Abstract
Objective
To compare use of chlorhexidine with use of iodine for preoperative skin antisepsis with respect to effectiveness in preventing surgical site infections (SSIs) and cost.
Methods
We searched the Agency for Healthcare Research and Quality website, the Cochrane Library, Medline, and EMBASE up to January 2010 for eligible studies. Included studies were systematic reviews, meta-analyses, or randomized controlled trials (RCTs) comparing preoperative skin antisepsis with chlorhexidine and with iodine and assessing for the outcomes of SSI or positive skin culture result after application. One reviewer extracted data and assessed individual study quality, quality of evidence for each outcome, and publication bias. Meta-analyses were performed using a fixed-effects model. Using results from the meta-analysis and cost data from the Hospital of the University of Pennsylvania, we developed a decision analytic cost-benefit model to compare the economic value, from the hospital perspective, of antisepsis with iodine versus antisepsis with 2 preparations of chlorhexidine (ie, 4% chlorhexidine bottle and single-use applicators of a 2% chlorhexidine gluconate [CHG] and 70% isopropyl alcohol [IPA] solution), and also performed sensitivity analyses.
Results
Nine RCTs with a total of 3,614 patients were included in the meta-analysis. Meta-analysis revealed that chlorhexidine antisepsis was associated with significantly fewer SSIs (adjusted risk ratio, 0.64 [95% confidence interval, [0.51–0.80]) and positive skin culture results (adjusted risk ratio, 0.44 [95% confidence interval, 0.35–0.56]) than was iodine antisepsis. In the cost-benefit model baseline scenario, switching from iodine to chlorhexidine resulted in a net cost savings of $16–$26 per surgical case and $349,904–$568,594 per year for the Hospital of the University of Pennsylvania. Sensitivity analyses showed that net cost savings persisted under most circumstances.
Conclusions
Preoperative skin antisepsis with chlorhexidine is more effective than preoperative skin antisepsis with iodine for preventing SSI and results in cost savings.
Surgical site infections (SSIs), which occur in up to 30% of all surgical procedures,1 are associated with significant morbidity and mortality, including increased length of hospitalization2 and increases of 2-fold to 5-fold in hospital costs.3,4 The use of preoperative skin antisepsis is an important intervention aimed at reducing the risk of SSI. It decreases the concentration of bacteria colonizing the skin, with the goal of sterilizing the surgical field. The use of skin antisepsis is recommended by professional and public health organizations, including the Royal College of Surgeons of England,5 the Centers for Disease Control and Prevention,3 the Association of peri-Operative Registered Nurses,6 and the National Association of Theater Nurses.7
The Food and Drug Administration has approved several agents for preoperative skin antisepsis. Iodine, which oxidizes sulfhydryl groups and affects microbial protein structure and function, has been used in the operating room for decades. Potential disadvantages of iodine include an average drying time of 3 minutes for optimal function8 and skin irritation. There has been increased interest in the use of chlorhexidine, which does not require a waiting time between application and first surgical incision and functions by destroying the bacterial cell membrane. A potential disadvantage of chlorhexidine is its cost in comparison with iodine. Both antiseptic agents have been shown to decrease bacterial counts, and their use varies at different institutions. However, the efficacy of these 2 agents in comparison to one another in preventing SSI across surgical procedures is not well known. Of the studies comparing these 2 skin antiseptics, only 1 study had sufficient statistical power to evaluate the outcome of SSI.9 We therefore performed a systematic review of the literature and a meta-analysis to compare the effect of chlorhexidine preoperative skin antisepsis and iodine preoperative skin antisepsis on decreasing the risk of SSI.
Methods
Literature Review and Meta-analysis
We searched the website of the Agency for Healthcare Research and Quality (AHRQ), the Cochrane Library, Medline, and EMBASE for studies published up to January 2010. To identify relevant studies, we used medical subject headings and keywords for study designs (eg, “systematic review,” “meta-analysis,” and “randomized controlled trial [RCT]”), interventions (“iodine,” “iodophor,” and “chlorhexidine”), and outcomes (“surgical wound infection” and “cellulitis”). A complete list of electronic search terms and strategies for systematic reviews and primary studies is given in the Appendix. So-called “gray” literature, such as conference abstracts, unpublished studies, or data obtained from personal communications, was not included. One reviewer (I.L.) screened all studies by title and abstract, retrieved potentially relevant articles in full text, and assessed them for inclusion. Articles selected for inclusion met the following criteria: (1) they were systematic reviews, meta-analyses, or RCTs; (2) they were in the English language; (3) they compared preoperative chlorhexidine versus iodine skin antisepsis; (4) they evaluated adult surgical patients; and (5) they assessed for at least one of the outcomes of interest (ie, SSI was the primary outcome of interest and positive skin culture result after application of antiseptic was the secondary outcome of interest). All types of surgical procedures were included. Studies evaluating chlorhexidine shower, bath, foot bath, or oral rinse prior to entry into the operating room were excluded for the purposes of this study. The reference lists of the included RCTs were also screened to identify additional relevant articles.
The following data were extracted for each included study: authors, year of publication, study design, study population (ie, what type of surgery the patients underwent), antiseptic preparations used in the intervention and the comparison groups, sample size, outcomes (ie, the number of patients with SSI or a positive skin culture result after application in each group), and effect size reported as risk ratio (RR) with a confidence interval (CI). If no effect size was reported in the trial, the original data was utilized to obtain an RR with a CI. Meta-analysis was performed to obtain a pooled estimate of effect using a fixed effects model. Study heterogeneity was evaluated using the I2 test (I2 test ≥50% was considered to indicate significant heterogeneity) and the P value of the χ2 test for heterogeneity (P ≤ .10 was considered to indicate significant heterogeneity).10 Publication bias was assessed using funnel plots, the Egger (weighted regression) method,11 and the Begg (rank correlation) method.12 All statistical analyses were performed with Review Manager, version 5.0.23 (Nordic Cochrane Centre) and Stata, version 10.0 (Stata).
