Abstract
Perinatal mood disorders, such as postpartum depression (PPD) are costly for society, with potentially serious consequences for mother and child. While multiple genes appear to play a role in PPD susceptibility, the contributions of specific genetic variations remain unclear. Previously implicated as a candidate gene, the estrogen receptor alpha gene (ESR1) is a key player in mediating hormonal differences during pregnancy and the postpartum period. This study addresses genetic factors in perinatal mood disorders, testing 9 polymorphisms in ESR1. 257 postpartum women were screened for mood disorders, including 52 women with PPD and 32 without any symptoms of mood disorders. We detected a significant association for the upstream TA microsatellite repeat with the Edinburgh Postnatal Depression Scale (p=0.007). The same variant was also associated with the occurrence of PPD. Separately, 11 candidate functional polymorphisms in 7 additional genes were genotyped to investigate gene-gene interaction with the ESR1 TA repeat, identifying a potential interaction with the serotonin transporter. Our results support a role for ESR1 in the etiology of PPD, possibly through the modulation of serotonin signaling. Our findings for ESR1 could have broad implications for other disorders and therapies that involve estrogens.
Keywords: Post-partum depression, Edinburgh Postnatal Depression Scale, ESR1 Estrogen receptor, Genetic variation, SNP
Introduction
Mood and anxiety disorders predominately affect women over men at a rate of 2 to 1, a sex difference likely determined by genomic as well as hormonal factors. Postpartum depression (PPD) affects between 10% and 15% of women (Steiner 1998; Steiner et al. 2003) and may constitute a major health-related concern for society. Postpartum depression, defined as depression within one year of delivery, is a serious, disabling disorder requiring medical attention; this condition should not be confused with “the baby blues”, a much less debilitating condition that affects 50%-80% of all new mothers. Perinatal depression is associated with both physiological and behavioral adverse effects on the offspring. Children of mothers with PPD are more likely to have failure-to-thrive, behavioral problems, and suboptimal cognitive and social development, relative to children with healthy mothers (Lazinski et al. 2008). These consequences, together with high risk of non-puerperal relapse in the mother (Bell et al. 1994), indicate that PPD constitutes a serious mental health risk for women.
Multiple genes may play a role in PPD susceptibility, and the contributions of specific genetic variations to the disorder remain unclear. The role of hormones in the onset of perinatal psychiatric disorders has long been hypothesized (Nott et al. 1976; Deecher et al. 2008), because the hormonal fluctuations that accompany pregnancy and childbirth are greater than those experienced at any other time in life. Estrogens can act on multiple central nervous system pathways through a variety of mechanisms (Beyer et al. 2003), such as affecting transcription by binding to intracellular estrogen receptor encoded by ESR1 and ESR2 in target tissues (Stahl 2001), and also through non-classical second messenger systems (McEwen 2001; Kugaya et al. 2003; Lokuge et al. 2010; Lokuge et al. 2011a). Among several pathways, estrogens modulate serotonin transmission (Bethea et al. 2002).
PPD and major depression (MDD) share similarities, supported by genetic and family studies in both populations (Treloar et al. 1999; Murphy-Eberenz et al. 2006; Forty et al. 2006; Payne et al. 2007; Sanjuan et al. 2008; Costas et al. 2010; Figueira et al. 2010; Doornbos et al. 2009; Xie and Innis 2009; Mahon et al. 2009) with a focus on genes involved in the serotonergic pathway (Sun et al. 2004; Yu et al. 2002; Bellivier et al. 2000; Caspi et al. 2003b; Cervilla et al. 2006; Murphy et al. 2004; Mrazek et al. 2008; Anguelova et al. 2003b, a), GABA (Amin et al. 2006; Epperson et al. 2006), and other neurotransmitter systems (Zill et al. 2002; Domschke et al. 2008). Also, ESR1 variants have been associated with major depression (Ryan et al. 2011, 2012) and anxiety (Comings et al. 1999). In a study of 1804 postpartum women, polymorphisms in ESR1 have been found to be associated with PPD (Costas et al. 2010), a finding that requires further testing in replication studies.
The aim of this pilot study was to conduct a targeted association analysis of postpartum depression and nine polymorphisms in the ESR1 gene. In the second part of our analysis, we genotyped eleven strong candidate polymorphisms in seven additional genes implicated in depression and other related mental disorders: COMT, DRD2, HTR2A, MAOA, SLC6A3 (DAT), SLC6A4 (SERT) and TPH2, to examine any impact on PPD and gene-gene interactions of these candidate genes with a variant in ESR1.
Methods
Subjects
A total of 257 women, including controls, were recruited at the Women's Health Concerns Clinic and on the maternity ward at St. Joseph's Healthcare, Hamilton, Ontario, Canada within the first 12 weeks postpartum. Referrals to the clinic of perinatal women with mood and/or anxiety disorders or reported stress were made by community-based health care providers. They were at least 18 years of age, had delivered a full term healthy infant following the index pregnancy and were able to communicate in English. Written and verbal consent was obtained from all participants.
Women were excluded if they: 1) Had a current diagnosis or a history of bipolar or psychotic disorder; 2) Presented with serious risk for suicide, homicide or infanticide; 3) Abused drugs and/or alcohol within the past 6 months; or 4) Showed signs of a concurrent serious medical condition.
Postpartum women were interviewed at the time of enrollment using the Structured Clinical Interview for DSM Disorders (SCID) (Spitzer et al. 1992) and the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998) to establish a psychiatric diagnosis, and the severity of depression was further assessed using the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al. 1987) and/or the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979). The EPDS is an effective screening tool for identifying women at risk for postpartum depression and provides an index of depression severity (higher EPDS scores indicate greater severity). The 10-item questionnaire is most often administered during pregnancy or within 8 weeks postpartum.
Of the 257 women, 225 were diagnosed with a mood disorder. 52 met criteria for “true” PPD, i.e. they had no prior history of a mood or anxiety disorder. They scored within the “severe depression” range on the MADRS (≥35) and/or on the EPDS (≥20). A further ninety-nine (99) women met criteria for recurrent major depressive disorder (MDD) and 74 women were diagnosed with postpartum adjustment disorder with mixed anxiety and depressed mood. The remaining thirty-two (32) postpartum mothers did not qualify for any current and/or past psychiatric disorder and were included as controls. The ethnicity of subjects reflects the demographics in the recruitment area and is mainly Caucasian (91%), with 2% Asian, 2% Hispanic and 4% other. Table 1 displays demographic data for the 184 subjects that were used for the association analyses.
Table 1.
