Abstract
Objective
To determine the extent of difference between better-eye visual field (VF) mean deviation (MD) and integrated VF (IVF) MD among Salisbury Eye Evaluation (SEE) subjects and a larger group of glaucoma clinic subjects and to assess how those measures relate to objective and subjective measures of ability/performance in SEE subjects.
Design
Retrospective analysis of population- and clinic-based samples of adults.
Participants
A total of 490 SEE and 7053 glaucoma clinic subjects with VF loss (MD ≤−3 decibels [dB] in at least 1 eye).
Methods
Visual field testing was performed in each eye, and IVF MD was calculated. Differences between better-eye and IVF MD were calculated for SEE and clinic-based subjects. In SEE subjects with VF loss, models were constructed to compare the relative impact of better-eye and IVF MD on driving habits, mobility, self-reported vision-related function, and reading speed.
Main Outcome Measures
Difference between better-eye and IVF MD and relationship of better-eye and IVF MD with performance measures.
Results
The median difference between better-eye and IVF MD was 0.41 dB (interquartile range [IQR], −0.21 to 1.04 dB) and 0.72 dB (IQR, 0.04–1.45 dB) for SEE subjects and clinic-based patients with glaucoma, respectively, with differences of ≥2 dB between the 2 MDs observed in 9% and 18% of the groups, respectively. Among SEE subjects with VF loss, both MDs demonstrated similar associations with multiple ability and performance metrics as judged by the presence/absence of a statistically significant association between the MD and the metric, the magnitude of observed associations (odds ratios, rate ratios, or regression coefficients associated with 5-dB decrements in MD), and the extent of variability in the metric explained by the model (R2). Similar associations of similar magnitude also were noted for the subgroup of subjects with glaucoma and subjects in whom better-eye and IVF MD differed by ≥2 dB.
Conclusions
The IVF MD rarely differs from better-eye MD, and similar associations between VF loss and visual disability are obtained using either MD. Unlike better-eye MD, IVF measurements require extra software/ calculation. As such, information from studies using better-eye MD can be more easily integrated into clinical decision-making, making better-eye MD a robust and meaningful method for reporting VF loss severity.
Visual field (VF) testing is central to the diagnosis and management of glaucoma and other diseases affecting the visual system. Understanding the relationship between VF loss and the disability resulting from glaucoma is important when assessing the impact of disease and guiding an appropriate treatment program.
At present, there are 2 opposing approaches to measure VF loss when relating it to measures of ability and performance. One common approach is to focus on VF loss in the better-seeing eye. This approach is based on the idea that loss of vision in 1 eye alone is generally not impactful because the fellow eye can compensate for the loss of visual function in 1 eye.1–7 An alternate approach considers that people largely function with binocular vision, so that the degree of overlapping VF loss between the 2 eyes is the more appropriate measure. Several systems have been developed to integrate monocular VFs to simulate this binocular VF.8,9 The inherent assumption of such integration is that the integrated VF (IVF) better reflects disability than parameters that can be judged by the individual right and left eye VFs alone.
There currently are few data comparing how better-eye and IVF measures relate to disability. Kulkarni et al10 recently found that of the 8 different VF staging systems they studied, the better-eye and IVF scores were the most correlated to the single objective and subjective measure of disability that they assessed. Furthermore, in their clinic-based population of patients with glaucoma, they found no difference between how well the better-eye and IVF scores correlated with these measures of disability. Data from the Salisbury Eye Evaluation (SEE), a population-based sample of older adults, offer a unique opportunity to address this question even more comprehensively, because standardized VFs were obtained in a population-based cohort and ability and performance were measured using a variety of subjective and objective criteria. In the current study, we examine whether individual assessment of the right and left VFs or assessment of the IVF better relates to objective and subjective ability and performance measures.
One possible explanation for why better-eye and IVF measures of VF might similarly predict disability is that the both measures rarely differ.11 Therefore, we also examine how often, to what extent, and at what levels of VF loss better-eye and IVF measures differ in SEE subjects and in a large database of VFs from a university glaucoma clinic. We specifically examine how different the associations between better-eye or IVF measures and visual disability are in the subgroup of individuals with differing levels of better-eye and IVF loss.
Methods
Data for analysis were derived from (1) the SEE Project, a prospective cohort study of community-dwelling adults begun in 1993 to study the impact of visual function on physical function; and (2) a database of VFs measured in patients with glaucoma at the Wilmer Eye Institute between 1996 and 2010.12,13 The data from SEE were from the fourth and final study round (conducted 8 years after baseline), the only study round in which threshold VF testing was performed. All SEE participants gave written informed consent, and study procedures were approved by the Johns Hopkins Institutional Review Board.
Measures of Ability and Performance
Driving habits were obtained using a previously described questionnaire.1 Subjects who described having previously driven a car, but reported not having driven a car over the previous 12 months, were considered to have stopped driving for this analysis.1
Mobility measures included stair-climbing speed, walking speed along a straight 4-m course, and performance in a standardized mobility course.7,14 Subjects were asked to walk the mobility course as quickly as possible while avoiding all obstacles. Mobility course performance metrics included walking speed and number of bumps.
Self-reported vision-related function was measured using the Activities of Daily Vision Scale15 instrument, as previously described.16 For this analysis, only the subscales that had adequate content validity, internal consistency, and discriminability were used as previously determined in this study population.17 Participants reported the extent to which they experienced difficulty with each survey item, with scores of 1 reflecting an inability to perform the task because of one’s vision and a score of 5 reflecting no difficulty with the task. If a person did not perform a task for reasons other than vision, the item was not scored.16 The overall and subscale scores were determined by averaging all relevant scored items and rescaling them to a 0 to 100 scale, with 0 representing an inability to perform all activities because of vision and 100 indicating no difficulty with any activity.17,18
Reading speed was evaluated by having subjects read short passages (6th–9th grade level) of nonscrolling text displayed on a computer screen. Reading was done out loud, with subjects asked to read as quickly as possible. The number of correctly read words over a 15-second interval was used to calculate reading speed. Words read incorrectly or out of order were not counted. Reading speed was evaluated for 4 text sizes ranging from 0.131° (pharmacy label print) to 0.525° (small newspaper heading size), with reading speed of size 2 text 0.26° (newspaper size print) analyzed.19 The commonly defined reading threshold of 90 words per minute was used for analysis.
