight loss from decreased oral intake. ENZ-3042 and scrambled control were reconstituted in H20. Response to treatment was measured weekly by retro-orbital bleeds to determine white blood cell counts and percentage of lymphoblasts by flow cytometry with antibodies recognizing CD19 and CD45. A subset of mice was killed at day 14 and RNA was prepared from the spleen and bone marrow to determine knockdown of target by quantitative RT-PCR. To compare the efficacy of ENZ-3042 with other cytotoxic and biological compounds using the same model, mice engrafted with ID 240 were also treated with temsirolimus 5 mg/kg intraperitoneally daily, 5 days a week (Wyeth Pharmaceuticals, Philadelphia, PA, USA), methotrexate 5–10 mg/kg intraperitoneally weekly (Mayne Pharmaceuticals, Paramus, NJ, USA) or the diterpenoid inhibitor of XIAP, triptolide26 0.15 mg/kg intraperitoneally daily, 5 days a week (Calbiochem, San Diego, CA, USA) as previously described.25
Results
Previously, we identified a cohort of genes that were differentially expressed at relapse compared with diagnosis in 35 pediatric ALL patients.1 Survivin was identified as a gene that was significantly upregulated in ALL relapse samples. In a follow-up study of 49 additional pairs (manuscript in preparation), survivin was ranked 22 out of a total of 148 differentially expressed genes (1.49-fold change, P=0.002) for patients with early relapse (27 samples <36 months from initial diagnosis) and 25 out of 180 genes differentially expressed (1.56-fold change, P=0.003) for patients with late relapse (22 samples ≥36 months from initial diagnosis), indicating that survivin is overexpressed in pediatric relapsed ALL.27 In addition to survivin, three other genes, securin, cyclin B1 and TOP2A, known to be involved in cell-cycle regulation and apoptosis, and implicated in cellular transformation, were also identified as significantly upregulated.1 To determine whether these genes are directly involved in mediating the well-known chemoresistance of relapsed ALL, we modulated expression of these transcripts in pre-clinical models.
To acutely knockdown gene expression, a series of shRNA constructs targeting each gene was developed. Transfection of the T-ALL cell line Molt4 with cyclin B1, TOP2A, securin and survivin shRNA vectors reduced endogenous mRNA expression by 70–90%, respectively, as determined by quantitative RT-PCR at 48 h post-transfection (Figure 1a). Western blot analysis confirmed that the levels of these proteins in the cell were also decreased (Figure 1b). A time course analysis determined that peak knockdown in survivin protein expression was achieved by 48 h post-transfection.
Figure 1.
Downregulation of relapse-associated genes by short-hairpin inhibitory RNA sequences. (a) shRNA constructs targeting survivin, securin, TOP2A and cyclin B1 effectively reduce mRNA expression as determined by RT-PCR at 48 h after transfection. Results are expressed relative to transfection with scrambled shRNA (control)±s.d. (b) Determination of concordant changes in protein expression by western blot at indicated time points in Molt4 cells.
The effect of downregulating expression of these genes on cell viability was determined by performing annexin-V PE/7AAD staining at 48 h following the transfection. The transfection of Molt4 cells with the green fluorescent protein-scrambled control shRNA caused, on average, 15% background apoptosis, which was subtracted from the analysis. Knocking down TOP2A and cyclin B1 expression with the shRNA constructs did not affect cell viability, nor did their knockdown potentiate cellular response to etoposide (Figure 2a), implying that overexpression of either TOP2A or cyclin B1 at relapse does not directly contribute to chemoresistance. Decreasing TOP2A antagonized etoposide at lower doses, consistent with the mechanism of action of this drug. In contrast, knockdown of endogenous securin induced 40% cell death and, in combination with low doses of etoposide (0.1 µm), increased apoptosis over chemotherapy alone (Figure 2a).
Figure 2.
Response of Molt4 cells to chemotherapy is altered by downregulation of target genes. (a) Molt4 cells transfected with securin, TOP2A, cyclin B1 or control shRNA constructs were incubated with increasing doses of etoposide for 24h followed by flow cytometric analysis for determination of percentage of annexin V-positive cells. (b) Transfection of control or survivin shRNA constructs into Molt4 cells followed by incubation with increasing doses of doxorubicin, etoposide, vincristine and L-asparaginase for 24–48h. Apoptosis was determined by flow cytometric analysis of annexin V-positive cells. Percentage of apoptosis is graphed relative to control shRNA in the absence of chemotherapeutic agent ±s.d. Each experiment was performed in triplicate (*P<0.01, **P<0.0001 comparing shRNA with control).