Population Demographics. Age and primary diagnoses of the 184 subjects used in the association study are shown. Abbreviations used for primary diagnosis include: (PPD) postpartum depression; (MDD) major depression; (GAD) generalized anxiety disorder; (Adj. Dis.) adjustment disorder. Ethnicity, country of birth, education, marriage and employment status are presented as averages from available data which is representative of the population as a whole.
| Primary Diagnosis | n | Average Age | StDev | Ethnicity | Country of Birth | ||
|---|---|---|---|---|---|---|---|
| Controls | 32 | 32.6 | 4.2 | Caucasian | 93% | Canada | 80% |
| PPD | 52 | 31.2 | 4.7 | Asian | 2% | UK | 6% |
| MDD | 52 | 30.5 | 5.4 | African Canadian | 1% | USA | 3% |
| GAD | 11 | 29.3 | 4.5 | Hispanic | 1% | Europe | 3% |
| Adj. Dis. | 26 | 31.1 | 5.0 | Other | 3% | Other | 8% |
| Others | 10 | 31.4 | 7.7 | ||||
|
| |||||||
| Highest Education | Marital Status | Employed Outside Home | |||||
|
| |||||||
| Grade School | 8% | Married | 71% | Yes | 66% | ||
| High School | 15% | Never Married | 9% | ||||
| College | 70% | Divorced | 20% | No | 34% | ||
| Post Graduate | 8% | ||||||
Sample preparation
Genomic DNA was extracted from whole blood. Cells were lysed with a sucrose Triton solution, providing a nuclear pellet for DNA purification. DNA was prepared by digestion of the pellet with SDS and proteinase K followed by NaCl “salting out” precipitation of proteins (Miller et al. 1988). The DNA in the supernatant was further purified and recovered by ethanol precipitation.
Genotyping of clinical cohort
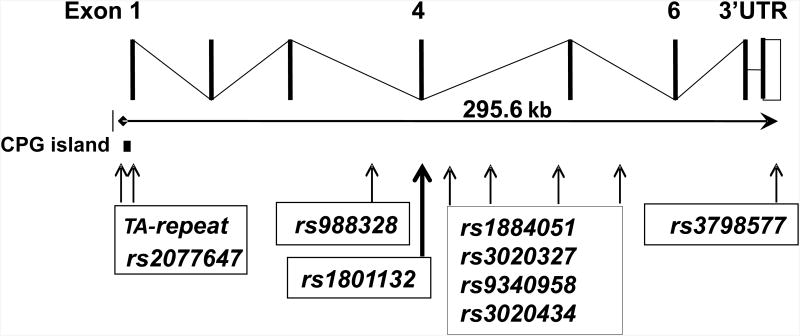
Samples were genotyped for nine ESR1 variants, including one microsatellite repeat polymorphism (Figure 1 and Table 2). Polymorphisms were selected to include variants in the transcribed region of the gene and the rest were distributed across the gene locus (Figure 1) with a concentration in introns 4 and 5. Selection made use of haplotype information and previously published association studies (Costas et al. 2010). The three SNPs in the transcribed region (rs2077647 in exon1; rs1801132 in exon4; and rs13798577 in the 3′ UTR) were genotyped by primer extension using a SNapShot kit (Applied Biosystems, Foster City, CA). The TA repeat was amplified by PCR with fluorescently labeled primers and amplicons were analyzed with capillary electrophoresis. The remaining SNPs were genotyped by restriction length fragment polymorphism (RFLP). A 100- 300 base pair region surrounding each SNP was amplified via PCR using one fluorescently labeled primer and one unlabeled primer. The resulting amplicons were digested overnight with a restriction endonuclease that selectively distinguished between the two SNP alleles. The fragments were analyzed by capillary electrophoresis (AB3730, Applied Biosystems). All ESR1 primer sequences and restriction enzymes are listed in Table 3. For the second analysis eleven polymorphisms in seven previously proposed candidate genes were genotyped (Table S1). DRD2, MAOA, DAT, SERT and TPH2 genotyping was conducted using methods described previously (Zhang et al. 2007; Pinsonneault et al. 2006; Pinsonneault et al. 2011; Lim et al. 2006; Lim et al. 2007; Smith et al. 2012). COMT rs4680 was genotyped using a TaqMan Assay (Cat #4362691, Applied Biosystems). Primers and applicable enzymes are listed in Table S2.
Figure 1.
Map of the coding portion of the ESR1 gene and the approximate locations of genotyped SNPs.
Table 2.
ESR1 SNP information. Genotyping information from All 257 subjects were included to determine genotype counts and allele frequencies.
| Marker | SNP | Minor Allele Freq. | Genotype DD | Genotype Dd | Genotype dd | Call Rate | HWE P n=257 |
|---|---|---|---|---|---|---|---|
| TA-repeat | S>L | 43% | 49 | 119 | 84 | 98% | 0.53 |
| rs2077647 | A>G | 43% | 49 | 124 | 82 | 99% | 0.87 |
| rs988328 | A>G | 14% | 5 | 59 | 173 | 92% | 1.00 |
| rs1801132 | C>G | 24% | 12 | 96 | 149 | 100% | 0.41 |
| rs1884051 | A>G | 17% | 11 | 58 | 166 | 91% | 0.05 |
| rs3020327 | G>A | 10% | 2 | 46 | 209 | 100% | 0.77 |
| rs9340958 | C>T | 7% | 3 | 28 | 203 | 91% | 0.11 |
| rs3020434 | C>T | 16% | 6 | 64 | 170 | 93% | 0.94 |
| rs3798577 | T>C | 48% | 63 | 118 | 73 | 99% | 0.22 |
Table 3.
Genotyping primers. A) Primers and restriction enzymes that were used for RFLP analysis. The TA repeat polymorphism did not require a restriction enzyme. B) Primers employed for primer extension reactions.
| A | |||
| Variant | Enzyme | Forward Primer | Reverse Primer |
| rs3020434 | Haelll | [6-FAM]-ATGAAGTTAGACCTTACAAAGCACATC | TCCTTGCCCCTCAGCTTG |
| rs 1884051 | Bccl | [6-FAM]-GAGGAGGGAGTGGATGTTGAG | AACCATAAAAATTATTCCATCTGAGC |
| rs3020327 | Xmnl | [6-FAM]-GGCATCTGTTCAAGGACAATTTC | GAGTCGTGTATCTTTTTGTCACCTATATAG |
| rs988328 | BsmAI | atagtcTCAGAAGAACCAGCCTATAAATAAAAACT | [6-FAM]-TTGAACTTATTTACCCAATTACCAAAG |
| rs9340958 | Csp6l | GATGTGCAACCTTATTAGTCATTAGGAA | [6-FAM]-CACCAGCAAAACATGAAAAGC |
| TA-Repeat | N/A | [6-FAM]-GACGCATGATATACTTCACC | GCAGAATCAAATATCCAGATG |
| B | |||
| Variant | Primer | Sequence | |
| rs1801132 | F | CAGTGCCTTGTTGGATGCTG | |
| rs1801132 | R | CCCTGTCTGCCAGGTTGGT | |
| rs1801132 | PER | GTAGGATCATACTCGGAATAGAGTAT | |
| rs3798577 | F | TGGTGTTGCATTTAGCCCTGG | |
| rs3798577 | R | AGCCACAACAATCCTGCACA | |
| rs3798577 | PEP | GGCATGGAGCTGAACAGTAC | |
| rs2077647 | F | GTTTCTGAGCCTTCTGCCCTG | |
| rs2077647 | R | TTCCCTTGGATCTGATGCAGT | |
| rs2077647 | PEF | CCTCCACACCAAAGCATC | |
Statistical analysis
Genetic data were analyzed with HelixTree© (Golden Helix, Inc., Bozeman, MT) and STATA 11 (StataCorp. College Station, TX). Two outcomes, and thus two separate analyses, were conducted. The first outcome considered was EPDS score, modeled continuously with linear regression (n=156). Single variants were tested for significance based on Wald p-values, under three genetic models (dominant, recessive, additive). Age was considered a confounder if the inclusion to the model changed the variant's coefficient by more than 10%. Two-variant main effect and interaction models were thoroughly investigated only for the univariate SNP with the lowest p value). The second outcome, PPD versus control (n=84), used logistic regression to model the association between PPD status in a 2-variant main effect model and interaction model selecting the two variants that scored the highest in a two variant interaction model in the first outcome. HelixTree© is a commercially available genetic statistical analysis program that includes genetic association, linkage disequilibrium (LD) analysis, haplotype estimation and regression analysis capabilities. Both Stata 11 and HelixTree© were employed for diplotype analysis and simple genetic association tests.