Visual Field Testing and Selection
The VF testing was performed in each eye using the Humphrey Field Analyzer II (Carl Zeiss Meditec, Dublin, CA) with the SITA Fast algorithm and 30-2 pattern for the SEE Project and the SITA Standard algorithm and 24-2 pattern for patients with glaucoma tested at the Wilmer Eye Institute. Because many subjects completed more than 1 VF test in each eye, it was necessary to choose a single VF for use in this analysis. For the SEE project, all VFs with >30% false-positives were excluded, and the first VF meeting the false-positive criteria and the Ocular Hypertension Treatment Study reliability criteria for false-negatives and fixation losses (<33% for each) was selected for analysis.20 Only fields with excess false-positives were excluded because recent studies suggest that of the 3 reliability indices, an excess of false-positives is by far the major threat to the reliability of the test result (Ramulu PY. Determinants of visual field reliability. Paper presented at: Association for Research in Vision and Ophthalmology Annual Meeting, May 7, 2013, Seattle, WA).21 If none of the fields met these false-negative and fixation loss criteria for a particular eye, the first field performed on that eye was selected (true for 28% of better-seeing eyes). For the clinic-based VFs, the last VF meeting the Ocular Hypertension Treatment Study reliability criteria was selected. The earliest VF meeting reliability criteria was chosen for the SEE subjects, but the latest VF meeting reliability criteria was chosen for the clinic patients, because the VFs in the SEE subjects were performed only in round 4 and were therefore all performed over a short time period, whereas the clinic-based patients with glaucoma had VFs performed over several years. The last VF was chosen for the clinic subjects because later VFs are more likely to have more advanced levels of VF loss, where disagreement between better-eye and IVF mean deviations (MDs) are theoretically more likely to occur.
Calculation of Binocular Summation Integrated Visual Field Mean Deviation
The IVFs were derived from the right and left VFs for each patient using the binocular summation method described by Nelson-Quigg et al.8 The 2 blind spot points in each eye, as well as points with threshold values <0, were assigned a threshold value of 0 for the individual eye VF. For the 24-2 clinic-based VFs, the 2 nonoverlapping, nasal-most points in the VF for each eye were excluded. Threshold values for all points in the selected left and right eye VFs were converted from logarithmic decibel values to nonlogarithmic values. The binocular sensitivity (SB) was then calculated for each point in the IVF using the quadratic summation equation , where SR represents the sensitivity (non-logarithmic threshold value) of a point in the right-eye VF and SL represents the sensitivity of the corresponding point in the left-eye VF. The SB for each point was then reconverted to its logarithmic decibel value to obtain the threshold value in the binocular summation IVF. This method of right and left eye VF integration was chosen because it seemed to best capture the true binocular VF in prior studies.8
The MD values were then calculated for the IVF. First, expected threshold (ET) values for each point in the left and right eye VFs were calculated using the formula ET = measured threshold – total deviation (TD). The ET values were then calculated from the monocular ET values using the same formula described to calculate the SB. The MD values were calculated by first subtracting the ET values from the measured threshold values at each point of the IVF to obtain the TD, summing these TD values, and then dividing this sum by 76 or 52 (the number of points in the 30-2 and 24-2 IVFs, respectively). Better-eye MD was obtained from the VFs directly.
Measurement of Covariates Related to Disability
Standardized forms and questionnaires were used to collect demographic and health-related information, including date of birth, race, sex, and total years of education in SEE subjects. Additional covariates, including a comorbidity index, the presence of depressive symptoms, and cognitive ability, were measured during the fourth round of the SEE study concurrent with the functional testing and VF testing described earlier. The comorbidity index was calculated by asking participants if they had been diagnosed with 1 of 15 different medical conditions and summing all positive responses.22 A positive response to any question from part D of the General Health Questionnaire23 was considered an indication of the presence of depressive symptoms. The Mini Mental State Examination (MMSE),24 with scores ranging from 0 to 30, was used to measure cognitive ability.
Definition of Glaucoma
All SEE subjects were formally evaluated for glaucoma as previously described on the basis of results of VF testing and examination of the optic nerve.25–32 Subjects were classified as having glaucoma if either eye was classified as having probable or definite glaucoma.
Analysis
Group differences in subjects with and without VF loss were compared using the t test (for age) and in bivariate logistic, log-linear, or linear regression models including age and the variable of interest.
Better-eye MD was visually compared with the IVF MD using Bland–Altman plots.33 Median and interquartile range (IQR) were used to describe the observed differences between better-eye and IVF MD. The difference was tested for statistical significance using the Wilcoxon signed-rank test.
Logistic, log-linear, and linear regression models were used to look at the association of each measure of VF loss with various ability and performance outcomes in participants with VF loss. Associations were adjusted for age, race, sex, education, MMSE score, number of comorbidities, and presence of depressive symptoms. In addition, driving habits and self-reported vision-related function were adjusted for average grip strength; measures of mobility were adjusted for average grip strength, height, body mass index, arthritis, use of a mobility aid, and visual acuity; and measures of reading speed were adjusted for visual acuity. When the analyses were limited to participants with VF loss and at least a 2-decibel (dB) difference between better-eye and IVF MD, associations were adjusted for fewer demographic and health variables because of the reduced sample size. Each association was adjusted for age, race, and sex. In addition, driving habits and self-reported vision-related function were adjusted for MMSE score and number of comorbidities; measures of mobility were adjusted for number of comorbidities; and measures of reading speed were adjusted for MMSE score.
For linear models, the R2 (proportion of variability in the metric explained by the model) was calculated. All analyses were performed using SAS software version 9.2 (SAS Inc., Cary, NC).