We tested whether a decrease in survivin expression would also lead to an increased sensitivity to other chemotherapy agents (doxorubicin, etoposide, L-asparaginase and vincristine). Chemotherapy was added 24 h after the cells were transfected with shRNA and chemotherapy doses, starting at a suboptimal concentration, were titrated up to a maximal cell-killing dose. Apoptosis assays were performed following 24–48 h of exposure to chemotherapy. The survivin shRNA construct alone induced a statistically significant decrease in cell viability as evidenced by 10–40% apoptosis in Molt4 cells in the absence of other drugs, (Figure 2b, **P<0.0001, 0 drug, all graphs). The survivin shRNA construct improved the cytotoxicity of the chemotherapy agents at lower concentrations, achieving a greater percentage of cell death than chemotherapy in the presence of control shRNA (Figure 2b, **P<0.0001). At the highest concentrations of chemotherapy, co-transfection with survivin shRNA had no additional effect, as maximal cell kill from chemotherapy had already been achieved.
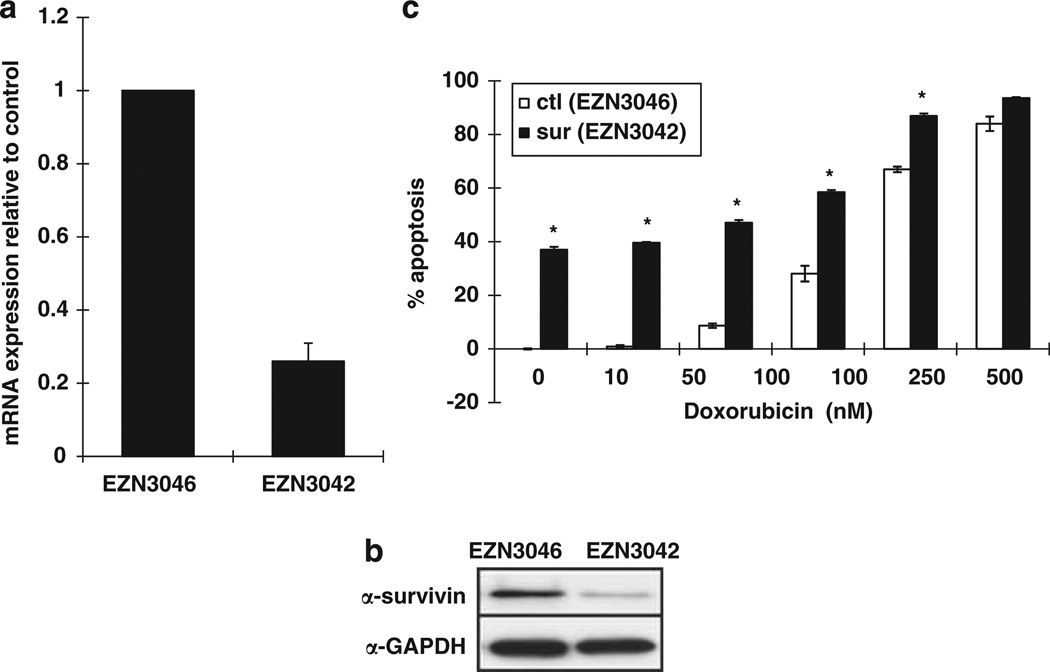
We also tested the cytotoxic effects of a locked nucleic acid oligonucleotide (LNA-ON) targeting survivin (EZN-3042) and its scrambled control (EZN-3046).22,28 LNA-ON compounds carry out modifications in the chemical structure of the oligonucleotide, which improve nuclease resistance and increase affinity for the target mRNA. EZN-3042 targets a 16mer region comprising the stop codon of the open-reading frame in exon 4 of the survivin transcript. We transfected three B-precursor ALL cell lines, Reh, RS4:11 and MV4;11, in addition to Molt4 cells, with EZN-3042 and assessed the effect on survivin expression and chemoresponse. Endogenous expression of survivin in the B-precursor ALL lines was higher, yet in the same range, as the expression of survivin in blasts purified from pediatric relapsed ALL patients (data not shown). EZN-3042 reduced survivin mRNA and protein expression by 60–80% depending on the cell line (Figures 3a and b). The control EZN-3046 had no effect on survivin expression levels. There are five splice-variants of survivin with differential effects on apoptosis;2,29 the main isoform, consisting of four exons, is antiapoptotic and most abundantly expressed in pediatric ALL samples and Molt4 and Reh cell lines (data not shown). ΔEx3 which is missing exon 3 and survivin-3B which results from an insertion of intron 3 are also antiapoptotic, whereas survivin 2B which includes part of intron 2 is pro-apoptotic. Survivin 2α, which contains a portion of intron 2 and is truncated, acts as a dominant negative of the survivin main form. Isoform-specific RT-PCR demonstrated that all five splice variants were knocked down by a least 50% with the greatest effect on survivin 2B and 2α (Figure 3c). Knockdown of survivin following transient transfection induced an average of 34, 16, 25 and 34% apoptosis above background in Molt4, Reh, MV4;11 and RS4;11 cell lines, respectively (Figures 3d and e; P<0.001). EZN-3042 significantly increased the amount of cell death in the presence of all chemotherapeutic agents tested in Molt4 cells (Figure 3d; P<0.0001). To evaluate the effect of combination therapy in B-precursor cell lines, we compared the IC50 values of the chemotherapeutic agents in the absence and presence of survivin LNA-ON. Using the Hill equation to estimate IC50 for each combination, we determined that treatment of B-precursor cells with the EZN-3042 potentiated the IC50 values for doxorubicin, prednisolone and etoposide on average 1.8, 1.7 and threefold, respectively (Figure 3e).