Results
Two hundred and fifty-seven (257) postpartum women were genotyped for 9 polymorphisms in the estrogen receptor. SNP information including call rate, allele frequency and Hardy-Weinberg equilibrium is listed in Table 3. We first conducted a linkage disequilibrium test and determined that the 9 SNPs defined approximately six haplotype blocks, i.e. three of the SNPs were found to be in high LD with another SNP (Figure 2). This finding enabled us to perform a correction for multiple testing for 6 distinct haplotype blocks. We next examined whether any of the genotyped ESR1 SNPs were associated with the Edinburgh Postnatal Depression Scale (EPDS).
Figure 2.
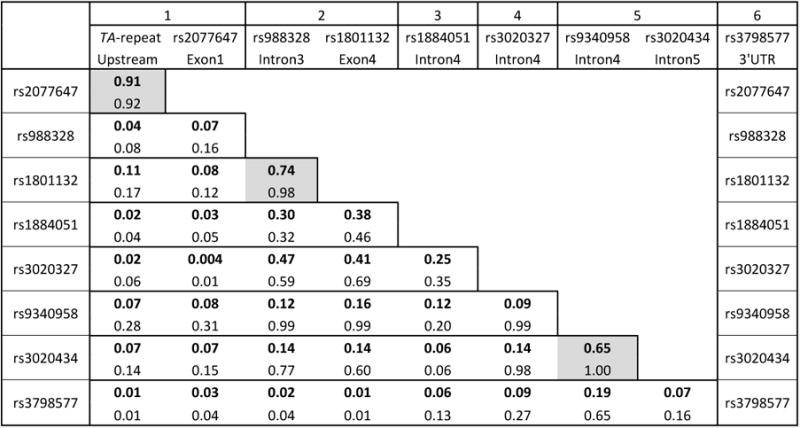
Pairwise linkage disequilibrium of 10 polymorphisms in the ESR1 gene. Numerical values in bold represent LD correlation R, on the top line of each box. The lower value, not in bold, is representative of D prime. Boxes shaded in gray highlight polymorphism pairs in sufficiently high LD to be defined as a haplotype block. Haplotype blocks are numbered in the top row, with SNP and location in the 2nd and 3rd row, respectively.
EPDS scores from the 156 women who were screened ranged from 2-30 and appeared normally distributed (Supplemental Fig. 1). The association p-values from dominant recessive and additive allele tests conducted using EPDS as the phenotypic variable are shown in Table 4. Both the ESR1 TA-repeat (L allele) and rs2077647 (G allele) were significantly associated with EPDS score in a dominant allele test (p=0.007 and p=0.03), however, only the TA-repeat withstood correction for multiple testing for the number of ESR1 haplotype blocks (p=0.04).
Table 4. Genetic association summary of ESR1 polymorphisms with EPDS score.
| Dominant | Additive | Recessive | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | P | Slope | Slope SE | P | Slope | Slope SE | P | Slope | Slope SE |
| TA-repeat | 0.007 | 2.87 | 1.04 | 0.06 | 1.32 | 0.69 | 0.81 | 0.30 | 1.26 |
| rs2077647 | 0.03 | 2.31 | 1.05 | 0.08 | 1.25 | 0.71 | 0.57 | 0.73 | 1.29 |
| rs988328 | 0.82 | -0.26 | 1.19 | 0.92 | -0.11 | 1.03 | 0.78 | 0.89 | 3.24 |
| rs1801132 | 0.21 | -1.25 | 1.00 | 0.37 | -0.71 | 0.80 | 0.81 | 0.47 | 1.96 |
| rs1884051 | 0.88 | -0.16 | 1.11 | 0.75 | 0.28 | 0.87 | 0.27 | 2.40 | 2.17 |
| rs3020327 | 0.46 | 0.88 | 1.18 | 0.31 | 1.11 | 1.08 | 0.17 | 6.15 | 4.43 |
| rs9340958 | 0.68 | 0.70 | 1.71 | 0.79 | 0.39 | 1.44 | 0.81 | -1.05 | 4.47 |
| rs3020434 | 0.19 | 1.54 | 1.16 | 0.29 | 1.11 | 1.04 | 0.69 | -1.47 | 3.66 |
| rs3798577 | 0.50 | 0.78 | 1.15 | 0.55 | 0.42 | 0.69 | 0.75 | 0.37 | 1.13 |
We next conducted an analysis using a second outcome: postpartum depression case control in a partially overlapping group of 84 subjects (52 with confirmed PPD and 32 control postpartum women) using the same tests. Of the 84, 56 subjects overlapped. The other 28 subjects (17 PPD and 11 controls) did not have EPDS data and therefore had not been included in the EPDS association test. In the dominant allele test, both the TA repeat and rs2077647 were significantly associated with PPD, with OR=3.05 (p=0.02) and 2.58 (p=0.04), respectively (Table 5). However, these associations did not survive correction for multiple testing.
Table 5.