Results
A total of 1250 subjects participated in SEE through round 4, and 1098 subjects (87.8%) completed 30-2 VF tests in both eyes, had VF results meeting reliability criteria, and had data for at least 1 ability and performance measure. A total of 490 of the 1098 SEE subjects had MD ≤−3 dB in at least 1 eye. These 490 participants had worse visual acuity and contrast sensitivity, were more likely to describe depressive symptoms, and were older, less educated, more cognitively impaired, and more likely to be AfricanAmerican than the 608 participants without VF loss in either eye (Table 1). In addition, we had access to completed VFs in both eyes for 13 955 clinic patients, 7053 of whom had MD ≤−3 dB in at least 1 eye. However, the assessment of the association of each measure of VF loss with various ability and performance outcomes was performed only in SEE participants.
Table 1.
Characteristics of Salisbury Eye Evaluation Round 4 Participants with Data on Visual Fields (VFs) and Function/Quality of Life by VF Loss
| Characteristics | Round 4 Participants
|
Age-Adjusted P Value* | |
|---|---|---|---|
| MD ≤ −3 dB in at Least 1 Eye (n = 490) | MD > − 3 dB in Both Eyes (n = 608) | ||
| Age (yrs), mean ± SD | 80.7±4.7 | 78.7±4.0 | <0.0001 |
| Male, n (%) | 183 (37) | 264 (43) | 0.13 |
| African American, n (%) | 166 (34) | 81 (13) | <0.0001 |
| Last grade completed, mean ± SD | 11.0±3.6 | 12.3±3.1 | <0.0001 |
| MMSE score, mean ± SD | 25.0±3.7 | 26.9±2.4 | <0.0001 |
| Depressed, n (%) | 43 (9) | 32 (5) | 0.01 |
| No. comorbid conditions, mean ± SD | 3.6±2.0 | 3.3±1.8 | 0.03 |
| logMAR acuity, mean ± SD | 0.13±0.25 | 0.00±0.11 | <0.0001 |
| Contrast sensitivity, mean ± SD† | 31.5±4.7 | 34.7±2.4 | <0.0001 |
| Better-eye VF MD, median (P5, P95) | −3.6 (−16.8 to −0.5) | −0.4 (−2.1 to +1.3) | <0.0001 |
| Worse-eye VF MD, median (P5, P95) | −6.2 (−23.8 to −3.2) | −1.2 (−2.8 to +0.4) | <0.0001 |
logMAR = logarithm of minimal angle of resolution; MD = mean deviation; MMSE = Mini Mental State Examination; P5 = 5th percentile; P95 = 95th percentile; SD = standard deviation.
Age difference was evaluated using the Student t test; all other P values were taken from bivariate logistic, log-linear, or linear regression models with age and the given characteristic included as covariates. All age-adjusted differences between the 2 groups were in the same direction as the unadjusted statistics shown.
Contrast sensitivity expressed as the number of letters correctly read on the Pelli-Robson chart in the better eye with best correction.
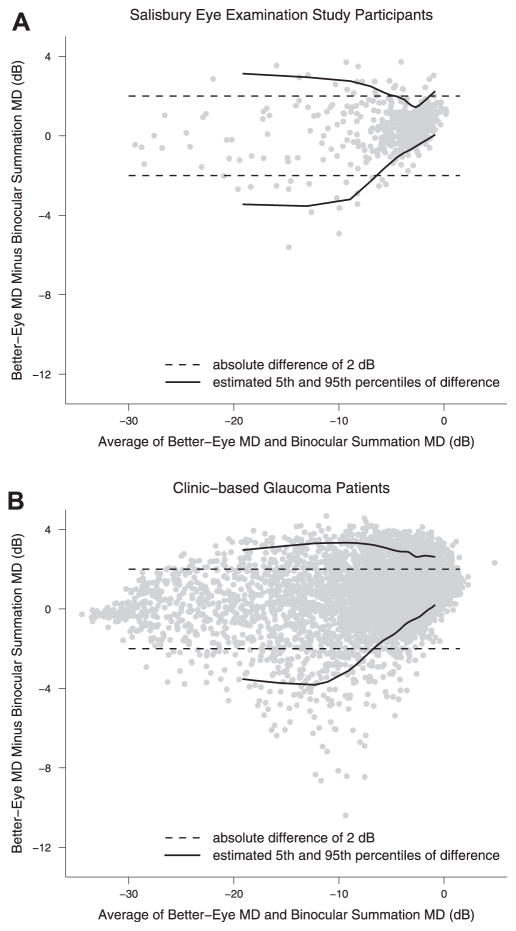
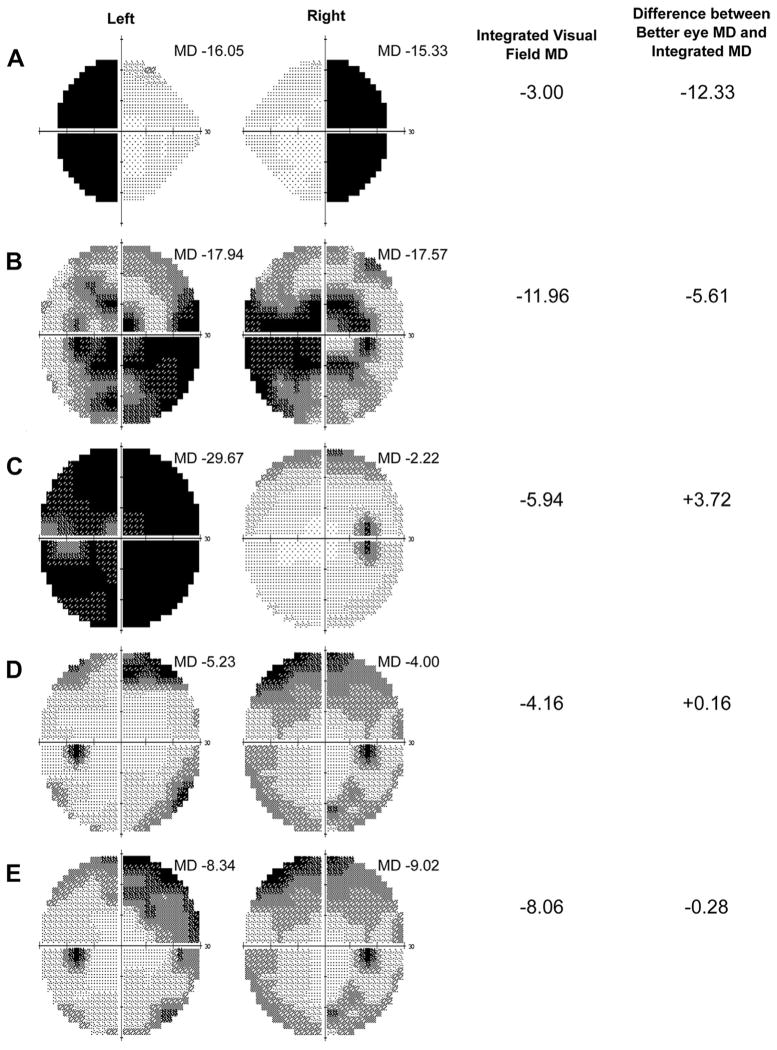