Figure 3.
The LNA EZN-3042 downregulatessurvivin expression and potentiates the chemotherapy killing curves in ALL cell lines. (a) Quantitative realtime PCR of total survivin mRNA expression in Molt4, Reh, MV4;11 and RS4;11 cells following transfection with antisurvivin (EZN-3042) LNA-ON or control (EZN-3046). Results are expressed as relative survivin mRNA expression compared with EZN-3046 treated (control) ±s.d. (n = 3). (b) Western blot analysis following transfection of Molt4 and Reh cells with EZN-3042 or EZN-3046. (c) Quantitative real-time PCR of survivin splice variants in Reh cells following transfection with antisurvivin (EZN-3042) LNA-ON or control (EZN-3046). Results are expressed as relative survivin mRNA expression compared with EZN-3046 treated (control) ±s.d. (n = 3). (d) Transfection of Molt4 cells with either the EZN-3042 or EZN-3046 alone or in combination with etoposide, doxorubicin or dexamethasone. Percentage of apoptosis is graphed relative to EZN-3046 in the absence of chemotherapeutic agent ±s.d. (*P<0.01, **P<0.0001). (e) Transfection of Reh, MV4;11 and RS4;11 cells with either EZN-3042 (filled shapes) or EZN-3046 (open shapes) in the presence of increasing doses of etoposide, doxorubicin and prednisolone. Percentage of survival is graphed relative to EZN-3046 in the absence of chemotherapeutic agent ±s.d. IC50 values for etopside (EZN3046, 3042 in µM: Reh 0.72, 9.3 × 10−4; MV4;11 0.6, 0.63 × 10−7; RS4;11 1.5, 0.28), doxorubicin (EZN-3046:3042 in nM: Reh 161, 85; MV4;11 253, 46; RS4;11 223, 145) and prednisolone (EZN-3046, 3042 in µg/ml: Reh 458, 248; MV4;11 922, 664; RS4;11 878, 483) in the presence and absence of survivin downregulation were determined by the Hill equation.
As EZN-3042 is administered by IV infusion, we tested the efficacy of EZN-3042 when added directly to the tissue culture media.23 EZN-3042 or its matched control were added to the tissue culture media of the Reh cells at a concentration of 10 µm, approximately three times the concentration used for transfections and, 48 h later, cells were analyzed for mRNA expression (Figure 4a), protein expression (Figure 4b) and induction of apoptosis (Figure 4c). A 70% downregulation of survivin mRNA expression and a 37% increase in apoptosis was observed (P<0.0001), suggesting that the EZN-3042 was taken up from the media and was functional within the cell. As seen with the transfection assays, EZN-3042 was able to augment the induction of apoptosis caused by doxorubicin alone (P<0.001, IC50 potentiation 1.3-fold). Control EZN-3046 did not cause knockdown of survivin expression or induction of apoptosis.
Figure 4.
Survivin gene silencing by gymnotic delivery of EZN-3042 to Reh cells. Expression of survivin (a) mRNA transcript and (b) protein was downregulated by 10µm EZN-3042 added directly to culture media. (c) Determination of cell survival following incubation of Reh cells with a 10 µM EZN-3042 or EZN-3046 in the presence of increasing doses of doxorubicin. Experiment was performed in triplicate (*P<0.005).
