Abstract
Both phosphatidylinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling and antiapoptotic Bcl-2 family members are critical for survival of acute myeloid leukemia (AML) cells. Here we demonstrate the antileukemic effects of simultaneous inhibition of PI3K by the selective class I PI3K inhibitor GDC-0941 and of Bcl-2 family members by the BH3 mimetic ABT-737 in the context of the bone marrow microenvironment, where hypoxia and interactions with bone marrow stromal cells promote AML cell survival and chemoresistance. The combination of GDC-0941 and ABT-737 profoundly downregulated antiapoptotic Mcl-1 expression levels, activated BAX, and induced mitochondrial apoptosis in AML cells co-cultured with bone marrow stromal cells under hypoxic conditions. Hypoxia caused degradation of Mcl-1 and rendered Mcl-1-overexpressing OCI-AML3 cells sensitive to ABT-737. Our findings suggest that pharmacologic PI3K inhibition by GDC-0941 enhances ABT-737–induced leukemia cell death even under the protective conditions afforded by the bone marrow microenvironment.
Keywords: acute myeloid leukemia, bone marrow microenvironment, hypoxia, apoptosis, GDC-0941, ABT-737
Introduction
Phosphatidyl inositol 3-kinase (PI3K), the major activator of the serine/threonine protein kinase Akt, plays crucial roles in promoting growth, survival, and proliferation of cancer cells[1]. Activation of the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway is one of the most frequent molecular events in acute myeloid leukemia (AML), documented in approximately 70% of patients[2]. PI3K phosphorylates phosphatidyl 4,5-bisphosphate to produce a second messenger, phosphatidylinositol 3,4,5-trisphosphate, which in turn promotes the activation of downstream kinases, including Akt and mTOR[1]. PI3Ks are divided into three classes, I, II, and III, according to their structural characteristics and substrate specificity. Of these, only the class I PI3Ks, comprising the p110α, β, and γ isoforms, are able to generate phosphatidylinositol 3,4,5-triphosphate through activation of phosphoinositide-dependent kinase 1, which subsequently phosphorylates and activates Akt[1]. Since this pathway is an attractive therapeutic target in cancer, novel PI3K isoform–specific inhibitors have been developed as potential chemotherapeutic drugs and have entered clinical trials[3]. GDC-0941 (Figure 1A) is a selective oral inhibitor of class I PI3K isoforms (median inhibitory concentrations [IC50]: 3nM for PI3K-α, 33nM for PI3K-β, 3nM for PI3K-δ, and 75nM for PI3K-γ); it has promising pharmaceutical properties[4] and currently is being evaluated in Phase I clinical trials[3, 4].
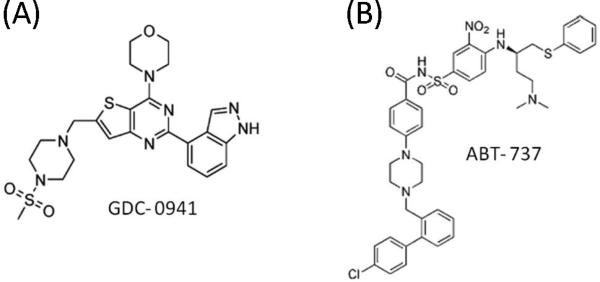
Figure 1.
Chemical structures of GDC-0941 and ABT-737.
The Bcl-2 family proteins are master regulators of apoptotic cell death through the mitochondrial pathway and have been identified as targets for cancer treatment[5]. Interactions between proapoptotic and antiapoptotic Bcl-2 family proteins dictate the fate of a cell following an apoptotic stimulus. The antiapoptotic members of the Bcl-2 family, Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1, share sequence homology in four alpha-helical Bcl-2 homology domains (BH), BH1-BH4[5], and are known to be regulated by the Stat5 or PI3K/Akt pathways[6, 7]. The multidomain proapoptotic proteins Bax, Bak, and Bad, the promoters of apoptosis, share homology in domains BH1 through BH3[5]. Overexpression of antiapoptotic Bcl-2 family proteins is frequent in human cancer, and several novel agents that target these antiapoptotic Bcl-2 family proteins have been developed[8]. ABT-737 (Figure 1B) is a potent and selective small-molecule antagonist of antiapoptotic Bcl-2 family proteins[9]. ABT-737 binds to the hydrophobic BH3-binding groove of antiapoptotic Bcl-2 family members Bcl-xL, Bcl-2, and Bcl-w with nanomolar affinity, and exerts its proapoptotic function by preventing antiapoptotic Bcl-2 family members from sequestering activating BH3 proteins[9]. ABT-737 sensitizes cancer cells to conventional cytotoxic drugs in vitro and in vivo[8], and we showed its single-agent activity in AML[10]. However, ABT-737 has poor affinity for antiapoptotic Mcl-1, a downstream target in the PI3K/Akt pathway, and increased cellular Mcl-1 levels were found to be associated with resistance to ABT-737[8, 10, 11]. Since Mcl-1 is critical for survival of human AML cells[12], targeting of Mcl-1 or regulators of its expression in combination with ABT-737 may be a useful strategy for the treatment of AML.