Better-eye MD was highly similar to IVF MD in both SEE and clinic-based subjects, even among those with MD ≤−3 in at least 1 eye (Fig 1). The median difference between the better-eye MD and the IVF MD (defined as better-eye MD minus IVF MD) was 0.15 dB (IQR, −0.15 to +0.53 dB) and 0.41 dB (IQR, −0.21 to 1.04 dB) for the full SEE study sample and SEE participants with MD ≤−3 dB in at least 1 eye, respectively (P <0.0001 when comparing the better-eye and IVF MDs in both the full SEE sample and SEE participants with VF loss, Wilcoxon signed-rank test). Absolute differences of ≥2 dB were noted between the better-eye MD and the IVF MD in 3.9% and 8.9% of these 2 groups, respectively. Differences of ≥2 dB between the better-eye VF MD and the IVF MD were more common among the 123 subjects with glaucoma (13.0%), subjects with MD of ≤−3 dB in both eyes (12.6%), and subjects with MD of ≤−6 dB in both eyes (24.2%). Examples of the varying degrees of representative integrated and better-eye VF MDs are shown in Figure 2.
Figure 1.
Bland–Altman plots comparing better-eye and integrated visual field (IVF) mean deviation (MD). Better-eye and IVF MD are compared among (A) Salisbury Eye Evaluation Study participants with an MD of ≤−3 decibels (dB) in at least 1 eye (N = 490) and (B) glaucoma clinic–based patients with an MD of ≤−3 dB in at least 1 eye (N = 7053).
Figure 2.
Examples of better-eye visual field (VF) and integrated VF (IVF) mean deviation (MDs) in representative VFs. Hypothetically, the largest difference between the better-eye and IVF MD is observed when the pattern of VF loss is in highly dissimilar locations in the 2 eyes, as in the case of bitemporal defects (A). The greatest difference between better-eye and IVF MD in the Salisbury Eye Evaluation (SEE) cohort observed in the negative direction (implying a better-eye MD worse than the IVF MD) was −5.6 decibels (dB), with corresponding VFs demonstrating a predominantly bitemporal defect, as shown in (B). The greatest difference between better-eye and IVF MD in the SEE cohort observed in the positive direction (implying a better-eye MD better than the IVF MD) was +3.7 dB, as was observed with highly asymmetric right and left eye VFs, as shown in (C). Visual field pairs representing the median difference between better-eye MD and IVF MD among subjects with an MD ≤−3 dB in both eyes and ≤−6 dB in both eyes are shown in (D) and (E), respectively. Better-eye VF MD is defined as higher (less negative or more positive) MD of right and left eyes, and IVF MD is derived from point-wise integration of right and left eye VFs.
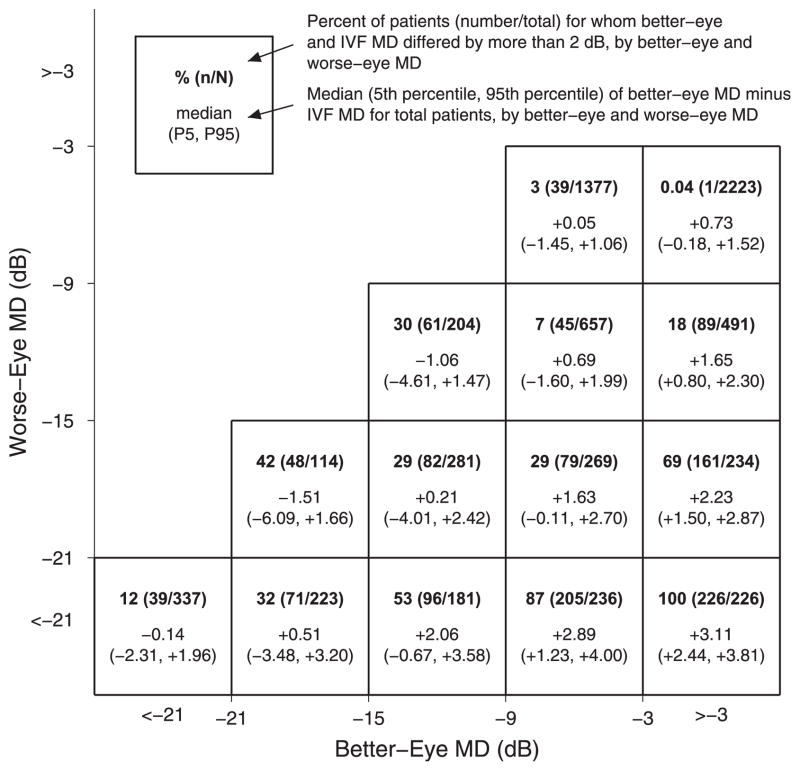
For the full clinic-based sample and clinic-based patients with MD ≤−3 dB in at least 1 eye (N = 7053), the median differences between the better-eye MD and the IVF MD were 0.23 dB (IQR, −0.08 to +0.79 dB) and 0.72 dB (IQR, 0.04–1.45 dB), respectively (P <0.0001 when comparing the better-eye and IVF MDs in both the full clinic sample and clinic patients with VF loss, Wilcoxon signed-rank test). Absolute differences of ≥2 dB were noted between the better-eye and the IVF MD in 8.9% and 17.6% of these 2 groups, respectively. Figure 3 demonstrates that differences of ≥2 dB between the better-eye and the IVF MD were more common when there were large differences in MD between the better eye and the worse eye.