Genetic association of summary of ESR1 polymorphisms to postpartum depression status.
| Marker | Dominant Allele Test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| MAF n=52 | MAF n=32 | Call Rate | Chi-Squared P | Odds Ratio | Confidence lnterval | DD Cases | DD Controls | Dd Cases | Dd Controls | dd Cases | dd Controls | |
| TA-repeat | 44% | 35% | 99% | 0.02 | 3.05 | 1.17-7.92 | 7 | 6 | 32 | 10 | 12 | 15 |
| rs2077647 | 46% | 34% | 100% | 0.04 | 2.58 | 1.01-6.59 | 7 | 6 | 31 | 11 | 13 | 15 |
| rs988328 | 17% | 15% | 94% | 0.91 | 1.06 | 0.39-2.86 | 1 | 0 | 14 | 9 | 33 | 21 |
| rs1801132 | 27% | 27% | 100% | 0.66 | 0.82 | 0.34-1.99 | 4 | 1 | 19 | 15 | 28 | 16 |
| rs1884051 | 19% | 18% | 93% | 0.89 | 0.93 | 0.34-2.53 | 3 | 1 | 12 | 8 | 34 | 19 |
| rs3020327 | 15% | 6% | 100% | 0.15 | 2.39 | 0.71-8.13 | 2 | 0 | 11 | 4 | 38 | 28 |
| rs9340958 | 6% | 9% | 92% | 0.82 | 0.86 | 0.22-3.34 | 0 | 1 | 6 | 3 | 42 | 24 |
| rs3020434 | 12% | 12% | 95% | 0.74 | 1.21 | 0.40-3.67 | 0 | 1 | 12 | 5 | 38 | 23 |
| rs3798577 | 46% | 52% | 100% | 0.84 | 0.91 | 0.35-2.34 | 13 | 11 | 21 | 11 | 17 | 10 |
Our next goal was to examine the effect of other candidate genes and gene-gene interactions with ESR1. For this purpose, we selected 11 functional polymorphisms in seven candidate genes for depression and other mental disorders. Table S1 lists the genes included in the analysis, including ESR1, while Table S3 contains information for each SNP genotyped. The majority of these SNPs were tested because they have previously been found to have regulatory effects (Lim et al. 2007; Zhang et al. 2007; Pinsonneault et al. 2011; Smith et al. 2012). These include catechol-O-methyl transferase (COMT), tryptophan hydroxylase 2 (TPH2), dopamine receptor D2 (DRD2), serotonin receptor 2A (HTR2A) and dopamine transporter (SLC6A3 or DAT). Additional variants from monoamine oxidase A (MAOA) and serotonin transporter (SLC6A4 or SERT) have been proposed to be functional (Heils et al. 1996; Pinsonneault et al. 2006; Kunnas et al. 1999).
As with the ESR1 analysis, single variant models were built using additive, dominant, and recessive genetic models. Age was examined as a possible confounding factor, but no evidence was found, and it itself is not significantly associated with EPDS scores. As we have prior knowledge guiding our targeted selection of these functional variants, we did not perform corrections for multiple testing. In addition to the ESR1 TA-repeat, three SNPs were significant at an unadjusted level of 0.10 under at least two genetic models and level 0.05 significant under at least one genetic model (Table S4). In the additive model the following polymorphisms were statistically significant at p<0.05: COMT rs4680 p=0.03 and MAOA rs1137070 p=0.05. In the recessive model COMT rs4680 was p=0.025 and HTR2A rs6314 was p=0.02.
Two-variant main effect and interaction models were thoroughly investigated only for the univariate SNP with the lowest p value (the ESR1 TA-repeat). For the gene-gene interaction models, both the ESR1 TA-repeat and the 2nd SNP were considered in the additive genetic mode. Each of the 11 SNPs were considered for the interaction with ESR1 TA-repeat. Table 6A shows the list of interaction p-values (Wald p-value) in ascending order. Three interaction models were significant at the 0.05 level and are highlighted with a box. These include interaction with one SNP in the dopamine transporter DAT, and two genes that are involved in serotonergic signaling: SLC6A4 (SERT) and the serotonin receptor 2A (HTR2A). The ESR1 TA-repeat interacted most significantly with SERTLPR. Since these data were unadjusted for multiple comparisons, only the most significant model was further examined.
Table 6.
Interaction models with EPDS A): EPDS interaction models testing the ESR1 TA repeat with the eleven remaining SNPs employing an “additive × additive” model. The Wald p-value of the interaction coefficient is listed in the final column. A box highlights any interaction that is significant at the 0.05 level.
B): ESR1 TA-repeat and SERTLPR interaction with EPDS score. Linear regression with EPDS score as outcome. The first two coefficients listed are the main effects of ESR1 TA-repeat and SERTLPR, respectively, and the third coefficient is the interaction. C): Means and Standard Deviations of EPDS score with subject counts, arranged by genotype. ESR1 TA-repeat genotypes are arranged by row, while the SERTLPR genotypes (underscored) are arranged by column.
| A
| |
|---|---|
| 2nd SNP | Interaction W p-value |
| SERTLPR | 0.007 |
| HTR2A rs6314 | 0.01 |
| SLC6A3 rs27072 | 0.01 |
|
| |
| MAOA rs1137070 | 0.10 |
| SLC6A3 rs6347 | 0.17 |
| MAOA pVNTR | 0.21 |
| TPH2 rs7305115 | 0.28 |
| HTR2A rs6311 | 0.48 |
| SLC6A3 In8VNTR | 0.52 |
| DRD2 rs2283265 | 0.58 |
| COMT rs4680 | 0.99 |
| B
| |||||
|---|---|---|---|---|---|
| Predictor | Coef. | Std. Err. | t | P-value | 95% CI |
| ESR1 TA-repeat | -0.92 | 1.07 | -0.86 | 0.39 | (-3.04, 1.19) |
| SERTLPR | -2.02 | 1.06 | -1.91 | 0.06 | (-4.12,0.07) |
| Interaction | 2.69 | 0.99 | 2.72 | 0.007 | (0.73,4.65) |
|
| |||||
| intercept | 15.52 | 1.19 | 13.08 | 0 | (13.18,17.87) |
| C
| |||||
|---|---|---|---|---|---|
| Mean SD | SERTLPR | ||||
| Frequency | L L | L S | S S | Total | |
| ESR1 TA-repeat | L L | 11.8 | 16.3 | 19.0 | 15.2 |
| 6.8 | 5.9 | 4.2 | 6.4 | ||
| 10 | 17 | 4 | 31 | ||
|
| |||||
| S L | 16.0 | 16.2 | 16.6 | 16.2 | |
| 6.4 | 5.4 | 4.9 | 5.6 | ||
| 24 | 35 | 11 | 70 | ||
|
| |||||
| S S | 15.0 | 12.1 | 11.8 | 13.1 | |
| 6.4 | 6.5 | 7.1 | 6.6 | ||
| 18 | 23 | 11 | 52 | ||
|
| |||||
| Total | 14.9 | 15.0 | 14.9 | 14.9 | |
| 6.5 | 6.1 | 6.35 | 6.3 | ||
| 52 | 75 | 26 | 153 | ||
In the best scoring interaction model, the interaction p-value was 0.007 for ESR1 TA-repeat – SERTLPR (Table 6B). The model p-value was 0.013, with R-squared 7%, which increased from 2.4% when only ESR1 was included in the model. The risk alleles in this interaction appear to be between the S allele of the SERTLPR and the L allele of the ESR1-TA repeat. Interpretations of the model are depicted in the 3×3 table (Table 6C), which includes means and standard deviations of EPDS and subject counts stratified by genotype. For clarity, SERTLPR genotypes are indicated with an underscore. The Total row shows that, ignoring the ESR1 TA-repeat, the mean EPDS is 14.9, 15.0, and 14.9 respectively for each SERTLPR genotype (“LL”, “LS”, and “SS”); reinforcing the fact that SERTLPR is not associated with EPDS by itself. However, among the ESR1 TA-repeat “LL” subjects (those homozygous for the minor L allele), the trend increases with SERTLPR genotype, from “LL” to “SS” (11.8, 16.3, 19.0), and for ESR1 TA-repeat “SS” subjects, the trend decreases from “LL” to “SS” (15.0, 12.1, 11.8). The subjects with the highest EPDS occur with SERT “SS” and ESR1 TA-repeat “LL” (value of 19.0 – though only 4 subjects had this genotype combination).