The quality of each included RCT was graded using a 9-point scale combining elements from the Jadad and the Chalmers scales.13,14 This scale evaluates 3 key areas: randomization (ie, was the study described as randomized and was randomization performed appropriately), blinding (ie, was the study described as double blinded, was the outcome assessor blinded, was the study participant blinded, and was the investigator blinded), and patient attrition (ie, was attrition described, was it less than 10%–15% of the assigned patients, and was it appropriately analyzed). We then assessed the quality of evidence for each outcome (ie, SSI and positive skin culture result after application) using the following criteria proposed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group: study quality, consistency, directness, precision, publication bias, large magnitude, dose-response, and unmeasured confounding.15
Cost-Benefit Analysis
Using TreeAge Pro 2009 (TreeAge Software), we developed a cost-benefit decision analytic model depicting the decision of whether to use chlorhexidine or iodine for a patient about to undergo surgery. The cost of implementing each strategy included the purchase costs of the antiseptic agents. For each antisepsis strategy, the patient then had a probability of subsequently developing an SSI based on the results of the meta-analysis. Patients who developed an SSI incurred a set of costs different from those incurred by patients who did not develop an infection.
A resource-use review of all surgical cases at the Hospital of the University of Pennsylvania (HUP) during fiscal year 2007 (FY2007) determined the mean costs associated with patients who did and patients who did not develop SSI. These cost calculations accounted for all direct variable supply costs associated with each surgical encounter, including use of rooms (eg, patient, operating, and procedure rooms), personnel (eg, physician, physical therapy, nursing, and technician personnel), and medical supplies (eg, reagents for laboratory tests, tubes and stoppers, slides, and imaging materials).
We used separate scenarios to compare the use of 2 different preparations of chlorhexidine (ie, a 113 g [4 oz] bottle of 4% chlorhexidine; and single-use applicators of a 2% chlorhexidine gluconate (CHG) and 70% isopropyl alcohol [IPA] solution) with a single preparation of iodine (118 mL 7.5% povidone-iodine surgical scrub). One-way and 2-way sensitivity analyses explored the effects of varying the SSI probability reduction from use of chlorhexidine (compared with use of iodine) and the incremental cost of an SSI (compared with no SSI). The incremental cost of use of chlorhexidine (based on the number of 2% CHG / 70% IPA single-use applicators used) was also evaluated. The range of the incremental cost of an SSI that we used in the sensitivity analyses was derived from a systematic review previously performed by our center; that study reported costs of healthcare-associated infections, including SSI, reported in the literature (converting to 2009 US$, and adjusting costs if possible).16
Results
Included Studies
Of 1,508 studies identified for title and abstract screening, 18 articles underwent review of the full text, and 9 RCTs were included in our analysis (Figure 1). The one systematic review identified in the Cochrane Library,17 which evaluated the effect of skin antiseptics in clean surgeries, was excluded because only 1 of the 7 RCTs included in that review evaluated use of chlorhexidine; that RCT18 was also included in our systematic review. No additional trials were identified after reviewing the reference lists of the included RCTs.
Figure 1.
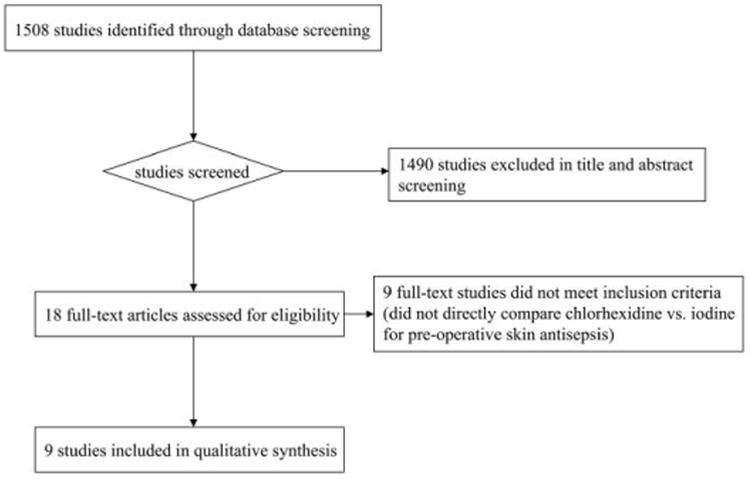
Flow diagram summarizing inclusion in the meta-analysis of studies on the use of chlorhexidine, compared with use of iodine, for preoperative skin antisepsis.
The 9 trials included had a total of 3,614 patients (Table 1).9,18-25 Five trials reported on patients who had undergone a single type of surgery,20-24 and 4 studies included a mix of surgical patients.9,18,19,25 The intervention group used chlorhexidine scrub and/or paint in varying concentrations (from 0.5% to 4%) with or without alcohol in all but 1 study, which evaluated a spray of chlorhexidine in IPA. The comparison group used povidone-iodine or iodophor scrub and/or paint in varying concentrations (from 0.7% to 10%) with or without alcohol. Four trials only reported results with respect to SSI,9,18,21,25 2 trials only reported on positive skin culture results,22,24 and 3 trials reported on both SSI and positive skin culture results.19-23 One trial that reported positive skin culture results from multiple sampling sites was excluded from the meta-analysis for this outcome.23
Table 1. Summary of Included Studies Comparing Use of Chlorhexidine with Use of Iodine for Preoperative Skin Antisepsis to Prevent Surgical Site Infection (SSI).