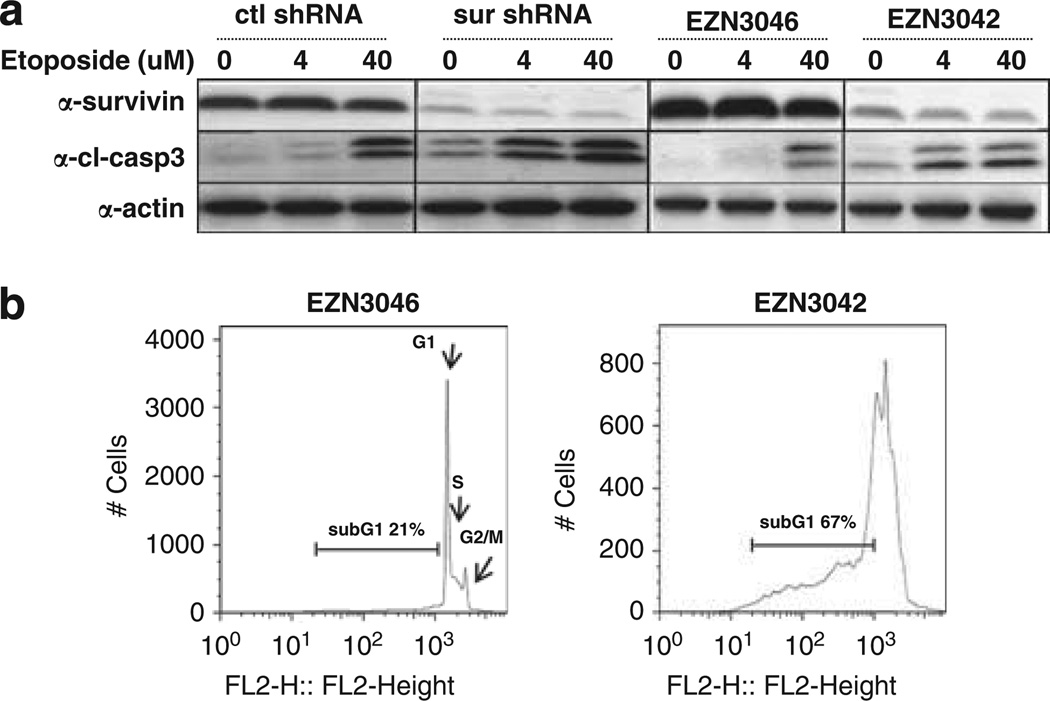
We examined cleavage of caspase-3 as a corollary to annexin-V staining to demonstrate that treatment with survivin shRNA or LNA-ON results in induction of apoptosis. We found that transfection of Molt4 cells with survivin shRNA or Reh cells with EZN-3042 increased basal levels of cleaved caspase-3 (Figure 5a). In addition, the level of caspase-3 cleavage in response to etoposide exposure was augmented with decreased survivin protein expression, (Figure 5a, 4 and 40 µm). To examine the effects of EZN-3042 or its control (EZN-3046) on the cellcycle profile, Molt4 cells were stained with propidium iodide and analyzed for DNA content by flow cytometry (Figure 5b). Abrogation of survivin expression in Molt4 cells resulted in cell-cycle arrest with an accumulation of cells in the apoptotic sub-G1 fraction. As shown Figure 5b, 67% of the cells transfected with EZN-3042 were in the sub-G1 phase compared with 21% of the control-transfected cells. Downmodulation of survivin also resulted in a shift in the proportion of cells in G1 (EZN-3042 6% vs EZN-3046 36%), S-phase (EZN-3042 23% vs EZN-3046 32%) and G2/M phase (EZN-3042 4% vs EZN-3046 11%). Taken together, these results support the hypothesis that repression of survivin expression by RNA interference mediates cell death via apoptosis.
Figure 5.
Downregulation of survivin results in increased caspase 3 activation and change in the cell-cycle profile. Western blot analysis of survivin, cleaved caspase 3 and actin expression following targeting of survivin mRNA with (a) shRNA in Molt4 cells or EZN-3042 in Reh cells and challenge with etoposide. (b) Propidium iodide staining and flow cytometric analysis of Molt4 cells 48h following transfection with survivin antisense EZN-3042 or scrambled control EZN-3046.
To determine if targeting survivin is effective in pediatric relapsed ALL in vivo, we generated xenografts from four primary human ALL samples, two pediatric (patient IDs 2365 and 4092) and two adult patients (patient IDs 200 and 240), all collected before the initiation of chemotherapy. Three xenografts (patient IDs 2365, 240 and 4092) were derived from patients with relapsed ALL and one from a patient with newly diagnosed ALL (patient ID 200). (See Supplementary Table 1 for details.) Mice were randomized to treatment with EZN-3042 vs scrambled control, after establishment of measurable disease (>5% peripheral blood blasts). A total of 4–10 mice were treated on each arm with each patient sample. Disease was measured at weekly intervals by flow cytometric analysis of peripheral blood until 50% of the control animals died due to disease burden after day 14. Mice generated from two of the four samples (patient IDs 2365 and 240) had a statistically significant decrease in peripheral blasts when treated with EZN-3042 as compared with control animals (P<0.05) (Figure 6a). The other two mice showed progressive disease despite treatment with EZN-3042. RT-PCR was performed on splenocytes from all four samples. A 65% downmodulation of survivin mRNA was documented in the xenograft that was the most responsive to treatment (patient ID 240, Figure 6b). In two of the other three samples, RT-PCR on the bone marrow demonstrated 70 and 80% survivin knockdown (patient IDs 4092 and 200, respectively, Figure 6b).
Figure 6.
Survivin antisense oligonucleotide is efficacious in ALL xenografts. NOD/severe combined immunodeficiencymice were xenografted with human ALL from patient samples. After establishment of disease, defined as greater than 5% blasts detected in peripheral blood, mice were randomized to treatment. Disease was evaluated at weekly intervals by flow cytometric analysis of peripheral blood detecting human CD19 + and CD45 + cells. Graphs depict mean absolute peripheral blood blast counts (white blood cells × % blasts by FACS analysis) from mice. Error bars depict standard error of mean. (a) Treatment with 25 mg/kg of EZN-3042 3 days a week (Monday, Wednusday and Friday) vs scrambled control EZN-3046 generated from the four patient samples at weekly intervals, demonstrating statistically significant difference (P<0.05) in two of four samples. (b) Determination of survivin mRNA expression in the spleen and bone marrow samples after 14 days of treatment with EZN-3042. Data graphed relative to expression in presence of control LNA-ON, corrected for B2-microglobulin levels. (c) Treatment of xenograft 240 with antisurvivin EZN-3042, methotrexate (MTX), temsirolimus (CCI) or triptolide vs untreated control. Graphs represent data collected at subsequent weeks normalized to starting mean absolute blast counts. Drug doses: EZN-3042 25 mg/kg 3 days a week (Monday, Wednesday and Friday); methotrexate 5–10 mg/kg weekly; CCI 5 mg/kg daily; triptolide 0.15 mg/kg/day IP. All chemotherapeutic drugs were administered intraperitoneally and each treatment:control combination was performed as a separate experiment.