Because the ESR1 TA-repeat interaction with the SERTLPR was significant for EPDS score, we also examined the same interaction in the occurrence of postpartum depression: PPD versus control (n=84). These two variants were chosen as they scored the highest in a two variant interaction model in the first outcome. We employed three methods to examine a gene interaction (Table 7). The first method used logistic regression to model the association between PPD status and the ESR1 TA-repeat and the SERT-LPR in a 2-variant main effect model and interaction model. While there was no statistical interaction between these two variants using the dominant genetic models (to account for the smaller sample size and fewer minor alleles), the main effects model indicates a relationship. In the main effects model, the OR for ESR1 TA-repeat L allele increased from 3.8 to 4.3 (p=0.007) when adjusted for SERTLPR genotype (Table 7A). In a second method, after dividing the cohort into cases and controls, all of the subjects that had at least one copy of the minor allele for both the TA-repeat (L) and the LPR(S) were summed. These numbers were inserted into a two by two contingency table. The remaining subjects, those lacking at least one minor allele in both variants, in each case/control category were summed for the other half of the table (Table 7B). Genotypes for both variant were available for 47 PPD subjects and 28 controls. For the other 9 subjects, complete genotype data were not available. We found that the combination of the minor alleles in SERTLPR (S allele) and ESR1 TA-repeat (L allele) was significantly elevated in PPD cases, 27 out of 47 compared to 5 out of 28 controls (Fisher's p=0.0008). The third method was a logistic regression of specific allele combination frequencies of case subjects compared to controls (Table 7C). The L,S diplotype representing the ESR1 TA L allele and the SERTLPRS allele, differed significantly (p=0.002) between the cases (21%) and controls (6%)
Table 7.
Three methods examining the interaction of the ESR1 TA-repeat and the SERTLPR in postpartum depression. A): Main effect model for ESR1 TA-repeat and SERTLPR: Dominant genetic models were used due to low sample size and low allele frequencies. Adjusting for the SERTLPR, the TA-repeat OR increases from 3.78 to 4.32. Adjusting for ESR1 TA-repeat, the OR for SERTLPR increases from 2.53 to 3.02 and now has level 0.05 significance. Comparisons made on the same 75 subjects. B): Fisher's exact test of a 2 × 2 contingency table of PPD subjects and controls indicating the number of subjects possessing at least one copy of both the SERTLPRS allele and TA-repeat L allele. The Fisher's exact p value is 0.0008. Only samples with genotypes in both variants were included in the analysis. C): Logistic regression analysis of the ESR1 TA repeat/SERTLPR interaction as a diplotype. ESR1 TA-repeat is listed first followed by SERTLPR, which is underscored. nd indicates that the p-value was not calculated but it was not significant.
| A. Main effect model | ||||
|---|---|---|---|---|
| Logistic regression Log likelihood = -43.94 |
N = 75 LR chi2(2) = 11.24 Prob > chi2 = 0.004 |
|||
|
| ||||
| Predictor | Odds Ratio | Std. Err. | p-value | 95% CI |
|
| ||||
| ESR1 TA-repeat | 4.32 | 2.33 | 0.007 | (1.50, 12.43) |
| SERTLPR | 3.02 | 1.65 | 0.043 | (1.04, 8.81) |
| B. Fisher's exact test
| ||||
|---|---|---|---|---|
| Subjects w/ both minor alleles | Others | Total | Fisher's exact p | |
| PPD | 27 | 20 | 47 | p=0.0008 |
|
| ||||
| Control | 5 | 23 | 28 | |
|
| ||||
| Total | 32 | 43 | 75 | |
| C. Diplotype analysis
| |||
|---|---|---|---|
| Cases n=47 | Controls n=28 | Univariate P | |
| L,L | 25% | 26% | 0.88 |
| L,S | 21% | 6% | 0.002 |
| S,L | 31% | 42% | 0.14 |
| S,S | 23% | 26% | nd |
|
| |||
| Sample: | 75 | Selected Markers: | |
| chisq: | 9.90 | ESR1 TA-repeat | |
| P-Value: | 0.02 | SERTLPR | |
Discussion
In a pilot genetic association analysis of postpartum depression and polymorphisms in ESR1, two variants in the ESR1 gene, the TA-repeat and rs2077647 were significantly associated with the Edinburgh Postnatal Depression Scale (EPDS), a screening tool for postpartum depression administered to 156 postpartum women. Additionally, these same ESR1 variants were associated with the occurrence of PPD, in a group of subjects and controls having only partial overlap with the EPDS results. In both cases the minor alleles were associated with poorer outcomes.
Limitations to our study include most notably small sample size which leads to low power. Another issue, inherent to examination of epistatic interactions, is multiple testing which was not corrected for in our interaction analyses. Instead we sought to minimize spurious results by limiting our investigation to known and proposed functional polymorphisms for the interaction study. Consequently, and as this is a pilot study, our findings must be viewed with caution. Nevertheless, in a broader context, our results further support a role for estrogen receptor alpha in the etiology of PPD, corroborating similar findings by others (Costas et al. 2010). In a prospective association study of 44 genes in Spanish women with postpartum depression, four SNPs in the fourth and fifth introns of ESR1 scored significantly (p=0.007 and 0.0008 respectively) (Costas et al. 2010). Although the two studies revealed different variants and different regions of the gene (LD between the regions is low: R=0.07, D'=0.28), they contribute to a growing body of evidence that ESR1 is involved in post-partum depression.
The ESR1 dinucleotide TA-repeat has been linked to bone density (Sano et al. 1995), harm avoidance (Gade-Andavolu et al. 2009) and mRNA expression (Kunnas et al. 1999). The ESR1 TA-repeat is in linkage disequilibrium with rs2077647, a synonymous SNP in exon 1. The TA-repeat and rs2077647 have been linked to arterial stiffness (Peter et al. 2009), and rs2077647 is part of a haplotype associated with child onset mood disorders (Mill et al. 2008). rs2077647 has also been associated with malignancies (Anghel et al. 2010).