| Antisepsis | No. of patients | No. of events | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Study | Study population | Test formulation | Comparison formulation | Overall | Test group | Control group | Outcome | Test group | Control group | RR (95% CI) | P |
| Darouiche et al,9 2010 | Patients undergoing clean-contaminated surgerya | Chlor 2% in 70% IPA | PVI 10% | 849 | 409 | 440 | SSI | 39 | 71 | 0.59 (0.41–0.85) | |
| Paocharoen et al,19 2009 | Patients with clean, clean contaminated, and contaminated wounds and ASA class 1 and 2 | Chlor 4% in 70% IPA scrub and paint | PVI scrub and paint | 500 | 250 | 250 | SSI | 5 | 8 | 0.62 (0.55–0.71) | |
| Saltzman et al,20 2009 | Patients undergoing shoulder surgery | CHG 2% and IPA 70% | Iodophor 0.7% and IPA 74% PVI 0.75% scrub and PVI 1.0% paint |
150 | 50 | 50 50 |
PC SSI PC SSI PC |
36 0 4 0 4 |
78 0 10 0 16 |
0.37(0.28–0.47) NA 0.40b NA 0.25 (0.09–0.70)b |
.01 <.0001 |
| Veiga et al,21 2008 | Patients undergoing elective and clean plastic surgery | Chlor 0.5% paint | PVI 10% paint | 250 | 125 | 125 | SSI | 0 | 4 | 0.11 (0.01–2.04)b | .06 |
| Culligan et al,22 2005 | Patients undergoing vaginal surgery | CHG 4% scrub | PVI 10% scrub | 50 | 23 | 27 | PC | 5 | 17 | 0.16 (0.04–0.59) | .003 |
| Ostrander et al,23 2005 | Patients undergoing surgery of the foot and ankle | CHG 2% and IPA 70% | Iodine 0.7% and IPA 74% | 85c | 40 | 45 | SSI PC (hallux) PC (toe) PC (control) |
1 12 9 4 |
0 29 20 10 |
3.37 (0.14–80.36)b 0.47 (0.28–0.78)b 0.51 (0.26–0.98)b 0.45 (0.15–1.32)b |
<.1 <.01 <.05 <.2 |
| Bibbo et al,24 2005 | Patients with intact, uninfected skin having clean, elective foot and ankle surgery | CHG 4% scrub (7-minute) and IPA 70% paint | PVI 7.5% scrub (7-min) with PVI 10% paint | 127 | 60 | 67 | PC | 23 | 53 | 0.48 (0.34–0.68)b | |
| Brown et al,25 1984 | Patients undergoing laparotomy, mastectomy, and cesarean delivery | CHG 0.5% spray in IPA 70% | PVI 0.75% scrub and paint | 737 | 378 | 359 | SSI | 23 | 29 | 0.75 (0.44–1.28)b | |
| Berry et al,18 1982 | Patients undergoing elective surgery | Chlor 0.5% in spirit paint | PVI 10% in IPA paint | 866 | 453 | 413 | SSI | 44 | 61 | 0.66 (0.46–0.95)b | .03 |
Note: ASA class, America Society of Anesthesiologists physical status classification; CHG, chlorhexidine gluconate; Chlor, chlorhexidine; CI, confidence interval; IPA, isopropyl alcohol; PC, positive skin culture result; PVI, povidone-iodine; RR, risk ratio.
Colorectal, small intestinal, gastroesophageal, biliary thoracic, gynecologic, or urologic operations performed under controlled conditions without substantial spillage or unusual contamination.
Calculated using RevMan.
Forty patients received chloroxylenol and were not included in the analyses.
In assessing individual study quality using the 9-point scale combining elements from the Jadad and Chalmers scales, there was 1 study with 1 point, 3 studies with 4 points, 4 studies with 5 points, and 1 study with 9 points. All studies were described as randomized trials, although only 5 studies described the randomization process. Only 1 study mentioned blinding of the investigator, study participant, or outcome assessor. Most studies had no patient attrition because the outcome reported on was positive skin culture result for a sample obtained immediately after application of the skin antiseptic. Only one study that evaluated SSI reported on patient attrition that occurred during the observation period.
SSI
Three trials reported significantly fewer SSIs with chlorhexidine, compared to iodine.9,18,19 Two other trials found nonsignificant decreases in the number of SSIs with chlorhexidine use,21,25 1 trial found a nonsignificant increase in the number of SSIs,23 and 1 trial reported no SSIs in either study group.20 We found no evidence of publication bias for the outcome of SSI, on the basis of visual inspection of funnel plots (Figure 2), the Egger method (P = .99), and the Begg method (P = .43).
Figure 2.
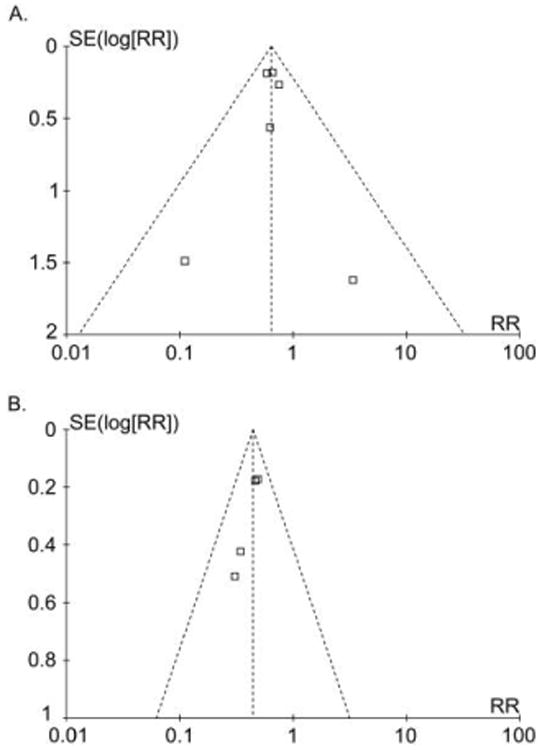
Funnel plots for surgical site infection (A) and for positive skin culture result (B) after application of chlorhexidine, compared with iodine, for preoperative skin antisepsis.