Mice generated from patient ID 240 were also treated with other chemotherapy agents with demonstrated activity against ALL, including methotrexate, temsirolimus (an mTOR inhibitor) and triptolide (an inhibitor of XIAP).25 EZN-3042 had similar activity to methotrexate and better activity than temsirolimus and triptolide when tested in this model (Figure 6c). No overt toxicity was found after treatment with EZN-3042 except that treated mice became dehydrated with time (5–15% reduction in body weight), leading to a dose reduction of EZN-3042 after 2 weeks of treatment.
Discussion
Survivin is a highly attractive target for therapy because it has a relatively high turnover rate and is selectively expressed in most human cancer cells with little expression in normal non-fetal tissues. Survivin-targeted therapies have been developed to treat different forms of cancer. Our interest in survivin originated from our gene expression microarray study of 35 pediatric ALL diagnosis/relapse-matched samples,1 which has recently been confirmed by analysis of an additional 49 diagnosis/relapse pediatric ALL pairs. This data show that survivin was 1 of only 14 genes whose upregulation is common to both early and late relapse cohorts.27 Relapsed disease is particularly difficult to treat, with only 5–50% of patients achieving a cure. The fact that survivin is upregulated at relapse in 70% of the patients studied to date, as compared with initial diagnosis, led us to hypothesize that targeting survivin would be an effective therapeutic strategy to treat relapsed/refractory ALL. To test this hypothesis we evaluated the effect of survivin inhibition in both in vitro and in vivo models and combined this strategy with several chemotherapeutic agents that are currently used to treat pediatric relapsed ALL.
Using both survivin shRNAs and LNA-ON, we showed that survivin expression can be significantly downregulated in both B-precursor and T-cell ALL cell lines when compared with controls. Moreover, downregulation of survivin correlated with increased apoptosis and an accumulation of cells in the sub-G1 stage of the cell cycle. This result is consistent with the previous reports in the literature demonstrating that sudden downregulation of survivin expression through various means, such as antisense oligonucleotides, small-interfering RNA, ribozymes or dominant-negative mutants, is often associated with spontaneous induction of apoptosis (reviewed in Pennati et al.3 and Altieri30). It has been proposed that tumor cells depend upon expression of survivin for tumor maintenance, and sudden loss of this pathway triggers a strong induction of apoptosis and growth arrest.31,32
Our microarray results showing upregulation of survivin expression at relapse suggests that it may have a role in chemoresistance.1 We also tested the impact of survivin shRNA and antisense oligonucleotide in combination with several different chemotherapeutic agents. We found that overall downregulation of survivin expression by RNA interference potentiated the effect of the other chemotherapeutic drugs as compared with controls. This is consistent with recent studies in which the survivin LNA-ON was shown to sensitize prostate and lung cancer cells to paclitaxel treatment both in vitro and in vivo.22,28
As the shRNA or LNA-ON did not generate a standard dose response curve, we could not directly assess drug combination effects using commonly available software (for example, Calcusyn, BioSoft, Ferguson, MO, USA). We instead examined the ability of antisurvivin compounds to potentiate the cytotoxicity of chemotherapy, comparing IC50 values with and without antisurvivin treatment. Our results are in accordance with similar studies reported in the literature where downregulation of survivin sensitized resistant cancer cell lines or xenografts derived from breast, lung, prostate, thyroid, liver and cervical cancer to several different pro-apoptotic stimuli including TRAIL, doxorubicin, TNF-α, 5-FU, methotrexate, etoposide and cisplatin.13,33–37
Finally, we demonstrated the efficacy of the survivin LNA-ON in mouse xenograft models of ALL. In this system EZN-3042 inhibited the growth of established tumors derived from two out of four different xenografts, as compared with the scrambled control. The best responder xenograft, ID 240, was derived from a relapsed ALL patient. For these mice, the average blast count at day 14 following EZN3042 treatment was the same as when treatment started, whereas for the control mice the blast count increased by greater than four fold. Moreover, there was a corresponding 65% downregulation of survivin expression in the spleen among LNA-ON-treated 240 mice. Interestingly, xenograft ID 2365, which harbors the Philadelphia chromosome translocation t(9;22), a particularly poor prognostic marker for pediatric ALL, also responded well to the treatment but we did not see a corresponding decrease in survivin expression in this sample. The lack of knockdown in this xenograft could have a number of explanations: (1) the drug is able to knockdown survivin in circulating blasts but not those protected in the bone marrow or spleen microenvironment; (2) we did not analyze this sample at the ideal time point, that is, 14 days may be too late and sensitive blasts are eradicated leaving resistant blasts possibly compensating by increasing survivin expression; (3) the activity of the drug is due to off target effects. It has been suggested that the efficacy of LNA-ON therapy may be related in part to non-target effects.38 Despite this, the LNA resulted in decreased leukemia burden in 50% of the samples, both of these representing tumors derived from relapsed ALL patients. In our experiments, the LNA-ON caused dehydration in the mice requiring the need to use a lower, potentially subtherapeutic dose. Indeed the EZN-3042 used in this study has been evaluated previously in mouse xenografts of solid tumors and has shown to both decrease survivin expression and result in significant inhibition of tumor growth.28 However, the doses used in those experiments for solid tumors (50 mg/kg by continuous infusion, 100 mg/kg q 2 days) far exceeded those used in this study for human leukemia. We had anticipated that tumor penetration for leukemia might be more efficient compared with solid tumors but future experiments will be needed to maximize the pharmacodynamic effects of EZN-3042 in acute leukemia. It is important to note that in these studies, EZN-3042 was administered in saline only. As LNA oligonucleotides are stable in plasma, highly potent and able to penetrate cells in the absence of transfection,23 these antisense molecules have ideal properties that allow them to be used in vivo.
