Abstract Abstract
The aim of this study was to characterize cytogenetically one population of the fish Moenkhausia sanctaefilomenae (Steindachner, 1907), with emphasis on the analysis of B chromosomes. The nucleolar activity in the B microchromosomes was characterized, and an analysis of mitotic instability of these microchromosomes was accomplished. The results showed a diploid chromosome number of 50 chromosomes. In all individuals, we observed the presence of B microchromosomes with intra- and inter-individual variability. The analysis of the nucleolus organizing regions (NORs) by silver nitrate staining demonstrated multiple NORs. We observed active sites of ribosomal DNA in the B microchromosomes, with a frequency of 20% in the analyzed cells, which shows gene activity in these chromosomal elements. The analysis of constitutive heterochromatin patterns showed that the B microchromosomes are heterochromatic or euchromatic, which demonstrates differentiation of DNA composition between these genomic elements. The calculation of the mitotic instability index implied that B chromosomes in this species might be in a final stage of instability.
Keywords: fish cytogenetic, NOR expression, supernumerary chromosomes, mitotic instability
Introduction
Moenkhausia Eigenmann, 1903 is considered as incertae sedis in Characidae and contains 65 valid species widely distributed in the Neotropical river basins (Lima et al. 2003). Although the genus Moenkhausia cannot be characterized as monophyletic, a group consisted of Moenkhausia oligolepis (Günther, 1864), Moenkhausia sanctaefilomenae (Steindachner, 1907), Moenkhausia cotinho Eigenmann, 1908, and Moenkhausia pyrophthalma Costa, 1994 shares a very similar color pattern (Costa 1994). Moenkhausia systematic is very complex and nowadays several studies have shown that it needs to be more thoroughly addressed (Benine et al. 2009).
Chromosome studies in the genus Moenkhausia are still restricted and cytogenetic data are available only for six species (Portela-Castro et al. 2001). In Moenkhausia sanctaefilomenae, a stable diploid number of 50 chromosomes and few karyotype variations among the different populations analyzed have been reported. Furthermore, some populations of Moenkhausia sanctaefilomenae can show a high inter- and intra-individual variability of the NOR (nucleolus organizer region) phenotypes, as well as conspicuous blocks of constitutive heterochromatin in the pericentromeric region of the chromosomes (Foresti et al. 1989, Portela-Castro et al. 2001, Portela-Castro and Júlio Jr. 2002). However, the occurrence of several B microchromosomes in the genome of this species is the most peculiar feature to be studied in this fish group (Foresti et al. 1989).
B chromosome includes a variety of extra chromosomes that display conspicuous heterogeneity in their nature, behavior, and evolutionary dynamics. This definition highlights some of the most universal properties of B chromosomes: their dispensability (that is, they are not necessary for the host to complete a normal life cycle); their origin from chromosomes (either from within the same species or from other species); and their remarkable differentiation relative to A chromosomes, with which they do not recombine (Camacho 2005).
B chromosomes are widely distributed among eukaryotes and their occurrence has been reported in 10 species of the fungi, nearly 1.300 plants (more than 1.400 when different ploidy levels of the same species are considered separately), and over 500 animals (Camacho 2005). In addition, B chromosomes have been described in 61 species of Neotropical fish to date (Carvalho et al. 2008).
In species of Moenkhausia, B chromosomes were documented for Moenkhausia sanctaefilomenae and Moenkhausia intermedia Eigenmann, 1908 (Portela et al. 1988, Foresti et al. 1989). Differently from other microchromosome-bearing fish species, which exhibit a low frequency and a sporadic occurrence (Hashimoto et al. 2008, Oliveira et al. 2009, Hashimoto et al. 2011), several microchromosomes can be found in the genome of Moenkhausia sanctaefilomenae and, in certain situations, the frequency can be related to sex (Portela-Castro et al. 2001).In fact, in Neotropical fish, it is possible to find both B macrochromosomes and B microchromosomes (Oliveira et al. 2009), but in both cases the presence of a large number of B chromosomes in the cells is rare, as was observed in Prochilodus lineatus (Valenciennes, 1836)and Moenkhausia sanctaefilomenae, which presented up to eight microchromosomes in the cells (Foresti et al. 1989, Voltolin et al. 2011).