Meta-analysis of 7 studies that evaluated the outcome of SSI reported that use of chlorhexidine significantly decreased the risk for SSI, compared with use of iodine (adjusted RR, 0.64 [95% CI, 0.51–0.80]) (Figure 3). No significant study heterogeneity was found (I2 test, 0%; χ2 test, P = .70). Based on the GRADE criteria, there is moderate quality evidence assessing the outcome of SSI (a deduction of 1 point was made for study quality).
Figure 3.
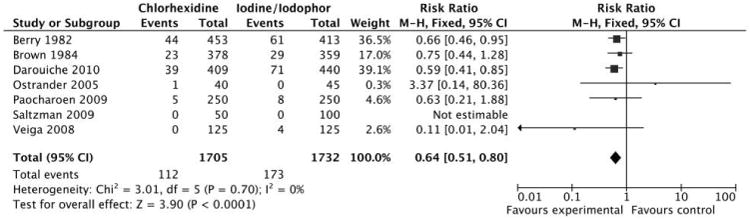
Meta-analysis of 7 studies that evaluated use of chlorhexidine, compared with use of iodine, for preoperative skin antisepsis with surgical site infection as the outcome.
Positive Skin Culture Results after Skin Antisepsis
Four studies reported significantly decreased numbers of positive culture results after chlorhexidine skin preparation, compared with iodine use.19,20,22,24 One other study, by Ostrander and colleagues,23 identified significantly decreased numbers of positive culture results for hallux and toe samples, and no difference for samples from the control site. This study was not included in the meta-analysis because the authors sampled 3 different skin sites with varying results.23 We found potential publication bias for the secondary outcome of positive skin culture result after skin preparation by visual inspection of funnel plots (Figure 2), the Egger method (P = .07), and the Begg method (P = .09).
Meta-analysis of 4 studies found that use of chlorhexidine significantly decreased the risk for a positive skin culture result after application, compared with iodine (adjusted RR, 0.44 [95% CI, 0.35–0.56]) (Figure 4). No significant study heterogeneity was found (I2 test, 0%; χ2 test, P = .78). Based on the GRADE criteria, there is moderate quality evidence available for the outcome of positive skin culture result (2 points were deducted for study quality and potential publication bias, and 1 point was added for large effect size).
Figure 4.
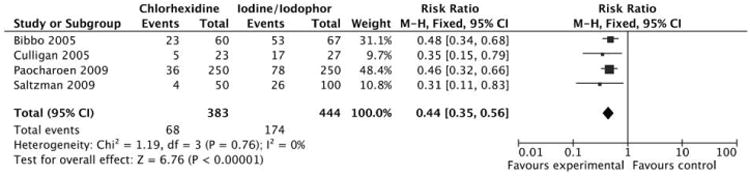
Meta-analysis of 4 studies that evaluated use of chlorhexidine, compared with use of iodine, for preoperative skin antisepsis with positive skin culture result as the outcome.
Cost-Benefit Analysis
In FY2007, there were 285 SSIs following a total of 21,869 surgeries performed at HUP. The mean cost per case for patients who did not develop SSI was $5,356, compared to $13,537 for patients who did develop SSI. The purchase prices of the antiseptic agents were: $6.0736 per 26-mL single-use applicator of 2% CHG / 70% IPA, $1.678 per 113-g bottle of 4% chlorhexidine, and $1.416 per 118-mL surgical scrub with 7.5% povidone-iodine (Table 2).
Table 2. Base Case Estimates and Ranges Used in Cost-Benefit Analysis Comparing Use of Chlorhexidine with Use of Iodine for Preoperative Skin Antisepsis.
| Description | Base case value (range) | References and/or source |
|---|---|---|
| Cost, US$ | ||
| Incremental cost of SSI | 8,181 (2,200–12,900) | [16, 36–39], HUPa |
| Iodine (118 mL) | 1.416 | HUPa |
| 4% chlorhexidine (113 g [4 oz]) | 1.678 | HUPa |
| 2% CHG / 70% IPA single-use applicators | 6.0736b | HUPa |
| Reduction in number of SSIs with chlorhexidine, % | 25 (15–35) | See Figure 3 |
Note: CHG, chlorhexidine gluconate; IPA, isopropyl alcohol; SSI, surgical site infection.
Hospital of the University of Pennsylvania (HUP) estimate.
Cost per single-use applicator.
Scenarios for single-use applicator of 2% CHG / 70% IPA
For the baseline case (in which we used a conservative 25% greater reduction in the number of SSIs with use of 2% CHG / 70% IPA single-use applicators), switching from use of iodine to use of the single-use applicators resulted in an incremental cost savings of $16 per case (the difference between $5,462 per case for iodine and $5,446 per case for the CHG / IPA applicators). On the basis of the annual surgical volume at HUP, this translated to a $349,904 annual cost savings to the hospital. Sensitivity analyses showed how the net cost savings from such a switch would change when varying the incremental reduction in the number of SSIs between 2% CHG / 70% IPA single-use applicators and iodine. When the incremental reduction in the number of SSIs was 35%, switching to 2% CHG / 70% IPA single-use applicators conferred a $26 cost savings per surgical case ($568,594 annual cost savings to HUP). When the incremental reduction was 15%, the cost savings per surgical case was $5 and the annual HUP cost savings was $109,345. The net cost savings threshold occurred at an incremental reduction of 10%; that is, if 2% CHG / 70% IPA single-use applicators yielded a reduction in the number of SSIs that was more than 10% the reduction yielded with iodine, switching to 2% CHG / 70% IPA single-use applicators provided net cost savings.