Figure 3.
Differences between better-eye visual field (VF) and integrated VF (IVF) mean deviation (MD) with varying degrees of better-eye and worse-eye VF damage. Data are shown for clinic-based patients with MD of ≤−3 decibels (dB) (N = 7053) in at least 1 eye. Within each box, the percentage of patients with a ≥2 dB difference between better eye and IVF MD is shown, along with the median and interquartile range of better-eye and IVF MD difference. P5 = 5th percentile; P95 = 95th percentile.
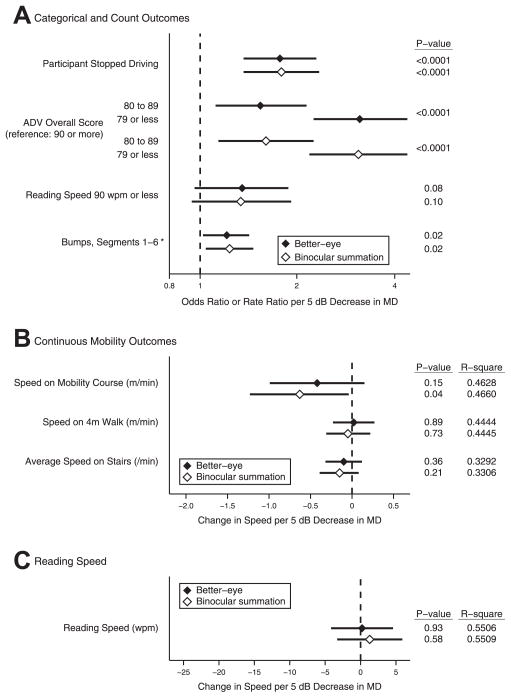
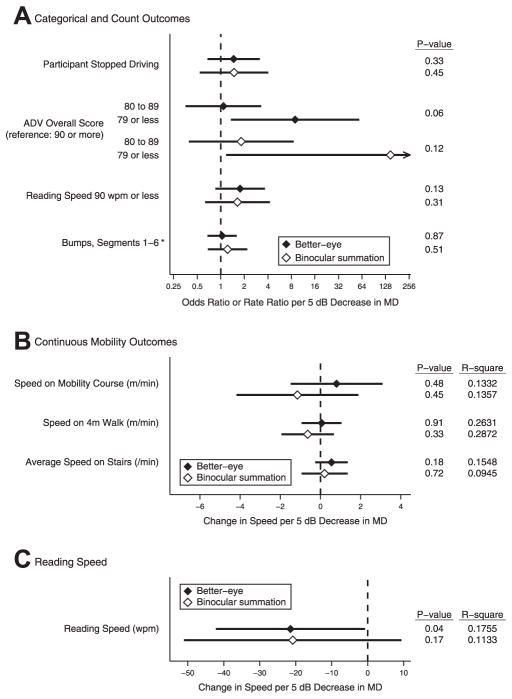
In SEE subjects with MD ≤−3 dB in at least 1 eye, the impact on ability and performance measures of a 5-dB decrement in the better-eye and IVF MD was assessed in multivariate regression models. A 5-dB decrement in each metric was associated with a similar increase in the likelihood of driving cessation, a lower Activities of Daily Vision score, a reading speed ≤90 words per minute, and a similar increase in the rate of bumps on the mobility course (Fig 4). In addition, a 5-dB decrement in the better-eye or binocular summation MD was associated with a similar decrease in walking speed through a mobility course, walking speed along a straight 4-m course, stair-climbing speed, and reading speed. For all metrics except mobility course walking speed, both better-eye MD and IVF MD resulted in similar conclusions regarding whether there was a statistically significant association between the VF loss and the ability and performance metric in question (based on P ≤0.05). A worse IVF MD, but not a worse better-eye MD, was associated with a slower speed on the mobility course (P = 0.04 vs. 0.15). However, the magnitude by which a 5-dB loss in IVF MD or better-eye MD affected walking speed was small and similar on a percentage basis (−0.63 m/min, 2.5% of average walking speed, for IVF MD vs. −0.42 m/min, 1.7% of average walking speed, for better-eye MD). For ability and performance metrics analyzed using linear regression models, similar R2 values were observed for models using the better-eye MD or IVF MD.
Figure 4.
Comparison of visual disability associated with better-eye visual field (VF) and integrated VF (IVF) loss, multivariable models, among Salisbury Eye Evaluation (SEE) participants with VF damage. Results show estimate and 95% confidence interval, P value, model R2 for association of (A) categoric and count outcomes, (B) continuous mobility outcomes, and (C) reading speed with better-eye and IVF mean deviation (MD) among SEE Study participants with MD of ≤−3 decibels (dB) in at least 1 eye (N = 490). Each association is adjusted for relevant covariables (described in “Methods”). *Rate ratio (all other estimates are odds ratios). ADV = Activities of Daily Vision; wpm = words per minute.
To determine whether better-eye MD truly predicts disability and IVF MD, the relationship between ability and performance measures and MD captured from the better-eye VF or the IVF was also assessed in the group of SEE subjects for whom the better-eye and IVF MD differed by ≥2 dB using multivariate models (N = 43) (Fig 5). A worse better-eye MD, but not a worse binocular summation MD, significantly slowed subjects’ reading speed (P = 0.04 vs. P = 0.17), although the magnitude of the effect was similar. A 5-dB loss in better-eye MD or IVF MD resulted in a decrease of 21.5 words per minute (22% of average reading speed) for better-eye MD versus 20.8 words/minute (21% of average reading speed) for IVF MD. No other ability and performance measure showed contradictory findings between better-eye MD and IVF MD (as judged by P ≤ 0.05 for 1 method of MD calculation only). For this group of subjects with discordant better-eye and IVF MDs, 5-dB decrements in the better-eye VF MD and the IVF MD were again associated with similar odds ratios, rate ratios, and linear regression coefficients describing vision-related disability. For all measures of ability and performance assessed, the confidence intervals were narrower for better-eye MD than for IVF MD models. In addition, for several metrics R2 values were significantly higher for better-eye MD models compared with IVF MD models.