When addressing possible gene-gene interactions, we detected interactions between several genes including those encoding serotonergic signaling proteins (SERT and HTR2A). Studied in more detail, a significant interaction was observed between the S allele of the SERTLPR and the L allele of the ESR1 TA-repeat with Edinburgh depression score (EPDS) in 154 subjects. We also detected the same interaction using a second outcome: PPD status in an overlapping cohort that included subjects without EPDS information. The S allele of the SERTLPR and the L allele of the ESR1 TA-repeat conferred risk in PPD cases (p=0.002). The SERTLPR itself was not significantly associated with PPD alone in either outcome, suggesting epistatic interactions. However, the judgment on the biological significance of the SERTLPR is still uncertain, given the mixed results of previous association studies (Caspi et al. 2003b; Lim et al. 2006). Taken together, the results of the different interaction models in our study suggest a role for ESR1 in serotonergic signaling. The SERTLPRS allele has been associated with lower SERT gene expression, resulting in decreased serotonin reuptake (Heils et al. 1996), but a molecular genetics analysis of the SERTLPR alleles has failed to reveal any detectable difference (Lim et al, 2006). The S allele has been correlated with depression and/or anxiety (Lesch et al. 1996; Caspi et al. 2003b), MDD during pregnancy (Scheid et al. 2007), and with PDD (Binder et al. 2010; Mehta et al. 2012). An additional study found a link between the SERTLPR and PPD, but the association was with the L allele (Sanjuan et al. 2008). SERT has also been associated with stress in depression (Caspi et al. 2003a) and anxiety (Lesch et al. 1996). While there is ample evidence for an effect of the SERTLPR on clinical phenotypes, the results in the current study still require caution in assigning causative relationships.
We also detected an interaction between ESR1 and two SNPs in the dopamine transporter (SLC6A3) although the interaction was not further investigated since it was not as strong as that with the SERTLPR. Recent reports have described a connection between dopamine signaling and depression (Chaudhury et al. 2013; Tye et al. 2013).
The idea that estrogen signaling may modify activity in serotonergic pathways has been proposed before (Lokuge et al. 2011b; Michopoulos et al. 2011). There have been numerous studies in animal models (McQueen et al. 1997; Pecins-Thompson et al. 1998; McQueen et al. 1999). In a study of ovariectomized rhesus macaques, chronic administration of estrogen led to reduced SLC6A4 mRNA expression (Pecins-Thompson et al. 1998), while a study in rats showed that estrogen leads to an increase in SLC6A4 expression (McQueen et al. 1997; McQueen et al. 1999). Mood disorders have been reported to be alleviated by estrogen administration (Fink et al. 1998; Halbreich et al. 1995; Ahokas et al. 2000). Ahokas et al. (Ahokas et al. 2000) showed that treatment of refractory PPD with estradiol produced an improvement in depressive symptoms that coincided with the rise in serum estradiol, and this occurred in women who had previously received and failed to respond to treatment with antidepressants or psychotherapy.
Estrogen has long been suspected to play a role in the onset of PPD. Increased incidence of depression has been linked to a fall in plasma estradiol concentrations. During pregnancy, significant increases in plasma estrogens occur with the placenta becoming the primary source of estrogens by week nine of gestation. On the other hand, women experience approximately a ten-fold drop in circulating levels of estradiol postpartum with the removal of the placenta at delivery (Albrecht and Pepe 1990). Women with a personal history of PPD are sensitive to changes in estradiol and progesterone levels when given exogenous hormones, whereas control subjects with no such psychiatric history are not (Bloch et al. 2000). This suggests that some women are more sensitive to the hormonal changes that accompany the perinatal period.
The results of our pilot study support a role for ESR1 in the etiology of postpartum depression. One mechanism may be through the modulation of serotonin signaling. Our findings for the estrogen receptor ESR1 have broad implications for other disorders and therapies that involve estrogens (e.g. the management of menopause-associated mood disorders), especially those where sex-differences are clearly pronounced (e.g. psychiatric disorders, cardiovascular diseases, and cancers).
Supplementary Material
Figure S1: A): A histogram of EPDS score distribution.): The Q-Q plot of linear regression with ‘EPDS’ as the outcome. All residuals appeared fine (plots C and D), and any log-transform of the outcome made the residuals significantly worse. C): A Q-Q plot for the residuals from the interaction model showing that normality is not violated. D): Residuals vs. fitted values demonstrating that the assumption of constant variance is not violated, and they also appear, for the most part, centered at zero. All model assumptions appear satisfied.
Table S1: Genes represented by SNP genotyping in our analysis.
Table S2: Genotyping primers for non-ESR1 variants except COMT rs4680, which was genotyped with a commercially available TaqMan assay (Life Technologies).
Table S3: Additional SNP information
Table S4: Genetic association summary of functional polymorphisms in the additional seven candidate genes with EPDS score.
Acknowledgments
The authors are grateful to Audrey Papp, Gloria Smith, and Marg Coote for preparation of DNA specimens. This study was supported by a pilot award from the Society for Women's Health Research Isis Fund Network (WS, MS, EH and JP), and the National Institutes of Health U01 GM092655 (WS). EH holds a Senior Career Research Chair in Women's Health from the Canadian Institutes of Health Research.
List of Abbreviations
- PPD
postpartum depression
- SNP
single nucleotide polymorphism
- LD
linkage disequilibrium
- MAF
minor allele frequency
- SCID
Structured Clinical Interview for DSM Disorders
- MINI
the Mini International Neuropsychiatric Interview
- MADRS
Montgomery-Asberg Depression Rating Scale
- EPDS
Edinburgh Postnatal Depression Scale
Footnotes
Competing Interests: The authors declare that they have no competing interests.