Another interesting characteristic observed in the B microchromosomes of Moenkhausia sanctaefilomenae is the polymorphism revealed by C-banding. Through this method, these microchromosomes can be characterized in different classes according to the pattern of constitutive heterochromatin; they can be partially and totally heterochromatic, and euchromatic (Foresti et al. 1989). Thus, such polymorphism indicates a distinct DNA composition between these microchromosomes, especially of repetitive DNA.
In the present study, we carried out cytogenetic analyses in one particular population of the fish Moenkhausia sanctaefilomenae focusing on two special features concerning the B microchromosomes: the occurrence of nucleolar activity in the B chromosome of this species and a study about the maintenance of microchromosomes in this population through the calculation of the mitotic instability index (MI).
Material and methods
The cytogenetic analyses were carried out in chromosomal preparations obtained from 15 specimens (8 males and 7 females) of Moenkhausia sanctaefilomenae. The individuals were collected from a population of the Batalha River (22°7.02'S, 49°16.01'W), belonging to Tietê River basin, São Paulo State, southeastern Brazil. The voucher specimens were identified and stored in the fish collection of the Laboratório de Genética de Peixes, UNESP, Bauru, SP, Brazil.
Before sacrifice, the animals were inoculated with yeast cell suspension to increase the number of metaphase cells (Oliveira et al. 1988). Chromosomal preparations were obtained from gill and kidney tissues using the technique described by Foresti et al. (1993). Silver staining (Ag-staining) of the nucleolus organizer regions followed the technique of Howell and Black (1980), and C-banding was performed according to Sumner (1972). The chromosomal morphology was determined on the basis of arm ratio, as proposed by Levan et al. (1964) and the chromosomes were classified as metacentric (m), submetacentric (sm), subtelocentric (st), and acrocentric (a).
The index to quantify the mitotic instability of B chromosome, MI, which was calculated as the sum of the absolute values of every deviation in B number with respect to the median (M), and normalized by dividing the median and the number of cells analyzed (N) so that the index is independent of the number of B and the sample size were performed by means of one-way ANOVA.
MI = (M-ni/fi)/M.N
where ni is the numer of B chromosome in the different types of cells that do not coincide with M, and fi is the number of cells of each particular type.
Results and discussion
In the individuals of Moenkhausia sanctaefilomenae, our results showed a diploid chromosome number of 50 chromosomes, with karyotypes composed of 6 m, 16 sm and 28 st (fundamental number FN = 100) (Fig. 1). No sex-related karyotype difference was observed. The diploid chromosome number and the karyotypes composed mainly of metacentric and submetacentric chromosomes seem to be a conserved characteristic observed for different Moenkhausia sanctaefilomenae populations (Foresti et al. 1989, Portela-Castro et al. 2001, Portela-Castro and Júlio Jr. 2002).
Figure 1.
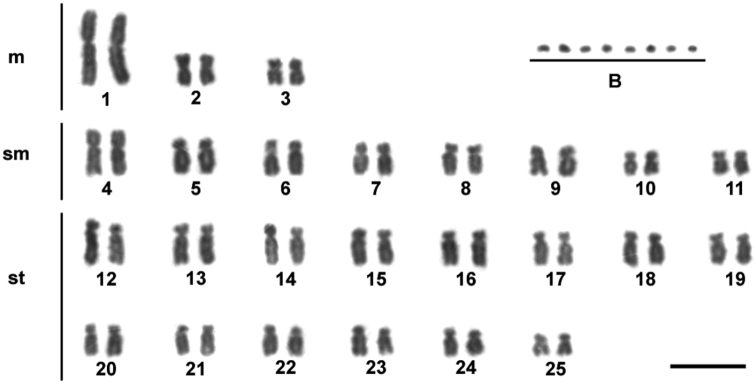
Giemsa-stained karyotype showing 2n = 50 chromosomes of one individual of Moenkhausia sanctaefilomenae. In evidence, eight B microchromosomes. Bars = 10 µm.