Figure 5.
Comparison of visual disability associated with better-eye visual field (VF) and integrated VF (IVF) loss, multivariable models, among Salisbury Eye Evaluation (SEE) participants in whom there was at least 2-decibel (dB) difference between the better-eye and the integrated mean deviations (MDs). Results show estimate and 95% confidence interval, P value, and model R2 for association of (A) categoric and count outcomes, (B) continuous mobility outcomes, and (C) reading speed with better-eye and IVF MDs among SEE Study participants with MD of ≤−3 dB in at least 1 eye and at least a 2-dB difference between the better-eye and the integrated MDs (N = 43). Each association is adjusted for relevant covariables (described in “Methods”). *Rate ratio (all other estimates are odds ratios). ADV = Activities of Daily Vision; wpm = words per minute.
Among participants with glaucoma in at least 1 eye (N = 123), no ability and performance measures showed contradictory findings (as judged by P ≤0.05 for 1 method of MD calculation only) between better-eye MD and IVF MD in multivariate models (data not shown). In this glaucoma subgroup, 5-dB decrements in the better-eye VF MD and the IVF MD were again associated with similar odds ratios, rate ratios, linear regression coefficients describing vision-related disability, and model R2 values.
We also compared better-eye MD and IVF MD derived using the best location method8 and found that better-eye MD was even less likely to differ by ≥2 dB from the IVF MD when IVF MD was calculated using the best location method as opposed to the binocular summation method. We also found similar associations between VF loss and visual disability when using the better-eye MD or the best location IVF MD.
Discussion
In this population-based sample of older Americans, better-eye VF MD infrequently differed by more than 2 dB from IVF MD, even among subjects with VF damage (defined as MD ≤−3 dB in at least 1 eye). This finding held true even in a large clinic-based sample of 7053 patients with glaucoma with VF loss. Among subjects with VF damage, both better-eye and IVF MD demonstrated similar associations with multiple metrics of ability and performance as judged by the presence/absence of a statistically significant association between the MD and the variable of interest, the magnitude of observed associations (odds ratios, rate ratios, or regression coefficients associated with a 5-dB decrement in MD), and the extent of variability in the ability and performance metric explained by the model (R2 values). Our results indicate that similar associations between VF loss and visual disability are obtained when VF loss is estimated through better-eye or IVF MD.
Our findings reinforce and extend the results from a recent study by Kulkarni et al,10 which reported that better-eye and IVF scores demonstrated similar correlations to objective and subjective measures of disability scores and that these 2 measures were the most correlated with disability among the 8 VF scoring systems assessed. Our findings suggest that IVF and better-eye MD not only account for a similar amount of the observed variability in ability and performance measures but also lead to similar estimates relating the extent of VF loss to disability, and they result in similar conclusions (based on P values) of whether the severity of VF loss is associated with disability. In addition, we examined a broad range of ability and performance measures within specific functional domains (i.e., reading, mobility, driving), suggesting that IVF and better-eye MD are likely to similarly capture disability in both domain-specific measures, as suggested in this article, and in composite measures of ability and performance, as suggested both in this article and by Kulkarni et al.10
The argument for using IVF in studies evaluating VF-related disability rests on 2 premises: (1) Overlapping VF loss between the 2 eyes and VF loss in the better eye are sometimes dissimilar, and (2) overlapping VF loss is more related to disability. A previous study by Asaoka et al11 compared better-eye with IVF MD and found that IVF MD calculated through the best location method tended to be on average 1.3 dB higher (less negative) in a group of subjects with normal tension glaucoma, with 24% of patients having a best location IVF MD that was at least 2 dB healthier than the MD in the better eye.11 We report the difference between better-eye MD and IVF MD using primarily the binocular summation method, which was shown by Nelson-Quigg et al8 to be the method of integrating left and right eye VFs that provides the best prediction of actual measured binocular VF MD. By using this method in subjects with VF damage, we found a statistically significant difference between IVF and better-eye MD, although the magnitude of this difference was small (0.41 and 0.72 dB healthier median better-eye MD in SEE and clinic-based subjects, respectively). However, similar results were found when the best location method was used to derive the IVF. A possible reason for the discrepancy between our findings and those of Asaoka et al11 is that they analyzed a group of subjects with normal tension glaucoma, whereas our analyses included broader groups of individuals. In addition, the severity of VF loss assessed from their referral hospital was greater than in our SEE population sample and may have a different distribution of VF loss severity compared with our population of clinic-based patients with glaucoma. Regardless of the interstudy differences, the results from both studies suggest that even among participants with VF loss, IVF and better-eye MD do not frequently differ.
As Figure 3 demonstrates, there are subgroups of patients in whom better-eye and IVF MD differ. For example, individuals in whom 1 eye has significantly more VF loss than the other eye always differ by at least 2 dB, although differences are never >4 dB (Fig 2C). The largest discrepancies between IVF and better-eye MD are found in patients with moderate/severe VF loss in which the VF loss in the 2 eyes is spatially disparate (Fig 2A). Of note, such differences are relatively uncommon in the population, with the greatest difference between IVF and better-eye MD noted to be −5.6 dB in our sample of > 1000 older adults (Fig 2B).
To determine whether better-eye and IVF MD are similarly associated with visual disability in the subset of individuals in whom the 2 measures differ, we performed additional subanalyses in the SEE subjects in whom the better-eye and IVF MD differed by ≥2 dB. Although the number of individuals assessed in these subanalyses was relatively small (N < 50 for all analyses), the observed results suggest that the actual effects of binocular VF loss, as measured via IVF MD, and better-eye VF loss are similar and that IVF MD is unlikely to predict disability substantially better than better-eye MD even in cases in which they differ. In fact, for all measures of ability and performance assessed in this subgroup, the confidence intervals were narrower for better-eye MD than for IVF MD models and R2 values tended to be greater for better-eye MD than for IVF MD models, suggesting that IVF MD may not even predict disability as well as better-eye MD.