A $2,200 incremental cost of SSI (ie, the difference in overall costs between a patient with an SSI and a patient without an SSI) yielded a net cost savings of $65,607 with iodine skin antisepsis. However, for an incremental cost per SSI of more than $3,000, switching from iodine to 2% CHG / 70% IPA single-use applicators generated net cost savings. For a $5,000 incremental SSI cost, this switch would result in $6 cost savings per surgical case and $131,214 annual cost savings for HUP; for a $12,900 incremental SSI cost, the savings were $31 and $677,939, respectively.
Switching to 2% CHG / 70% IPA single-use applicators continued to generate net cost savings even when increasing the number of applicators used from 2 to 4. Use of 3 applicators per case lowered cost savings to $9 per case and $196,821 annually for HUP, and using 4 applicators per case further reduced costs savings to $3 per case and $65,607 annually for HUP.
Figure 5 shows the results of 2-way sensitivity analyses when varying incremental cost and incremental reduction in the number of SSIs for different numbers of applicators used and the frontiers at which use of the 2% CHG / 70% IPA single-use applicator is more cost-beneficial than use of iodine.
Figure 5.
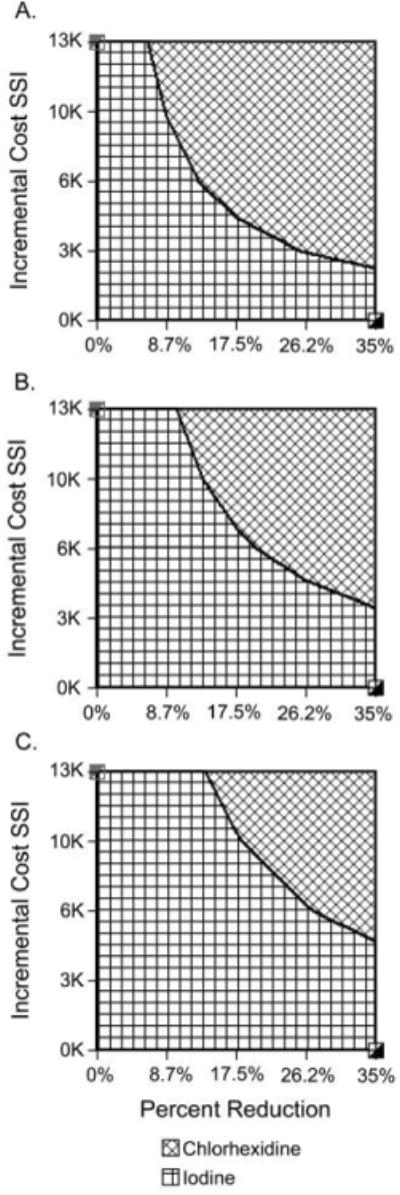
Two-way sensitivity analyses comparing use of single-use applicators of a 2% chlorhexidine gluconate (CHG) and 70% isopropyl alcohol (IPA) solution with use of iodine for preoperative skin antisepsis and varying the incremental cost of surgical site infection (SSI) and the incremental reduction in the number of SSIs. A, Analyses with use of 2 applicators. B, Analysis with use of 3 single-use applicators. C, Analysis with use of 4 applicators.
Scenarios with bottles of 4% chlorhexidine
Switching from use of iodine to use of 113 g bottles of chlorhexidine introduced even greater net cost savings. For the baseline case (in which we used a conservative 25% greater reduction in the number of SSIs with use of chlorhexidine), use of chlorhexidine yielded an incremental cost savings of $26 per surgical case (the difference between the mean cost of $5,436 per surgical case for chlorhexidine and $5,462 per surgical case for iodine). This translates to an annual cost savings of $568,594 for HUP.
The greater cost savings for switching from use of iodine to use of bottles of chlorhexidine (compared with switching to 2% CHG / 70% IPA single-use applicators) persisted when sensitivity analyses varied the incremental reduction in the number of SSIs. For an incremental SSI reduction of 35%, switching from iodine to chlorhexidine bottles yielded a cost savings of $37 per surgical case and $809,153 annual cost savings to HUP. For a lower incremental SSI reduction of 10%, the switch yielded $10 cost savings per surgical case and $218,690 annual cost savings for HUP. As long as chlorhexidine was more effective than iodine in reducing the incidence of SSI (ie, the incremental SSI reduction was greater than 0%), switching from iodine to chlorhexidine provided net cost savings.
We obtained similar findings when we varied the incremental SSI cost in sensitivity analyses: for the switch from iodine to chlorhexidine, a $2,200 incremental SSI cost conferred a cost savings of $7 per case and $153,083 annual cost savings for HUP. When the incremental SSI cost moved to $12,900, switching saved $42 per case ($918,498 annually for HUP). As long as the incremental SSI cost was greater than $0, switching to use of 113 g bottles of chlorhexidine provided net cost savings. Figure 6 shows the results of 2-way sensitivity analyses varying both the incremental SSI cost and the incremental SSI reduction, and shows that the switch to chlorhexidine generated cost savings for most combinations of these parameters.
Figure 6.