In adults it has been widely reported that survivin expression is upregulated in many solid tumors including lung, breast, colon and ovarian carcinoma, and in hematological malignancies such as lymphoma and acute leukemia. The role of survivin in pediatric cancers has also been investigated, and survivin overexpression has been evaluated in central nervous system tumors, neuroblastoma, Wilms tumor, sarcomas, leukemia and lymphoma (reviewed in Fangusaro et al.17). Higher expression of survivin has correlated with more advanced grades of disease and with a poorer prognosis.39–41 Our demonstration that attenuation of survivin expression potentiated several chemother-apeutic agents, is consistent with studies by Zhou and colleagues42 who showed that inhibition of survivin increases the cytotoxic effect of nutlin-3, a small molecule inhibitor of MDM2, in pediatric ALL.
The networks that lead to increased survivin expression in relapsed ALL have not yet been characterized. Survivin is the target of many pathways that regulate its expression. For example, survivin is negatively regulated by PTEN, p53, BRCA1 and Rb, which are often mutated and inactivated in a variety of cancers, and upregulated by Notch signaling, WNT/β - catenin, STAT3 and NF-κB. As survivin is associated with many different signaling pathways involved in cell proliferation and cell survival, it could be considered an unique target for therapeutic intervention, either by direct targeting or through the targeting of pathways regulating survivin expression.30
Antisense reagents targeting survivin are beginning to be tested in the clinical setting. Another survivin antisense oligonucleotide LY2181308 was well tolerated in a phase I trial in adult patients and resulted in reduced survivin expression in tumor biopsy specimens following drug treatment.43 Consequently, a phase II trial for LY2181308 in combination with idarubicin and cytarabine for adult patients with relapsed or refractory acute myeloid leukemia was initiated (http://ClinicalTrials.gov NCT006230321). A phase 1 clinical trial evaluating EZN-3042 with or without docetaxel in adults with advanced solid tumors or lymphoma was recently completed.44 Based on the data described in this manuscript, a multi-institutional phase I trial evaluating the safety, tolerability and biological activity of EZN-3042 in combination with standard re-induction chemotherapy for children with relapsed B-precursor ALL was recently opened within the Therapeutic Advances in Childhood Leukemia and Lymphoma consortium (http://ClinicalTrials.gov: NCT01186328). Survivin mRNA and protein expression will be measured on this trial before, and following the administration of EZN-3042 as a single agent, to determine the efficiency of survivin inhibition in vivo. Hematological dose limiting toxicity due to antisense treatment as well as standard chemotherapy will be monitored throughout the course of treatment. Although survivin is expressed in normal CD34 + stem cells and has a role in maintenance of progenitor cells in the bone marrow and driving normal erythropoiesis,4 dose limiting hematologic toxicity has not been observed in other trials using various survivin antagonists to date.43–45 This will be the first clinical trial of a survivin antisense agent in pediatric patients with relapsed leukemia, and it is hoped that bringing antisense technology to this patient population will close the gap on the last 20% of patients who do not currently achieve durable remission. In summary, survivin is a well-established cancer target. Our study validates this approach in pre-clinical leukemia models and provides a strong biological rationale for targeting survivin in relapsed ALL.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Health (NIH; 5 RO1 CA 140729-02, 5 P30 CA 01608730), the Pediatric Cancer Foundation, the Penelope London Foundation, Silber Pediatric Leukemia Fund and the Walter Family Pediatric Leukemia Fund. LH is supported by the American Society of Hematology Research Training Award for Fellows and a St Baldrick’s Foundation Fellowship. DTT is supported by a Larry and Helen Hoag Foundation Clinical Translational Research Career Development Award and the Leukemia and Lymphoma Society. Enzon Pharmaceuticals provided EZN-3042 and EZN-3046.