Several plausible explanations exist for why the degree of binocular VF loss (estimated through integration of right and left eye VFs) and better-eye VF loss have a similar effect on ability and performance measures. First, large differences are relatively uncommon. As mentioned earlier, large (>4 dB) differences in the better-eye and IVF MDs are only observed in cases in which the location of the right and left eye VF loss is highly spatially dissimilar, such as bitemporal defects (Fig 2) or discordant horizontal hemifield defects. Discordant horizontal hemifield defects affect an individual as a result of vision loss outside the area measured by static perimetry (in the case of bitemporal defects) or because a lack of overlapping regions of the VF impairs image fusion and depth perception.34,35 Second, IVFs as derived in the current study only capture the amount of overlapping VF loss when looking straight ahead at a focal distance of 33 cm. The real world is more complicated, with frequent changing of focal distance and gaze direction. Such differences may mitigate the extent to which the IVF captures the binocular VF, thus missing a truly greater relative impact of binocular VF loss over better-eye VF loss. Finally, it is possible that binocular VF loss (as approximated by IVF MD) is indeed a better predictor of disability than VF loss determined through better-eye MD, but the current study was not sufficient to test this hypothesis given that fewer than 50 subjects had better-eye and IVF MDs discordant by ≥2 dB.
Study Limitations
There are several challenges in comparing the ability of 2 VF loss metrics with regard to their ability to adequately capture vision-related disability. First, there is neither a gold standard regarding how VF loss severity should be represented nor a single measure to capture vision-related disability. As such, numerous metrics of ability and performance were evaluated as part of the SEE, including objective measures of reading and mobility and self-reported vision-related function. Indeed, we found that IVF but not better-eye MD loss predicted a statistically significant decrease in walking speed among subjects with VF loss, whereas better-eye but not IVF MD predicted a statistically significant decrease in reading speed in subjects in whom the 2 MDs differed by ≥2 dB. However, the magnitude by which IVF and better-eye VF loss affected even these 2 measures was similar. Nonetheless, it remains possible that IVF MD may differ in the ability to capture other measures of ability and performance previously demonstrated to increase with severity of VF loss but that were not evaluated in the current study.36–43 Second, a significant limitation is that even a large study such as ours cannot fully assess all patterns of VF damage, and there may be specific patterns of VF loss in which IVF MD captures disability better than better-eye MD. Finally, there is no clear method for arguing that 1 VF loss metric/ability and performance measure relationship is more “correct” than another. However, the associations between IVF MD and better-eye MD with ability and performance were similar across numerous metrics of ability and performance as judged by the presence/absence of a statistically significant P value in multivariable regression models, similar regression coefficients or odds/rate ratios reflecting vision-related disability, and similar R2 values for linear regression models of continuous ability and performance measures, thus providing the strongest possible suggestion that these 2 measures of VF loss do indeed capture vision-related disability to a similar extent. Additional limitations of the SEE data are that they focused on older patients who had VF testing; results may differ in younger subjects or in those with extensive experience with VF testing.
In conclusion, in addition to the findings presented, there is a strong clinical rationale for supporting the use of better-eye MD in studies aiming to capture disability related to VF loss, particularly given the accumulating evidence that better-eye and IVF MD are similarly associated with visual disability. Methods for estimating IVF loss are obtained only with extra software or calculation, unlike better-eye VF MD, which can be obtained easily from standard VF reports. As such, information from studies using better-eye VF MD can be more easily integrated into clinical decision-making, making better-eye MD a robust and meaningful method for reporting VF loss severity. Evidence supporting the use of IVF MD preferentially to better-eye VF MD is lacking.
Footnotes
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Ramulu PY, West SK, Munoz B, et al. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116:1846–53. doi: 10.1016/j.ophtha.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nah YS, Seong GJ, Kim CY. Visual function and quality of life in Korean patients with glaucoma. Korean J Ophthalmol. 2002;16:70–4. doi: 10.3341/kjo.2002.16.2.70. [DOI] [PubMed] [Google Scholar]
- 3.Kotecha A, O’Leary N, Melmoth D, et al. The functional consequences of glaucoma for eye-hand coordination. Invest Ophthalmol Vis Sci. 2009;50:203–13. doi: 10.1167/iovs.08-2496. [DOI] [PubMed] [Google Scholar]
- 4.Warrian KJ, Lorenzana LL, Lankaranian D, et al. The assessment of disability related to vision performance-based measure in diabetic retinopathy. Am J Ophthalmol. 2010;149:852–60. doi: 10.1016/j.ajo.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 5.McGwin G, Xie A, Mays A, et al. Visual field defects and the risk of motor vehicle collisions among patients with glaucoma. Invest Ophthalmol Vis Sci. 2005;46:4437–41. doi: 10.1167/iovs.05-0750. [DOI] [PubMed] [Google Scholar]
- 6.Sumi I, Shirato S, Matsumoto S, Araie M. The relationship between visual disability and visual field in patients with glaucoma. Ophthalmology. 2003;110:332–9. doi: 10.1016/S0161-6420(02)01742-6. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DS, Freeman E, Munoz B, et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114:2232–7. doi: 10.1016/j.ophtha.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Nelson-Quigg JM, Cello K, Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41:2212–21. [PubMed] [Google Scholar]