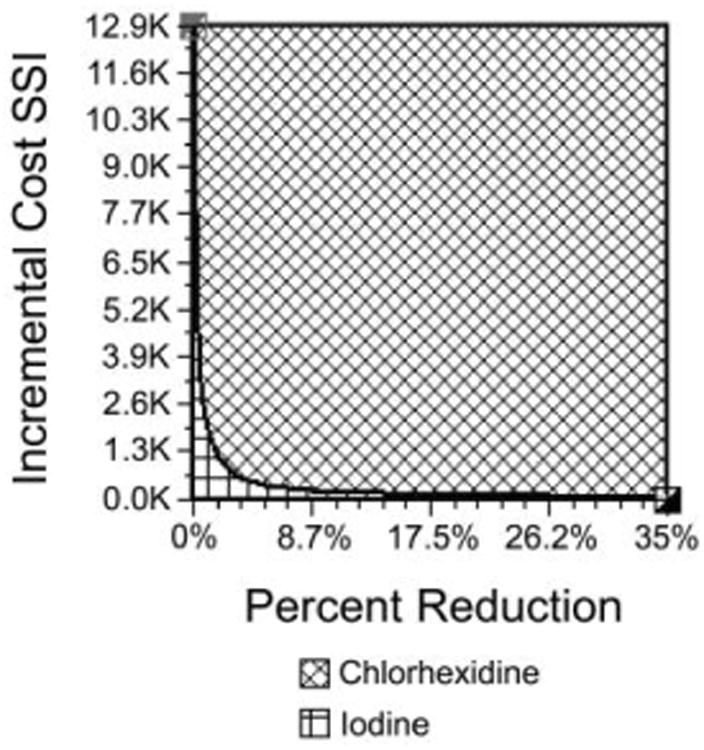
Two-way sensitivity analyses comparing use of 4% chlorhexidine bottles (113 g [4 oz]) with use of iodine for preoperative skin antisepsis and varying the incremental cost of surgical site infection (SSI) and the incremental reduction in the number of SSIs.
Discussion
We report that there is moderate-quality evidence supporting the use of chlorhexidine over iodine for preoperative skin antisepsis to prevent SSI. Additionally, there is moderate quality evidence that use of chlorhexidine is associated with fewer positive skin culture results after application.
There was a 36% reduction in the number of SSIs among patients who received preoperative skin antisepsis with chlorhexidine, compared with those who received iodine. This estimate was similar for most studies, regardless of the surgical procedure involved, the concentration of chlorhexidine used, and whether the chlorhexidine preparation included alcohol. Three studies had point estimates differing from the pooled estimate.20,21,23 These studies had very few events: there were a total of 4 SSIs in the study by Veiga and colleagues,21 1 SSI in the study by Ostrander and colleagues,23 and no SSIs in the study by Saltzmann and colleagues.20 This dramatic reduction associated with chlorhexidine use has potentially significant implications. One drawback of preoperative skin antisepsis with chlorhexidine has been cost-related. However, given the high costs and increased length of hospitalization associated with SSI, preventing these infections would likely result in significantly decreased lengths of stay after surgery and overall cost savings.
In this era of cost containment, demonstrating the economic value of an infection control intervention is pivotal to driving its adoption. Our study suggests that switching from iodine to the more expensive chlorhexidine can actually provide net cost savings for a hospital or healthcare system—compelling evidence to make such a switch. These findings were fairly robust with respect to changes in key variables, such as the efficacy of chlorhexidine in preventing SSIs, the incremental cost of SSI, and the incremental cost of chlorhexidine.
Use of chlorhexidine was also associated with an overall 56% reduction in positive skin culture results after skin preparation. This reduction was likely greater than the reduction in number of SSIs, because preoperative skin antisepsis is typically used to sterilize the surgical field in hopes of preventing surgical wound contamination, which can then result in SSI. Despite the adequate efficacy of skin antiseptics, SSI may still occur in several ways. Wounds can become contaminated from another source during surgery (eg, by gastrointestinal flora during abdominal surgery) or can become contaminated after surgery, after the effects of the skin antiseptic have worn off.
Our findings are comparable to those reported in studies evaluating the use of skin antiseptics for decreasing the incidence of catheter-associated bloodstream infection. The purpose of using chlorhexidine and iodine for catheter-site care is similar to the purpose for their use preoperatively: to decrease the level of bacterial contamination of the skin. Chaiyakunapruk and colleagues26 conducted a meta-analysis of 8 RCTs and reported that catheter-site care with chlorhexidine was associated with significantly decreased risk for catheter-related bloodstream infection, compared with use of iodine (RR, 0.49 [95% CI, 0.28–0.88]). They hypothesized that chlorhexidine may have higher efficacy because protein-rich biomaterials found in blood and on skin may decrease the antimicrobial effects of povidone-iodine but not those of chlorhexidine,27,28 and chlorhexidine may have a longer duration of activity.29
There are several potential limitations to our study. First, this systematic review did not include studies published in languages other than English and did not include “gray” literature. The effect of excluding trials published in languages other than English in systematic reviews and meta-analyses remains uncertain, with conflicting reports as to whether it affects overall results or effect sizes.11,30-33 Additionally, there is conflicting evidence regarding whether the quality of studies published in languages other than English may differ on the basis of the intervention studied.34,35 Given these uncertainties, coupled with the difficulties associated with obtaining and accurately translating manuscripts published in languages other than English, we elected to limit our systematic review to studies published in English. We also excluded “gray” literature (such as conference abstracts, unpublished studies, or data obtained from personal communications) because these have not undergone peer review and thus the validity of their results may be less certain.
Second, there were 3 studies that contributed more than 75% of the patients in the meta-analysis. However, almost all of the studies favored use of chlorhexidine, and there was no significant study heterogeneity identified using the I2 test and the χ2 test. Third, only a single reviewer screened the potential articles for inclusion. However, we verified that all relevant articles were captured by reviewing the reference lists of the systematic review captured by the search of the Cochrane Library, and the reference lists of all included RCTs. These additional measures did not yield any additional studies for inclusion. Last, the base case estimates for our cost analysis were derived from our institution (HUP). However, to ensure generalizability of our results to other institutions, we performed 1-way and 2-way sensitivity analyses using ranges derived from the existing literature, and obtained similar results.
Use of chlorhexidine for preoperative skin antisepsis is associated with a 36% reduction in the number of SSIs, compared with use of iodine. Although chlorhexidine is more costly than iodine, this dramatic reduction in the number of SSIs will likely result in greater overall cost savings with chlorhexidine use. Given the clinical and economic benefits, use of chlorhexidine should be considered over use of iodine for standard preoperative skin antisepsis. However, further studies are needed to evaluate what preparation of chlorhexidine (eg, what concentration and whether the preparation includes alcohol) is most effective in decreasing the incidence of SSI.