Footnotes
Conflict of interest
LMG and IDH are employees of Enzon Pharmaceuticals. The remaining authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Bhojwani D, Kang H, Moskowitz NP, Min DJ, Lee H, Potter JW, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2006;108:711–717. doi: 10.1182/blood-2006-02-002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knauer SK, Bier C, Schlag P, Fritzmann J, Dietmaier W, Rodel F, et al. The survivin isoform survivin-3B is cytoprotective and can function as a chromosomal passenger complex protein. Cell Cycle. 2007;6:1502–1509. [PubMed] [Google Scholar]
- 3.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–1139. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 5.Engels K, Knauer SK, Metzler D, Simf C, Struschka O, Bier C, et al. Dynamic intracellular survivin in oral squamous cell carcinoma: underlying molecular mechanism and potential as an early prognostic marker. J Pathol. 2007;211:532–540. doi: 10.1002/path.2134. [DOI] [PubMed] [Google Scholar]
- 6.Fortugno P, Beltrami E, Plescia J, Fontana J, Pradhan D, Marchisio PC, et al. Regulation of survivin function by Hsp90. Proc Natl Acad Sci USA. 2003;100:13791–13796. doi: 10.1073/pnas.2434345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hattori M, Sakamoto H, Satoh K, Yamamoto T. DNA demethylase is expressed in ovarian cancers and the expression correlates with demethylation of CpG sites in the promoter region of c-erbB-2 and survivin genes. Cancer Lett. 2001;169:155–164. doi: 10.1016/s0304-3835(01)00499-2. [DOI] [PubMed] [Google Scholar]
- 8.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, et al. High expression of survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 9.Capalbo G, Rodel C, Stauber RH, Knauer SK, Bache M, Kappler M, et al. The role of survivin for radiation therapy. Prognostic and predictive factor and therapeutic target. Strahlenther Onkol. 2007;183:593–599. doi: 10.1007/s00066-007-1800-4. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Arifin MT, Hama S, Kajiwara Y, Sugiyama K, Yamasaki F, et al. Survivin subcellular localization in high-grade astrocytomas: simultaneous expression in both nucleus and cytoplasm is negative prognostic marker. J Neurooncol. 2007;82:193–198. doi: 10.1007/s11060-006-9267-1. [DOI] [PubMed] [Google Scholar]
- 11.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26:2678–2684. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- 12.Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004;279:15196–15203. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 13.Tirro E, Consoli ML, Massimino M, Manzella L, Frasca F, Sciacca L, et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res. 2006;66:4263–4272. doi: 10.1158/0008-5472.CAN-05-3248. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Gu L, Li F, Zhu Y, Woods WG, Findley HW. DNA damage induces a novel p53-survivin signaling pathway regulating cell cycle and apoptosis in acute lymphoblastic leukemia cells. J Pharmacol Exp Ther. 2002;303:124–131. doi: 10.1124/jpet.102.037192. [DOI] [PubMed] [Google Scholar]
- 15.Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921–1925. [PubMed] [Google Scholar]
- 16.Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13(15 Part 1):4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 17.Fangusaro JR, Caldas H, Jiang Y, Altura RA. Survivin: an inhibitor of apoptosis in pediatric cancer. Pediatr Blood Cancer. 2006;47:4–13. doi: 10.1002/pbc.20805. [DOI] [PubMed] [Google Scholar]
- 18.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 19.Smith SD, Wheeler MA, Plescia J, Colberg JW, Weiss RM, Altieri DC. Urine detection of survivin and diagnosis of bladder cancer. JAMA. 2001;285:324–328. doi: 10.1001/jama.285.3.324. [DOI] [PubMed] [Google Scholar]
- 20.Watanuki-Miyauchi R, Kojima Y, Tsurumi H, Hara T, Goto N, Kasahara S, et al. Expression of survivin and of antigen detected by a novel monoclonal antibody, T332, is associated with outcome of diffuse large B-cell lymphoma and its subtypes. Pathol Int. 2005;55:324–330. doi: 10.1111/j.1440-1827.2005.01832.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter BZ, Andreeff M. Targeting survivin in leukemia. Oncol Rev. 2008;1:195–204. [Google Scholar]
- 22.Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA, et al. SPC3042: a proapoptotic .survivin inhibitor. Mol Cancer Ther. 2008;7:2736–2745. doi: 10.1158/1535-7163.MCT-08-0161. [DOI] [PubMed] [Google Scholar]