References
- Ahokas A, Aito M, Rimon R. Positive treatment effect of estradiol in postpartum psychosis: a pilot study. J Clin Psychiatry. 2000;61:166–169. doi: 10.4088/jcp.v61n0303. [DOI] [PubMed] [Google Scholar]
- Albrecht E, Pepe G. Placental steroid hormone biosynthesis in primate pregnancy. EndocrRev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol Biochem Behav. 2006;84(4):635–643. doi: 10.1016/j.pbb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Anghel A, Narita D, Seclaman E, Popovici E, Anghel M, Tamas L. Estrogen receptor alpha polymorphisms and the risk of malignancies. Pathol Oncol Res. 2010;16(4):485–496. doi: 10.1007/s12253-010-9263-9. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol Psychiatry. 2003a;8(6):574–591. doi: 10.1038/sj.mp.4001328. [DOI] [PubMed] [Google Scholar]
- Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry. 2003b;8(7):646–653. doi: 10.1038/sj.mp.4001336. [DOI] [PubMed] [Google Scholar]
- Bell A, Land N, Milne S, Hassanyeh F. Long-term outcome of post-partum psychiatric illness requiring admission. JAffectDisord. 1994;31:67–70. doi: 10.1016/0165-0327(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Szoke A, Henry C, Lacoste J, Bottos C, Nosten-Bertrand M, Hardy P, Rouillon F, Launay JM, Laplanche JL, Leboyer M. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biol Psychiatry. 2000;48(4):319–322. doi: 10.1016/s0006-3223(00)00891-x. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225S0091302201902250. [pii] [DOI] [PubMed] [Google Scholar]
- Beyer C, Pawlak J, Karolczak M. Membrane receptors for oestrogen in the brain. Journal of Neurochemistry. 2003;87:545–550. doi: 10.1046/j.1471-4159.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- Binder EB, Jeffrey Newport D, Zach EB, Smith AK, Deveau TC, Altshuler LL, Cohen LS, Stowe ZN, Cubells JF. A serotonin transporter gene polymorphism predicts peripartum depressive symptoms in an at-risk psychiatric cohort. J Psychiatr Res. 2010;44(10):640–646. doi: 10.1016/j.jpsychires.2009.12.001S0022-3956(09)00273-8. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt P, Danaceau M, Murphy J, Nieman L, Rubinow D. Effects of gonadal steroids in women with a history of postpartum depression. AmJPsychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt T, Taylor A, Craig I, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003a;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003b;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Rivera M, Molina E, Torres-Gonzalez F, Bellon JA, Moreno B, de Dios Luna J, Lorente JA, de Diego-Otero Y, King M, Nazareth I, Gutierrez B. The 5-HTTLPR s/s genotype at the serotonin transporter gene (SLC6A4) increases the risk for depression in a large cohort of primary care attendees: the PREDICT-gene study. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(8):912–917. doi: 10.1002/ajmg.b.30455. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings D, Muhleman D, Johnson P, MacMurray J. Potential role of the estrogen receptor gene (ESR1) in anxiety. Mol Psychiatry. 1999;4(4):374–377. doi: 10.1038/sj.mp.4000503. [DOI] [PubMed] [Google Scholar]
- Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, Baca-Garcia E, Canellas F, Estivill X, Guillamat R, Guitart M, Gutierrez-Zotes A, Garcia-Esteve L, Mayoral F, Molto MD, Phillips C, Roca M, Carracedo A, Vilella E, Sanjuan J. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44(11):717–724. doi: 10.1016/j.jpsychires.2009.12.012. S0022-3956(09)00287-8. [pii] [DOI] [PubMed] [Google Scholar]
- Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal depression scale. BrJPsychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33(1):3–17. doi: 10.1016/j.psyneuen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Domschke K, Dannlowski U, Ohrmann P, Lawford B, Bauer J, Kugel H, Heindel W, Young R, Morris P, Arolt V, Deckert J, Suslow T, Baune BT. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol. 2008;18(10):751–759. doi: 10.1016/j.euroneuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Doornbos B, Dijck-Brouwer DA, Kema IP, Tanke MA, van Goor SA, Muskiet FA, Korf J. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1250–1254. doi: 10.1016/j.pnpbp.2009.07.013. doi:S0278-5846(09)00241-3 [pii] 10.1016/j.pnpbp.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186(3):425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Figueira P, Malloy-Diniz L, Campos SB, Miranda DM, Romano-Silva MA, De Marco L, Neves FS, Correa H. An association study between the Val66Met polymorphism of the BDNF gene and postpartum depression. Arch Womens Ment Health. 2010;13(3):285–289. doi: 10.1007/s00737-010-0146-6. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25(10):764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, Dean L, Dave S, Farmer A, McGuffin P, Brewster S, Craddock N, Jones I. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163(9):1549–1553. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Gade-Andavolu R, Macmurray J, Comings DE, Calati R, Chiesa A, Serretti A. Association between the estrogen receptor TA polymorphism and Harm avoidance. Neurosci Lett. 2009;467(2):155–158. doi: 10.1016/j.neulet.2009.10.028S0304-3940(09)01338-X. [pii] [DOI] [PubMed] [Google Scholar]
- Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37(7):434–441. doi: 10.1016/0006-3223(94)00181-2. doi:0006-3223(94)00181-2 [pii] [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160(8):1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Kunnas TA, Holmberg-Marttila D, Karhunen PJ. Analysis of estrogen receptor dinucleotide polymorphism by capillary gel electrophoresis with a population genetic study in 180 Finns. Hum Hered. 1999;49(3):142–145. doi: 10.1159/000022862. doi: 22862 [pii] 22862. [DOI] [PubMed] [Google Scholar]
- Lazinski M, Shea A, Steiner M. Effects of maternal stress on offspring development. Arch Womens Ment Health. 2008;11:363–375. doi: 10.1007/s00737-008-0035-4. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lim J, Papp A, Pinsonneault J, Sadee W, Saffen D. Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTLPR. Mol Psychiatry. 2006;11:649–662. doi: 10.1038/sj.mp.4001797. [DOI] [PubMed] [Google Scholar]
- Lim J, Pinsonneault J, Sadee W, Saffen D. Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons. Mol Psychiatry. 2007;12(5):491–501. doi: 10.1038/sj.mp.4001923. [DOI] [PubMed] [Google Scholar]
- Lokuge S, Frey B, Foster J, Soares C, Steiner M. Neurobiological basis of depression in women: new insights into the link between estrogen and serotonin. Journal of Clinical Psychiatry. 2011a;72:e1563–e1569. doi: 10.4088/JCP.11com07089. [DOI] [PubMed] [Google Scholar]
- Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. The rapid effects of estrogen: a mini-review. Behav Pharmacol. 2010;21(5-6):465–472. doi: 10.1097/FBP.0b013e32833da5c3. [DOI] [PubMed] [Google Scholar]
- Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. 2011b;72(11):e1563–1569. doi: 10.4088/JCP.11com07089. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Payne JL, MacKinnon DF, Mondimore FM, Goes FS, Schweizer B, Jancic D, Coryell WH, Holmans PA, Shi J, Knowles JA, Scheftner WA, Weissman MM, Levinson DF, DePaulo JR, Jr, Zandi PP, Potash JB. Genome-wide linkage and follow-up association study of postpartum mood symptoms. Am J Psychiatry. 2009;166(11):1229–1237. doi: 10.1176/appi.ajp.2009.09030417. doi:appi.ajp.2009.09030417 [pii] 10.1176/appi.ajp.2009.09030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Estrogens effects on the brain: multiple sites and molecular mechanisms. JApplPhysiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res Mol Brain Res. 1997;45(1):13–23. doi: 10.1016/s0169-328x(96)00233-1. S0169328X96002331 [pii] [DOI] [PubMed] [Google Scholar]
- McQueen JK, Wilson H, Sumner BE, Fink G. Serotonin transporter (SERT) mRNA and binding site densities in male rat brain affected by sex steroids. Brain Res Mol Brain Res. 1999;63(2):241–247. doi: 10.1016/s0169-328x(98)00281-2. doi:S0169328X98002812 [pii] [DOI] [PubMed] [Google Scholar]