Acknowledgments
We thank Michael Mercincavage for his assistance in providing data for the cost benefit analysis and Jeffrey Miller for his assistance in developing the search strategies.
Financial support. Investigator time was supported by the National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) grant 1U54GM088491–0109 and the Pennsylvania Department of Health (BYL). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix. Electronic Search Strategies
Table A1. Search Strategy Used to Find Systematic Reviews.
| Source and search | Keywords or syntax |
|---|---|
| 1. AHRQ | Chlorhexidine; surgical-site infection |
| 2. Cochrane Library | Chlorhexidine; surgical-site infection |
| 3. Medline | |
| Search 1 | Exp Detergents/ |
| Search 2 | Exp Povidone-iodine/ |
| Search 3 | Exp Chlorhexidine/ |
| Search 4 | Exp Disinfection/ |
| Search 5 | Betadine.mp |
| Search 6 | Exp Surgical Wound Infection/ |
| Search 7 | Exp Preoperative Care/ |
| Search 8 | Exp Perioperative Care/ |
| Search 9 | Exp Skin Care/ |
| Search 10 | 1 OR 2 OR 3 OR 4 OR 5 |
| Search 11 | 6 OR 7 OR 8 OR 9 |
| Search 12 | 10 AND 11 |
| Search 13 | Limit 12 to (English language AND humans AND “all adults (19 plus years)” AND (meta analysis OR practice guideline OR “review”) |
| 3. EMBASE | |
| Search 1 | “detergent”/exp |
| Search 2 | “povidone”/exp |
| Search 3 | “chlorhexidine”/exp |
| Search 4 | “disinfectant agent”/exp |
| Search 5 | “surgical infection”/exp |
| Search 6 | “wound infection”/exp |
| Search 7 | “postoperative infection”/exp |
| Search 8 | “preoperative care”/exp |
| Search 9 | “skin care”/exp |
| Search 10 | 1 OR 2 OR 3 OR 4 |
| Search 11 | 5 OR 6 OR 7 OR 8 OR 9 |
| Search 12 | 10 AND 11 |
| Search 13 | Limit 12 to ([Cochrane review]/lim OR [meta analysis]/lim OR [systematic review]/lim) AND ([adult]/limt OR [aged]/lim) AND [humans]/lim AND {English]/lim |
Table A2. Search Strategy Used to Find Primary Studies.
| Source and search | Syntax |
|---|---|
| 1. MEDLINE | |
| Search 1 | Exp Anti-infective Agents, Local/ |
| Search 2 | Exp Disinfectants/ |
| Search 3 | Exp Chemoprevention/ |
| Search 4 | Exp Antisepsis/mt [Methods] |
| Search 5 | Exp Chlorhexidine |
| Search 6 | Exp Iodine/ |
| Search 7 | Exp Povidone-Iodine/ |
| Search 8 | Exp iodophors/ |
| Search 9 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 |
| Search 10 | Exp Skin Care/ |
| Search 11 | Exp Cellulitis/ |
| Search 12 | Exp Surgical Wound Infection/ |
| Search 13 | Exp Bacterial infections/su [Surgery] |
| Search 14 | Exp Preoperative Care/ |
| Search 15 | Exp Perioperative Care/ |
| Search 16 | Exp Bacterial Infections/pc [Prevention & Control] |
| Search 17 | 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 |
| Search 18 | 9 AND 17 |
| Search 19 | Limit 18 to (English language AND humans AND (clinical trial OR randomized controlled trial)) |
| 2. EMBASE | |
| Search 1 | “antiinfective agent”/exp/dd_tp |
| Search 2 | “disinfectant agent”/exp/dd_tp |
| Search 3 | “chemoprophylaxis”/exp |
| Search 4 | “antisepsis”/exp |
| Search 5 | “chlorhexidine”/exp |
| Search 6 | “iodine”/exp |
| Search 7 | “povidone iodine”/exp |
| Search 8 | “iodophor”/exp |
| Search 9 | “skin care”/exp |
| Search 10 | “cellulitis”/exp |
| Search 11 | “surgical infection”/exp |
| Search 12 | “bacterial skin disease”/exp |
| Search 13 | “preoperative care”/exp |
| Search 14 | “perioperative period”/exp |
| Search 15 | “antibiotic prophylaxis”/exp |
| Search 16 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 |
| Search 17 | #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 |
| Search 18 | #16 AND #17 |
| Search 19 | 18 (Limit to article AND human AND English AND embase AND ([adult]/lim OR [aged]/lim)) |
Footnotes
Potential conflicts of interest. All authors report no potential conflicts of interest relevant to this article.
References
- 1.Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technol Assess. 2001;5(22):1–194. doi: 10.3310/hta5220. [DOI] [PubMed] [Google Scholar]
- 2.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 3.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Clinical Excellence (NICE) [Accessed October 1, 2010];Surgical site infection: draft full guideline. 2006 http://www.nice.org.uk/CG74.
- 5.Leaper DJ, Orr C, Maung Z, White A. Inflammation and Infection: STEP 2000 Module II. Royal College of Surgeons of England. Blackwell Science; 2001. [Google Scholar]
- 6.AORN. Standards, Recommended Practices, and Guidelines. Denver: 2006. [DOI] [PubMed] [Google Scholar]
- 7.National Association of Theatre Nurses (NATN) NATN standards and recommendations for safe perioperative practice. Harrogate: NATN; 2004. [DOI] [PubMed] [Google Scholar]
- 8.3M DuraPrep surgical solution (iodine povacrylex [0.7% available iodine] and isopropyl alcohol, 74% w/w) patient preoperative skin preparation. http://solutions.3m.com/wps/portal/3M/en_US/SH/SkinHealth/products/catalog/?PC_7_RJH9U5230GE3E02LECFTDQG207_nid=GSF83Z3YYXbeJLRV63SXXBgl.