Extra chromosomes were observed in the genomes of all individuals of Moenkhausia sanctaefilomenae, which were characterized as B microchromosomes (Fig. 1). We detected inter- and intra-individual variation in relation to the number of B chromosomes in the cells, with specimens bearing up to eight microchromosomes. Metaphase counts for 13 individuals showing the variation in supernumerary chromosome numbers are presented in Table 1. The modal numbers were of 2 and 3 microchromosomes. Such variation is in accordance with the pioneer study of Foresti et al. (1989), who also analyzed a population from the Tietê River basin. On the other hand, the specimens from the Paraná River analyzed by Portela-Castro et al. (2001), showed differences because the presence of 0–2 microchromosomes were reported only in males. These polymorphisms concerning the distribution of B chromosomes indicate a process of genetic divergence in distinct populations that likely occurs in some species restricted to small tributaries and streams, as reported for species of Astyanax (Moreira-Filho and Bertollo 1991, Vicari et al. 2008, Hashimoto et al. 2011).
Table 1.
Metaphase counts for 13 specimens of Moenkhausia sanctaefilomenae demonstrating the variation in B microchromosome numbers.<br/>
| Specimen identification | Number of B microchromosomes per cell | Number of cells counted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 849 | 6 | 12 | 22 | - | - | - | - | - | - | 40 |
| 852 | 2 | 32 | 36 | 10 | - | - | - | - | - | 80 |
| 853 | 9 | 70 | - | - | - | - | - | - | - | 79 |
| 857 | 3 | 3 | 6 | 10 | 9 | 9 | 2 | - | - | 42 |
| 887 | - | 3 | 12 | 22 | 4 | 2 | 7 | 13 | 2 | 65 |
| 888 | - | 6 | 6 | 41 | 5 | 10 | - | - | - | 68 |
| 889 | - | 3 | 12 | 63 | 11 | 3 | - | - | - | 92 |
| 1233 | - | 4 | 10 | 24 | 26 | 29 | 13 | 4 | - | 110 |
| 1235 | 1 | 6 | 31 | 79 | 15 | 8 | - | - | - | 140 |
| 1240 | 8 | 31 | 33 | 26 | 3 | - | - | - | - | 101 |
| 1241 | 9 | 137 | 175 | 4 | - | - | - | - | - | 325 |
| 1242 | 24 | 85 | 27 | - | - | - | - | - | - | 136 |
| 1246 | 5 | 25 | 4 | 5 | 4 | 1 | - | - | - | 44 |
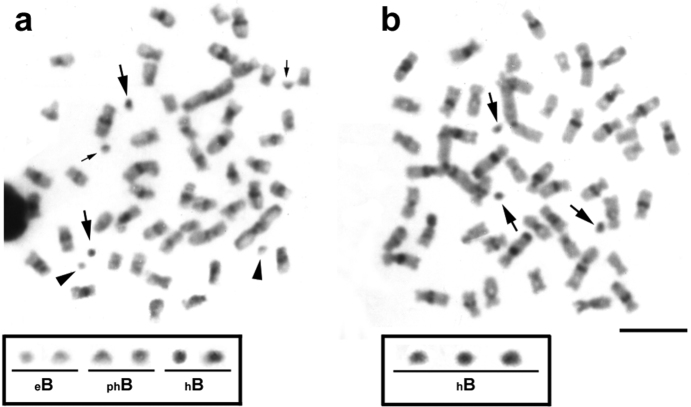
Analysis of the constitutive heterochromatin patterns by C-banding showed heterochromatic blocks in the centromeric and pericentromeric regions in the majority of the chromosomes (Fig. 2a, b). Such general heterochromatin pattern was also observed in previous analyses for other Moenkhausia sanctaefilomenae populations (Foresti et al. 1989, Portela-Castro et al. 2001, Portela-Castro and Júlio Jr. 2002), demonstrating that these chromosomal regions present a highlyconservative distribution in this species. The supernumerary chromosomes showed different C-banding patterns. We observed euchromatic (Fig. 2a) as well as partially or totally heterochromatic microchromosomes (Fig. 2a, b), evidencing that these B chromosomes can have a different DNA composition, mainly of repetitive sequences. This is a common feature also reported for B chromosomes in other characid species (Néo et al. 2000, Jesus et al. 2003, Moreira-Filho et al. 2004).