- Mehta D, Quast C, Fasching PA, Seifert A, Voigt F, Beckmann MW, Faschingbauer F, Burger P, Ekici AB, Kornhuber J, Binder EB, Goecke TW. The 5-HTTLPR polymorphism modulates the influence on environmental stressors on peripartum depression symptoms. J Affect Disord. 2012;136(3):1192–1197. doi: 10.1016/j.jad.2011.11.042S0165-0327(11)00759-2. [pii] [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Wilson ME. Estradiol and progesterone modify the effects of the serotonin reuptake transporter polymorphism on serotonergic responsivity to citalopram. Exp Clin Psychopharmacol. 2011;19(6):401–408. doi: 10.1037/a00250082011-17877-001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Kiss E, Baji I, Kapornai K, Daróczy G, Vetró A, Kennedy J, Kovacs M, Barr C. Association study of the estrogen receptor alpha gene (ESR1) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1323–1326. doi: 10.1002/ajmg.b.30751. [DOI] [PubMed] [Google Scholar]
- Miller S, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mrazek DA, Rush AJ, Biernacka JM, O'Kane DJ, Cunningham JM, Wieben ED, Schaid DJ, Drews MS, Courson VL, Snyder KA, Black JL, 3rd, Weinshilboum RM. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Eberenz K, Zandi PP, March D, Crowe RR, Scheftner WA, Alexander M, McInnis MG, Coryell W, Adams P, DePaulo JR, Jr, Miller EB, Marta DH, Potash JB, Payne J, Levinson DF. Is perinatal depression familial? J Affect Disord. 2006;90(1):49–55. doi: 10.1016/j.jad.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61(11):1163–1169. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- Nott P, Franklin M, Armitage C, Gelder M. Hormonal changes and mood in the puerperium. BrJPsychiatry. 1976;128:379–383. doi: 10.1192/bjp.128.4.379. [DOI] [PubMed] [Google Scholar]
- Payne JL, Roy PS, Murphy-Eberenz K, Weismann MM, Swartz KL, McInnis MG, Nwulia E, Mondimore FM, MacKinnon DF, Miller EB, Nurnberger JI, Levinson DF, DePaulo JR, Jr, Potash JB. Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord. 2007;99(1-3):221–229. doi: 10.1016/j.jad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res. 1998;53(1-2):120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Peter I, Kelley-Hedgepeth A, Huggins GS, Housman DE, Mendelsohn ME, Vita JA, Vasan RS, Levy D, Benjamin EJ, Mitchell GF. Association between arterial stiffness and variations in oestrogen-related genes. J Hum Hypertens. 2009;23(10):636–644. doi: 10.1038/jhh.2009.1jhh20091. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault J, Papp A, Sadee W. Allelic expression of monoamine oxidase A (MAOA) in human brain: Evidence for cis-acting genetic and epigenetic factors. Hum Mol Gen. 2006;15(17):2636–2649. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, Bertolino A, Malhotra AK, Gu HH, Sadee W. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36(8):1644–1655. doi: 10.1038/npp.2011.45npp201145. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carriere I, Peres K, Rouaud O, Scarabin PY, Ritchie K, Ancelin ML. Oestrogen receptor polymorphisms and late-life depression. Br J Psychiatry. 2011;199(2):126–131. doi: 10.1192/bjp.bp.111.091751. doi:199/2/126 [pii] 10.1192/bjp.bp.111.091751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carriere I, Peres K, Rouaud O, Scarabin PY, Ritchie K, Ancelin ML. Estrogen receptor alpha gene variants and major depressive episodes. J Affect Disord. 2012;136(3):1222–1226. doi: 10.1016/j.jad.2011.10.010. doi:S0165-0327(11)00654-9 [pii] 10.1016/j.jad.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, Gutierrez-Zotes A, Gornemann I, Canellas F, Baca-Garcia E, Jover M, Navines R, Valles V, Vilella E, de Diego Y, Castro JA, Ivorra JL, Gelabert E, Guitart M, Labad A, Mayoral F, Roca M, Gratacos M, Costas J, van Os J, de Frutos R. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193(5):383–388. doi: 10.1192/bjp.bp.107.045427. doi:193/5/383 [pii] 10.1192/bjp.bp.107.045427. [DOI] [PubMed] [Google Scholar]
- Sano M, Inoue S, Hosoi T, Ouchi Y, Emi M, Shiraki M, Orimo H. Association of estrogen receptor dinucleotide repeat polymorphism with osteoporosis. Biochem Biophys Res Commun. 1995;217(1):378–383. doi: 10.1006/bbrc.1995.2787. doi:S0006-291x(85)72787-8 [pii] 10.1006/bbrc.1995.2787. [DOI] [PubMed] [Google Scholar]
- Scheid JM, Holzman CB, Jones N, Friderici KH, Nummy KA, Symonds LL, Sikorskii A, Regier MK, Fisher R. Depressive symptoms in mid-pregnancy, lifetime stressors and the 5-HTTLPR genotype. Genes Brain Behav. 2007;6(5):453–464. doi: 10.1111/j.1601-183X.2006.00272.x. doi:GBB272 [pii] 10.1111/j.1601-183x.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smith RM, Papp AC, Webb A, Ruble CL, Munsie LM, Nisenbaum LK, Kleinman JE, Lipska BK, Sadee W. Multiple Regulatory Variants Modulate Expression of 5-Hydroxytryptamine 2A Receptors in Human Cortex. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.028. doi:S0006-3223(12)00854-2 [pii] 10.1016/j.biopsych.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stahl S. Effects of estrogen on the central nervous system. JClinPsychiatry. 2001;62:317–318. doi: 10.4088/jcp.v62n0501. [DOI] [PubMed] [Google Scholar]
- Steiner M. Perinatal mood disorders: position paper. PsychopharmacolBull. 1998;34:301–306. [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Sun HS, Tsai HW, Ko HC, Chang FM, Yeh TL. Association of tryptophan hydroxylase gene polymorphism with depression, anxiety and comorbid depression and anxiety in a population-based sample of postpartum Taiwanese women. Genes Brain Behav. 2004;3(6):328–336. doi: 10.1111/j.1601-183X.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- Treloar SA, Martin NG, Bucholz KK, Madden PA, Heath AC. Genetic influences on post-natal depressive symptoms: findings from an Australian twin sample. Psychol Med. 1999;29(3):645–654. doi: 10.1017/s0033291799008387. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740nature11740. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Innis SM. Association of fatty acid desaturase gene polymorphisms with blood lipid essential fatty acids and perinatal depression among Canadian women: a pilot study. J Nutrigenet Nutrigenomics. 2009;2(4-5):243–250. doi: 10.1159/000255636. doi:000255636 [pii] 10.1159/000255636. [DOI] [PubMed] [Google Scholar]
- Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7(10):1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee M, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104(51):20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Engel R, Baghai TC, Juckel G, Frodl T, Muller-Siecheneder F, Zwanzger P, Schule C, Minov C, Behrens S, Rupprecht R, Hegerl U, Moller HJ, Bondy B. Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine transporter and analysis in major depression. Neuropsychopharmacology. 2002;26(4):489–493. doi: 10.1016/S0893-133X(01)00386-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: A): A histogram of EPDS score distribution.): The Q-Q plot of linear regression with ‘EPDS’ as the outcome. All residuals appeared fine (plots C and D), and any log-transform of the outcome made the residuals significantly worse. C): A Q-Q plot for the residuals from the interaction model showing that normality is not violated. D): Residuals vs. fitted values demonstrating that the assumption of constant variance is not violated, and they also appear, for the most part, centered at zero. All model assumptions appear satisfied.
Table S1: Genes represented by SNP genotyping in our analysis.
Table S2: Genotyping primers for non-ESR1 variants except COMT rs4680, which was genotyped with a commercially available TaqMan assay (Life Technologies).
Table S3: Additional SNP information
Table S4: Genetic association summary of functional polymorphisms in the additional seven candidate genes with EPDS score.