- 9.Crabb DP, Viswanathan AC, McNaught AI, et al. Simulating binocular visual field status in glaucoma. Br J Ophthalmol. 1998;82:1236–41. doi: 10.1136/bjo.82.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni KM, Mayer JR, Lorenzana LL, et al. Visual field staging systems in glaucoma and the activities of daily living. Am J Ophthalmol. 2012;154:445–51. doi: 10.1016/j.ajo.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Asaoka R, Crabb DP, Yamashita T, et al. Patients have two eyes!: binocular versus better eye visual field indices. Invest Ophthalmol Vis Sci. 2011;52:7007–11. doi: 10.1167/iovs.11-7643. [DOI] [PubMed] [Google Scholar]
- 12.Rubin GS, West SK, Munoz B, et al. SEE Project Team. A comprehensive assessment of visual impairment in a population of older Americans: the SEE Study. Invest Ophthalmol Vis Sci. 1997;38:557–68. [PubMed] [Google Scholar]
- 13.Muñoz B, West S, Rubin GS, et al. SEE Project Team. Who participates in population based studies of visual impairment? The Salisbury Eye Evaluation Project experience. Ann Epidemiol. 1999;9:53–9. doi: 10.1016/s1047-2797(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 14.Turano KA, Broman AT, Bandeen-Roche K, et al. SEE Project Team. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81:298–307. doi: 10.1097/01.opx.0000134903.13651.8e. [DOI] [PubMed] [Google Scholar]
- 15.Mangione CM, Phillips RS, Seddon JM, et al. Development of the “Activities of Daily Vision Scale”: a measure of visual functional status. Med Care. 1992;30:1111–26. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Freeman EE, Muñoz B, West SK, et al. Glaucoma and quality of life: the Salisbury Eye Evaluation. Ophthalmology. 2008;115:233–8. doi: 10.1016/j.ophtha.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 17.Valbuena M, Bandeen-Roche K, Rubin GS, et al. SEE Project Team. Self-reported assessment of visual function in a population-based study: the SEE Project. Invest Ophthalmol Vis Sci. 1999;40:280–8. [PubMed] [Google Scholar]
- 18.Rubin GS, Bandeen-Roche K, Huang GH, et al. SEE Project Team. The association of multiple visual impairments with self-reported visual disability: SEE Project. Invest Ophthalmol Vis Sci. 2001;42:64–72. [PubMed] [Google Scholar]
- 19.Ramulu PY, West SK, Munoz B, et al. Glaucoma and reading speed: the Salisbury Eye Evaluation project. Arch Ophthalmol. 2009;127:82–7. doi: 10.1001/archophthalmol.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CA, Keltner JL, Cello KE, et al. Ocular Hypertension Study Group. Baseline visual field characteristics in the Ocular Hypertension Treatment Study. Ophthalmology. 2002;109:432–7. doi: 10.1016/s0161-6420(01)00948-4. [DOI] [PubMed] [Google Scholar]
- 21.Montolio FG, Wesselink C, Gordijn M, Jansonius NM. Factors that influence standard automated perimetry test results in glaucoma: test reliability, technician experience, time of day, and season. Invest Ophthalmol Vis Sci. 2012;53:7010–7. doi: 10.1167/iovs.12-10268. [DOI] [PubMed] [Google Scholar]
- 22.Rozzini R, Sabatini T, Barbisoni P, Trabucchi M. How to measure comorbidity in elderly persons [letter] J Clin Epidemiol. 2004;57:321–2. doi: 10.1016/j.jclinepi.2003.08.001. author reply 323. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychol Med. 1979;9:139–45. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Friedman DS, Jampel HD, Muñoz B, West SK. The prevalence of open-angle glaucoma among blacks and whites 73 years and older: the Salisbury Eye Evaluation Glaucoma Study. Arch Ophthalmol. 2006;124:1625–30. doi: 10.1001/archopht.124.11.1625. [DOI] [PubMed] [Google Scholar]
- 26.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–74. [PubMed] [Google Scholar]
- 27.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–9. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 28.Coffey M, Reidy A, Wormald R, et al. Prevalence of glaucoma in the west of Ireland. Br J Ophthalmol. 1993;77:17–21. doi: 10.1136/bjo.77.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in the Netherlands: the Rotterdam Study. Ophthalmology. 1994;101:1851–5. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 30.Wensor MD, McCarty CA, Stanislavsky YL, et al. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105:733–9. doi: 10.1016/S0161-6420(98)94031-3. [DOI] [PubMed] [Google Scholar]
- 31.Leske MC, Connell AM, Schachat AP, Hyman L Barbados Eye Study Group. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–9. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 32.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–48. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. [PubMed] [Google Scholar]
- 34.Trauzettel-Klosinski S. Rehabilitation of lesions in the visual pathways [in German] Klin Monbl Augenheilkd. 2009;226:897–907. doi: 10.1055/s-0028-1109874. [DOI] [PubMed] [Google Scholar]
- 35.Trauzettel-Klosinski S. Rehabilitation in neuroophthalmology. In: Lorenz B, Borruat FX, Krieglstein GK, Weinreb RN, editors. Pediatric Ophthalmology, Neuro-Ophthalmology, Genetics. Berlin: Springer-Verlag; 2008. pp. 301–319. Essentials in Ophthalmology. [Google Scholar]
- 36.Hochberg C, Maul E, Chan ES, et al. Association of vision loss in glaucoma and age-related macular degeneration with IADL disability. Invest Ophthalmol Vis Sci. 2012;53:3201–6. doi: 10.1167/iovs.12-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramulu PY, van Landingham SW, Massof RW, et al. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012;119:1352–8. doi: 10.1016/j.ophtha.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20:92–8. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramulu PY, Maul E, Hochberg C, et al. Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology. 2012;11:1159–66. doi: 10.1016/j.ophtha.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith ND, Crabb DP, Garway-Heath DF. An exploratory study of visual search performance in glaucoma. Ophthalmic Physiol Opt. 2011;31:225–32. doi: 10.1111/j.1475-1313.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 41.Owsley C, McGwin G., Jr Vision and driving. Vision Res. 2010;50:2348–61. doi: 10.1016/j.visres.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black AA, Wood JM, Lovie-Kitchen JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011;88:1275–82. doi: 10.1097/OPX.0b013e31822f4d6a. [DOI] [PubMed] [Google Scholar]
- 43.Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–97. doi: 10.1097/OPX.0b013e31817882db. [DOI] [PubMed] [Google Scholar]