Figure 2.
Metaphases from specimens of Moenkhausia sanctaefilomenae after C-banding technique. In (a), metaphase shows euchromatic (eB), partially heterochromatic (phB) and totally heterochromatic (hB) microchromosomes. In (b), metaphase demonstrates only heterochromatic B chromosomes. The boxes show enlarged B chromosomes. Major and minor arrows indicate totally and partially heterochromatic B microchromosomes, respectively. Arrowheads exhibit euchromatic B microchromosomes. Bars = 10 µm.
The Ag-impregnation revealed intra- (Fig. 3a, b) and inter-individual (Fig. 3c, d) variability for the NOR phenotypes in metaphases of Moenkhausia sanctaefilomenae, ranging from two to five Ag-positive sites, distributed in the interstitial and terminal regions of distinct chromosomes (Fig. 3e). However, the chromosomes 6 always presented active Ag-NORs, and consequently, were considered the major NOR-bearing chromosomes. The minor NORs showed a very variable pattern of activity. Such NOR features were previously reported by Foresti et al. (1989).
Figure 3.
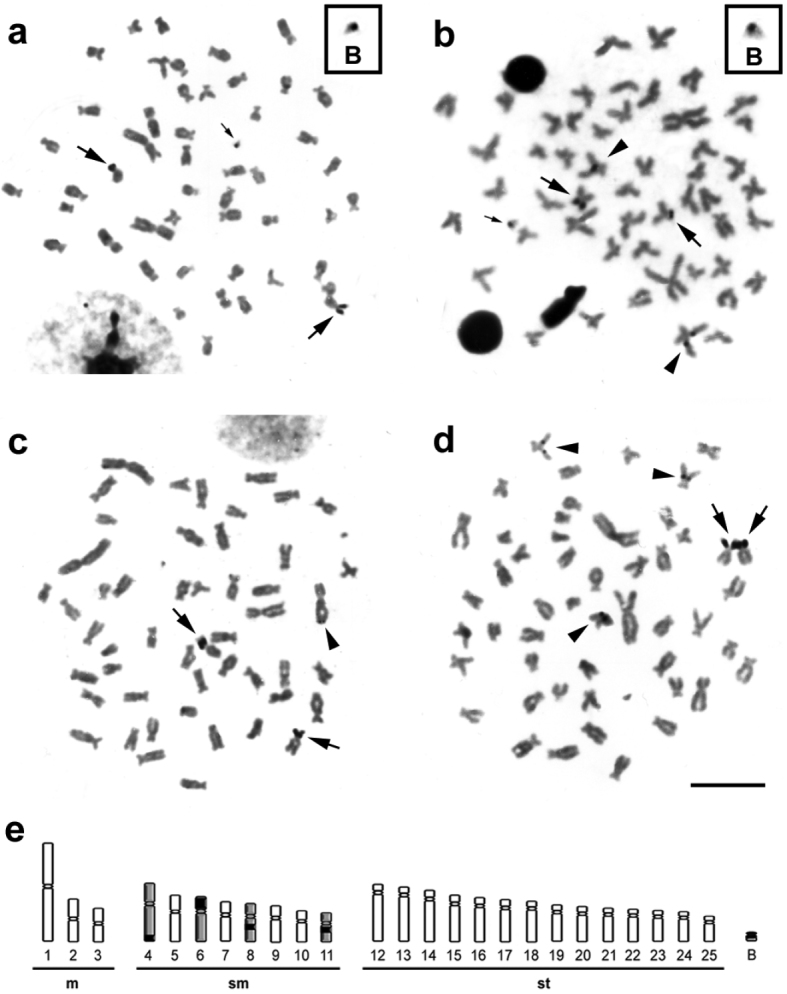
Metaphases from specimens of Moenkhausia sanctaefilomenae submitted to the silver coloration. In (a) and (b), metaphases of one individual show intra-individual variability of active NORs. The boxes exhibit enlarged B chromosomes with nucleolar activity. In (c) and (d), metaphases of different samples demonstrate inter-individual variability for the NORs. In (e), schematic representation shows the NOR-bearing chromosomes (4, 6, 8, 11 and B). Major arrows indicate major NOR-bearing chromosomes (chromosomes 6). Minor arrows show nucleolar activity in the B microchromosomes (a) and (b). Arrowheads exhibit minor NORs demonstrating a variable pattern of activity in different chromosomes. Bars = 10 µm.