- 23.Stein CA, Hansen JB, Lai J, Wu S, Voskresenskiy A, Hog A, et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38:e3. doi: 10.1093/nar/gkp841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min DJ, Moskowitz NP, Brownstein C, Lee H, Horton TM, Carroll WL. Diverse pathways mediate chemotherapy-induced cell death in acute lymphoblastic leukemia cell lines. Apoptosis. 2006;11:1977–1986. doi: 10.1007/s10495-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 25.Teachey DT, Sheen C, Hall J, Ryan T, Brown VI, Fish J, et al. mTOR inhibitors are synergistic with methotrexate: an effective combination to treat acute lymphoblastic leukemia. Blood. 2008;112:2020–2023. doi: 10.1182/blood-2008-02-137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan L, Bhojwani D, Wang J, Morrison D, Yang J, Zhang Y, et al. Upregulation of genes involved in folate metabolism characterize late but not early relapse in childhood acute lymphoblastic leukemia. Blood [abstract] 2009;114:689. [Google Scholar]
- 28.Sapra P, Wang M, Bandaru R, Zhao H, Greenberger LM, Horak ID. Down-modulation of survivin expression and inhibition of tumor growth in vivo by EZN-3042, a locked nucleic acid antisense oligonucleotide. Nucleosides Nucleotides Nucl Acids. 2010;29:97–112. doi: 10.1080/15257771003597733. [DOI] [PubMed] [Google Scholar]
- 29.Hu H, Shikama Y, Matsuoka I, Kimura J. Terminally differentiated neutrophils predominantly express Survivin-2 alpha, a dominantnegative isoform of survivin. J Leukoc Biol. 2008;83:393–400. doi: 10.1189/jlb.0507282. [DOI] [PubMed] [Google Scholar]
- 30.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 31.Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, et al. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, et al. Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi N, Asano K, Suzuki H, Yamamoto T, Tanigawa N, Egawa S, et al. Adenoviral infection of survivin antisense sensitizes prostate cancer cells to etoposide in vivo . Prostate. 2005;65:10–19. doi: 10.1002/pros.20232. [DOI] [PubMed] [Google Scholar]
- 34.Li QX, Zhao J, Liu JY, Jia LT, Huang HY, Xu YM, et al. Survivin stable knockdown by siRNA inhibits tumor cell growth and angiogenesis in breast and cervical cancers. Cancer Biol Ther. 2006;5:860–866. doi: 10.4161/cbt.5.7.2893. [DOI] [PubMed] [Google Scholar]
- 35.Nakao K, Hamasaki K, Ichikawa T, Arima K, Eguchi K, Ishii N. Survivin downregulation by siRNA sensitizes human hepatoma cells to TRAIL-induced apoptosis. Oncol Rep. 2006;16:389–392. [PubMed] [Google Scholar]
- 36.Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 37.Yonesaka K, Tamura K, Kurata T, Satoh T, Ikeda M, Fukuoka M, et al. Small interfering RNA targeting survivin sensitizes lung cancer cell with mutant p53 to adriamycin. Int J Cancer. 2006;118:812–820. doi: 10.1002/ijc.21350. [DOI] [PubMed] [Google Scholar]
- 38.Kim R, Emi M, Matsuura K, Tanabe K. Antisense and nonantisense effects of antisense Bcl-2 on multiple roles of Bcl-2 as a chemosensitizer in cancer therapy. Cancer Gene Ther. 2007;14:1–11. doi: 10.1038/sj.cgt.7700986. [DOI] [PubMed] [Google Scholar]
- 39.Fangusaro JR, Jiang Y, Holloway MP, Caldas H, Singh V, Boue DR, et al. Survivin, survivin-2B, and survivin-deItaEx3 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome. Br J Cancer. 2005;92:359–365. doi: 10.1038/sj.bjc.6602317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takamizawa S, Scott D, Wen J, Grundy P, Bishop W, Kimura K, et al. The survivin: fas ratio in pediatric renal tumors. J Pediatr Surg. 2001;36:37–42. doi: 10.1053/jpsu.2001.20000. [DOI] [PubMed] [Google Scholar]
- 41.Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, et al. High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2004;10:3737–3744. doi: 10.1158/1078-0432.CCR-03-0642. [DOI] [PubMed] [Google Scholar]
- 42.Zhu N, Gu L, Li F, Zhou M. Inhibition of the Akt/survivin pathway synergizes the antileukemia effect of nutlin-3 in acute lymphoblastic leukemia cells. Mol Cancer Ther. 2008;7:1101–1109. doi: 10.1158/1535-7163.MCT-08-0179. [DOI] [PubMed] [Google Scholar]
- 43.Talbot D, Davies J, Callies S, Andre V, Lahn M, Ang J, et al. First human dose study evaluating safety and pharmacokinetics of LY2181308, an antisense oligonucleotide designed to inhibit survivin. J Clin Oncol. 2008;26:3518. [Google Scholar]
- 44.Tolcher AW, Patnaik A, Papadopoulos KP, Agnew J, Lokiec FM, Rezai K, et al. Results of a phase 1, open-label, dose-escalation study evaluating the safety and tolerability of EZN-3042, a survivin mRNA antagonist, administered with or without docetaxel in adult patients with advanced solid tumors or lymphoma. Proceedings of the 102nd Annual Meeting of AACR. 2011 LB-409 (abstract) [Google Scholar]
- 45.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.