- 9.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362(1):18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 10.Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: 2008. p. 501. [Google Scholar]
- 11.Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350(9074):326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]
- 12.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers TC, Smith H, Jr, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2(1):31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of reasonably preventable healthcare associated infections and associated mortality and costs; Program and abstracts of the 19th Annual Scientific Meeting of the Soceity for Healthcare Epidemiology of America; 2009. [Google Scholar]
- 17.Edwards PS, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2004;(3):003949. doi: 10.1002/14651858.CD003949.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Berry AR, Watt B, Goldacre MJ, Thomson JW, McNair TJ. A comparison of the use of povidone-iodine and chlorhexidine in the prophylaxis of postoperative wound infection. J Hosp Infect. 1982;3(1):55–63. doi: 10.1016/0195-6701(82)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.Paocharoen V, Mingmalairak C, Apisarnthanarak A. Comparison of surgical wound infection after preoperative skin preparation with 4% chlorhexidine [correction of chlohexidine] and povidone iodine: a prospective randomized trial. J Med Assoc Thai. 2009;92(7):898–902. [PubMed] [Google Scholar]
- 20.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009;91(8):1949–1953. doi: 10.2106/JBJS.H.00768. [DOI] [PubMed] [Google Scholar]
- 21.Veiga DF, Damasceno CA, Veiga-Filho J, Figueiras RG, Vieira RB, Florenzano FH, et al. Povidone iodine versus chlorhexidine in skin antisepsis before elective plastic surgery procedures: a randomized controlled trial. Plast Reconstr Surg. 2008;122(5):170e–171e. doi: 10.1097/PRS.0b013e318186cd7f. [DOI] [PubMed] [Google Scholar]
- 22.Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J. A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. 2005;192(2):422–425. doi: 10.1016/j.ajog.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Ostrander RV, Botte MJ, Brage ME. Efficacy of surgical preparation solutions in foot and ankle surgery. J Bone Joint Surg Am. 2005;87(5):980–985. doi: 10.2106/JBJS.D.01977. [DOI] [PubMed] [Google Scholar]
- 24.Bibbo C, Patel DV, Gehrmann RM, Lin SS. Chlorhexidine provides superior skin decontamination in foot and ankle surgery: a prospective randomized study. Clin Orthop. 2005;438:204–208. doi: 10.1097/01.blo.0000167832.47464.22. [DOI] [PubMed] [Google Scholar]
- 25.Brown TR, Ehrlich CE, Stehman FB, Golichowski AM, Madura JA, Eitzen HE. A clinical evaluation of chlorhexidine gluconate spray as compared with iodophor scrub for preoperative skin preparation. Surg Gynecol Obstet. 1984;158(4):363–366. [PubMed] [Google Scholar]
- 26.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792–801. doi: 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 27.Zamora JL, Price MF, Chuang P, Gentry LO. Inhibition of povidone-iodine's bactericidal activity by common organic substances: an experimental study. Surgery. 1985;98(1):25–29. [PubMed] [Google Scholar]
- 28.Larson E, Bobo L. Effective hand degerming in the presence of blood. J Emerg Med. 1992;10(1):7–11. doi: 10.1016/0736-4679(92)90003-c. [DOI] [PubMed] [Google Scholar]
- 29.Ayliffe GA. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23–27. doi: 10.1017/s0195941700058756. [DOI] [PubMed] [Google Scholar]
- 30.Gregoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias? J Clin Epidemiol. 1995;48(1):159–163. doi: 10.1016/0895-4356(94)00098-b. [DOI] [PubMed] [Google Scholar]
- 31.Chalmers TC, Berrier J, Sacks HS, Levin H, Reitman D, Nagalingam R. Meta-analysis of clinical trials as a scientific discipline. II: Replicate variability and comparison of studies that agree and disagree. Stat Med. 1987;6(7):733–744. doi: 10.1002/sim.4780060704. [DOI] [PubMed] [Google Scholar]
- 32.Chalmers TC, Levin H, Sacks HS, Reitman D, Berrier J, Nagalingam R. Meta-analysis of clinical trials as a scientific discipline. I: Control of bias and comparison with large co-operative trials. Stat Med. 1987;6(3):315–328. doi: 10.1002/sim.4780060320. [DOI] [PubMed] [Google Scholar]
- 33.Pham B, Klassen TP, Lawson ML, Moher D. Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol. 2005;58(8):769–776. doi: 10.1016/j.jclinepi.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Fortin P, Jadad AR, Juni P, Klassen T, Le Lorier J, et al. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet. 1996;347(8998):363–366. doi: 10.1016/s0140-6736(96)90538-3. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41):1–90. doi: 10.3310/hta7410. [DOI] [PubMed] [Google Scholar]
- 36.Herwaldt LA, Cullen JJ, Scholz D, French P, Zimmerman MB, Pfaller MA, et al. A prospective study of outcomes, healthcare resource utilization, and costs associated with postoperative nosocomial infections. Infect Control Hosp Epidemiol. 2006;27(12):1291–1298. doi: 10.1086/509827. [DOI] [PubMed] [Google Scholar]
- 37.Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Complications and costs after high-risk surgery: where should we focus quality improvement initiatives? J Am Coll Surg. 2003;196(5):671–678. doi: 10.1016/S1072-7515(03)00122-4. [DOI] [PubMed] [Google Scholar]
- 38.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 39.Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9(2):196–203. doi: 10.3201/eid0902.020232. [DOI] [PMC free article] [PubMed] [Google Scholar]