Indeed, NOR expression was detected in a B chromosome of one individual, which carried only this microchromosome (Fig. 3a, b). We analyzed 60 cells by Ag-staining and observed that about 20% had active ribosomal DNA sites in the B chromosome of this individual. Moreover, this supernumerary chromosome showed to be euchromatic by C-banding. The nucleolar region is a dynamic cell compartment involved in the control of numerous cellular functions that can be visualized after Ag coloration, when the genes present activities in the interphase that anticipates the mitosis (Roussel et al. 1996, Caperta et al. 2007, Hiscox 2007). Therefore, Ag-staining provides a simple and reliable method to detect ribosomal RNA (rRNA) gene transcription (Bakkali et al. 2001, Teruel et al. 2009). B chromosomes in several species carry rRNA genes (Camacho 2005), including fish species (Baroni et al. 2009, Poletto et al. 2010), and in most of the cases, rRNA has been detected by Ag-staining evidencing the presence of active genes, as demonstrated in the present study. However, further analysis using FISH technique will be necessary to detect positions of additional rDNA genes not only during their activity.
The fact that the NORs located in the chromosomes 6 were always active can suggest that a process of nucleolar dominance can influence the rRNA gene transcription in order to provide the proper amount of rRNA for ribosome assembly. Nucleolar dominance is an epigenetic phenomenon common in interspecific hybrids, in which ribosomal RNA genes set inherited from one parental are rather transcribed in relation to the other (Hashimoto et al. 2009). Nucleolar dominance can also be a consequence of the regulatory process that controls the effective dosage of rRNA genes in pure species (non-hybrid) (Pikaard 2000). Nowadays, the mechanisms by which whole NORs or rRNA genes subsets are selected for inactivation still remains unclear (Preuss and Pikaard 2007).
The chromosome context appears to be important for NOR activity, as deduced from changes in the on/off activity status following chromosome rearrangements moving NORs to new locations (Pikaard 2000). The present findings show that the B chromosome plays an important role in the genome organization of Moenkhausia sanctaefilomenae, and will be useful for further analyses to determine whether the frequency of B chromosomes expressing their NOR is changing over time and how the B chromosome context can influence A chromosome NOR activity.
In relation to the mitotic instability and maintenance of B chromosomes in Moenkhausia sanctaefilomenae, we compared the results reported by Foresti et al. (1989) with the data described in this study, because both populations were collected from the Tietê River basin (Brazil). In both populations, a pattern of mitotic instability for all analyzed individuals was observed. The analysis of the standard maintenance of these B chromosomes by calculating the mitotic instability index (MI) revealed that the Moenkhausia sanctaefilomenae population analyzed by Foresti et al. (1989) showed a MI = 0.6; however, the Moenkhausia sanctaefilomenae population analyzed in the present study showed a MI = 0.2. Taking account the high variability of B chromosomes in the genomes of these Moenkhausia sanctaefilomenae specimens, further studies are still necessary to verify if these B chromosomes might be underway towards the neutralization stage, in accordance with the life cycle of B chromosomes described by Camacho et al. (1997).
In fish, the possibility of neutralization through mitotic stabilization of B-chromosomes was also observed in Prochilodus lineatus, in the population from the Mogi-Guaçu River (Brazil) (Oliveira et al. 1997). Afterwards, in this same population, Cavallaro et al. (2000) found a drastic temporal decline in the degree of B mitotic instability; Voltolin et al. (2010) showed that the stabilization process was continuous for over 15 years; and currently, the population of Prochilodus lineatus from the Mogi-Guaçu River presents a total mitotic stability index (MI = 0) and the B chromosomes were considered completely neutralized.
In Neotropical fish, most of the studies about B chromosomes are still descriptive, because many species have not yet been cytogenetically analyzed. Thus, studies focusing B chromosomes in Neotropical fish are extremely necessary to better understand this intriguing class of chromosomes, as has been done for some species, such as Prochilodus lineatus and Astyanax species (Moreira-Filho et al. 2004, Voltolin et al. 2010, 2011, Hashimoto et al. 2011), towards which efforts are more thoroughly addressed. Thus, our results show that B chromosomes of Moenkhausia sanctaefilomenae are excellent models and also that extensive studies in this species are essential to improve the knowledge of the diversification of B chromosomes.
Acknowledgements
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Bakkali M, Cabrero J, López-León MD, Perfectti F, Camacho JPM. (2001) Population differences in the expression of nucleolus organizer regions in the grasshopper Eyprepocnemis plorans. Protoplasma 217: 185-190.10.1007/BF01283399 [DOI] [PubMed] [Google Scholar]
- Baroni S, Lopes CE, Almeida-Toledo LF. (2009) Cytogenetic characterization of Metynnis maculatus (Teleostei; Characiformes): the description in Serrasalminae of a small B chromosome bearing inactive NOR-like sequences. Caryologia 2: 95-101 [Google Scholar]
- Benine RC, Mariguela TC, Oliveira C. (2009) New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotropical Ichthyology 7: 161-168.10.1590/S1679-62252009000200005 [Google Scholar]
- Camacho JPM, Shaw MW, López-Léon MD, Pardo MC, Cabrero J. (1997)Population dynamics of a selfish B chromosome neutralized by the standard genome in the grasshopper Eyprepocnemis plorans. The American Naturalist 149: 1030-1050.10.1086/286037 [DOI] [PubMed] [Google Scholar]
- Camacho JPM. (2005) B Chromosomes. In: Gregory TR. (Ed.). The evolution of the genome.Elsevier, San Diego: 223-286.10.1016/B978-012301463-4/50006-1
- Caperta AD, Neves N, Viegas W, Pikaard CS, Preuss S. (2007) Relationships between transcription, silver staining, and chromatin organization of nucleolar organizers in Secale cereale. Protoplasma 232: 55-59.10.1007/s00709-007-0277-4 [DOI] [PubMed] [Google Scholar]
- Carvalho AR, Martins-Santos IC, Dias AL. (2008) B chromosomes: an update about their occurrence in freshwater Neotropical fishes (Teleostei). Journal of Fish Biology 72: 1907-1932.10.1111/j.1095-8649.2008.01835.x. [Google Scholar]
- Cavallaro ZI, Bertollo LAC, Perfectti F, Camacho JPM. (2000) Frequency increase and mitotic stabilization of a B chromosome in fish Prochilodus lineatus. Chromosome Research 8: 627-634.10.1023/A:1009242209375 [DOI] [PubMed] [Google Scholar]
- Costa WJEM. (1994) Description of two new species of the genus Moenkhausia (Characiformes: Characidae) from the central Brazil. Zoologischer Anzeiger 232: 21-29 [Google Scholar]
- Foresti F, Almeida-Toledo LF, Toledo SA. (1989) Supranumerary chromosome system C-banding pattern characterization and multiple Nucleolus Organizing Region in Moenkhausia sanctaefilomenae (Pisces, Characidae). Genetica 79: 107-114.10.1007/BF00057927 [Google Scholar]
- Foresti F, Oliveira C, Almeida-Toledo LF. (1993) A method for chromosome preparations from large specimens of fishes using in vitro short treatment with colchicine. Experientia 49: 810-813.10.1007/BF01923555 [Google Scholar]
- Hashimoto DT, Gonçalves VR, Bortolozzi J, Foresti F, Porto-Foresti F. (2008) First report of a B chromosome in a natural population of Astyanax altiparanae (Characiformes, Characidae). Genetics and Molecular Biology 31: 275-278.10.1590/S1415-47572008000200021 [Google Scholar]
- Hashimoto DT, Laudicina A, Bortolozzi J, Foresti F, Porto-Foresti F. (2009) Chromosomal features of nucleolar dominance in hybrids between the Neotropical fish Leporinus macrocephalus and Leporinus elongatus (Characiformes, Anostomidae). Genetica 137: 135-140.10.1007/s10709-009-9366-y [DOI] [PubMed] [Google Scholar]
- Hashimoto DT, Ferguson-Smith MA, Rens W, Foresti F, Porto-Foresti F. (2011) Chromosome mapping of H1 histone and 5S rRNA gene clusters in three species of Astyanax (Teleostei, Characiformes). Cytogenetic and Genome Research 134: 64-71.10.1159/000323512 [DOI] [PubMed] [Google Scholar]
- Hiscox JA. (2007) RNA viruses: hijacking the dynamic nucleolus. Nature Reviews Microbiology 5: 119-127.10.1038/nrmicro1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WM, Black DA. (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36: 1014-1015.10.1007/BF01953855 [DOI] [PubMed] [Google Scholar]
- Jesus CM, Galetti Jr PM, Valentini SR, Moreira-Filho O. (2003) Molecular characterization and chromosomal location of two families of satelite DNA in Prochilodus lineatus (Pisces, Prochilodontidae), a species with B chromosomes. Genetica 118: 25-32.10.1023/A:1022986816648 [DOI] [PubMed] [Google Scholar]
- Levan A, Fregda K, Sandbreg AA. (1964) Nomeclature for centromeric position on chromosomes. Hereditas 52: 201-220.10.1111/j.1601-5223.1964.tb01953.x [Google Scholar]
- Lima FCT, Malabarba LR, Buckup PA, Silva JFP, Vari RP, Harold A, Benine R, Oyakawa OT, Pavanelli CS, Menezes NA, et al. (2003) Characidae – genera incertae sedis in Characidae. In: Reis RE, Kullander SO, Ferraris Jr CJ. (Eds). Check List of the freshwater fishes of the South and Central America.EDIPUCRS. Porto Alegre: 106-169
- Moreira-Filho O, Bertollo LAC. (1991) Astyanax scabripinnis (Pisces, Characidae): A species complex. Brazilian Journal of Genetics 14: 331-357 [Google Scholar]
- Moreira-Filho O, Galletti Jr. PM, Bertollo LAC. (2004) B chromosomes in the fish Astyanax scabripinnis (Characidae, Tetragonopterinae): An overview in natural population. Cytogenetic and Genome Research 106: 230-234.10.1159/000079292 [DOI] [PubMed] [Google Scholar]
- Néo DM, Bertollo LAC, Moreira-Filho O. (2000) Morphological differentiation and possible origin of B chromosome in natural Brazilian population of Astyanax scabripinnis (Pisces, Characidae). Genetica 108: 211-215.10.1023/A:1004157901097 [DOI] [PubMed] [Google Scholar]
- Oliveira C, Almeida-Toledo LF, Foresti F, Britski HA, Filho SAT. (1988) Chromosome formulae of neotropical freswater fishes. Brazilian Journal of Genetics 11: 577-624 [Google Scholar]
- Oliveira C, Saboya SMR, Foresti F, Senhorini JA, Bernardino G. (1997) Heredity 79: 473-476.10.1038/hdy.1997.186 [Google Scholar]
- Oliveira C, Foresti F, Hilsdorf AWS. (2009) Genetics of neotropical fish: from chromosomes to populations. Fish Physiology and Biochemistry 35: 81-100.10.1007/s10695-008-9250-1. [DOI] [PubMed] [Google Scholar]
- Pikaard CS. (2000) The epigenetics of nucleolar dominance. Trends in Genetics 16: 495-500.10.1016/S0168-9525(00)02113-2 [DOI] [PubMed] [Google Scholar]
- Poletto AB, Ferreira IA, Martins C. (2010) The B chromosomes of the African cichlid fish Haplochromis obliquidens harbor 18S rRNA gene copies. BMC Genetics 11: 1. 10.1186/1471-2156-11-1 [DOI] [PMC free article] [PubMed]
- Portela ALBS, Galetti Jr. PM, Bertollo LAC. (1988) Considerations on the chromosome evolution of Tetragonopterinae. Brazilian Journal of Genetics 11: 307-313 [Google Scholar]
- Portela-Castro ALBS, Julio HF, Nishiyama PB. (2001) New occurrence of microchromosomes B in Moenkhausia sanctaefilomenae (Pisces, Characidae) from the Paraná River of Brazil: analysis of the synaptonemal complex. Genetica 110: 277-283.10.1023/A:1012742717240 [DOI] [PubMed] [Google Scholar]
- Portela-Castro ALB, Júlio–Júnior HF. (2002) Karyotype relationships among species of the subfamily Tetragonopterinae (Pisces, Characidae): Cytotaxonomic and evolution aspects. Cytologia 67: 329-336.10.1508/cytologia.67.329 [Google Scholar]
- Preuss S, Pikkard CS. (2007) rRNA gene silencing and nucleolar dominance: Insights into a chromosome-scale epigenetic on/off swich. Biochimica et Biophysica Acta 1769: 383-392.10.1016/j.bbaexp.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P, Andre C, Comai L, Hernandez-Verdun D. (1996) The rDNA transcription machinery is assembled during mitosis in active NORs and absent in inactive NORs. The Journal of Cell Biology 133: 235-246.10.1083/jcb.133.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner AT. (1972) A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75: 304-306.10.1016/0014-4827(72)90558-7 [DOI] [PubMed] [Google Scholar]
- Teruel M, Cabrero J, Perfectti F, Acosta MJ, Sanchez A, Camacho JPM. (2009) Chromosoma 119: 291-301.10.1007/s00412-008-0197-x [DOI] [PubMed] [Google Scholar]
- Vicari MR, Noleto RB, Artoni RF, Moreira-Filho O, Bertollo LAC. (2008) Comparative cytogenetics among species of the Astyanax scabripinnis complex. evolutionary and biogeographical inferences. Genetics and Molecular Biology 31: 173-179.10.15902/S1415-47572008000200002 [Google Scholar]
- Voltolin TA, Senhorini JA, Oliveira C, Foresti F, Bortolozzi J, Porto-Foresti F. (2010) B-chromosome frequency stability in Prochilodus lineatus (Characiformes, Prochilodontidae). Genetica 138: 281-284.10.1007/s10709-009-9420-9 [DOI] [PubMed] [Google Scholar]
- Voltolin TA, Senhorini JA, Foresti F, Bortolozzi J, Porto-Foresti F. (2011) Intraspecific crosses resulting in the first occurrence of eight and nine B chromosomes in Prochilodus lineatus (Characiformes, Prochilodontidae). Genetics and Molecular Biology 34: 220-224.10.1590/S1415-47572011005000009 [DOI] [PMC free article] [PubMed] [Google Scholar]