Hypoxia and interactions with bone marrow (BM) stromal cells are essential components of the leukemic BM microenvironment in promoting AML cell survival and chemoresistance[13]. We recently showed that leukemia progression is associated with vast expansion of hypoxia in bone marrow microenvironment, and that hypoxia contributes to chemoresistance of leukemic cells[13, 14]. Hypoxia-inducible factor-1 alpha (HIF-1α), one of the best characterized markers of hypoxia, is a transcription factor that controls gene products involved in energy metabolism, angiogenesis, apoptosis, and cell-cycle regulation and has become recognized as a strong promoter of tumor growth[15]. Hypoxia activates the PI3K/Akt signaling pathway[16], which may be one mechanism for leukemic cells to adapt and survive under conditions of hypoxia[17]. Notably, HIF-1α–independent downregulation of Mcl-1 has been reported to sensitize tumor cells to ABT-737-induced cell death under hypoxic conditions in colorectal carcinoma and small cell lung cancer[18].
In light of the known roles in cell survival of PI3K/Akt/mTOR signaling and antiapoptotic Bcl-2 family members, the potential modulation of Mcl-1 by PI3K pathway, and the possible Mcl-1 downregulation under hypoxic conditions, we hypothesized that GDC-0941 and ABT-737 would effectively suppress AML cell survival in the hypoxic BM microenvironment. In this study, we investigated the antileukemic efficacy of simultaneous blockade of PI3K and antiapoptotic Bcl-2 pathways, which may constitute a targeted approach to eradicating chemoresistant AML cells sequestered in hypoxic BM niches.
Materials and methods
Cell lines and culture conditions
Acute myeloid leukemia cell lines were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C in 5% CO2. MOLM-13 cells were kindly provided by Hayashibara Biochemical Laboratories (Okayama, Japan). HL-60 and MV4; 11cells were purchased from the American Type Culture Collection (Rockville, MD), and OCI-AML3 cells were from DSMZ (Braunschweig, Germany). Primary AML samples were obtained after informed consent in accordance with institutional guidelines set forth by Aichi Medical University per Declaration of Helsinki principles. Mononuclear cells were purified by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density-gradient centrifugation, and nonadherent cell were resuspended in RPMI 1640 medium supplemented with 10% FBS at a density of 6×106 cells/mL. Clinical characteristics of patients are summarized in Supplementary Table 1.
Mesenchymal stem cells (MSCs) obtained from healthy BM donors and AML patients were cultured at a density of 5,000 to 6,000 cells/cm2 in minimum essential medium alpha supplemented with 20% FBS, 1% L-glutamine, and 1% penicillin-streptomycin as described elsewhere[19]. The isolated, cultured MSCs at passage 3 comprised a single phenotypic population, as determined by flow cytometric analysis, positive for SH2 and SH3 and negative for markers of hematopoietic lineage. Passage 3 or 4 MSCs were used for the co-culture experiments. To study the effect of BM stroma on AML cells, MOLM-13 and HL-60 cells were cultured at a density of 5×105, with or without a layer of MSCs plated at a density of 0.2×105 cells/cm2. Co-cultured AML cells were separated from the MSC monolayer by careful pipetting with ice-cold phosphate-buffered saline solution (PBS), repeated twice. After the AML cells were collected, to rule out the possibility of contamination with MSCs, MSC monolayers were examined by microscopy (×100) to confirm that the monolayer was not damaged and that fewer than 10 leukemic cells per visual field remained attached. To verify lack of significant contamination in collected leukemic cells, the expression of CD45 was measured by flow cytometry as a discriminator between leukemic cells and MSCs[19]. Data were acquired and analyzed using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). In indicated experiments, co-cultures were performed in the presence of the class I PI3K isoform inhibitor GDC-0941 (Genentech Inc, South San Francisco, CA) and/or the BH-3 mimetic agent ABT-737 (Abbott Laboratories, IL). Chemical structures are shown in Figure 1. The MEK inhibitor U0126 was purchased from Calbiochem (San Diego, CA). For hypoxia experiments, leukemic cells from AML cell lines were cultured in a 1% O2 environment for at least 7 days to assure their continuous proliferation and survival.
Isolation of CD34+ cells
Normal bone marrow CD34+ cells were obtained with informed consent from patients undergoing routine diagnostic procedures for nonmyeloid hematopoietic disorders. CD34+ cells were isolated from mononuclear cell preparations, as previously described [20].
Lentiviral transduction of Mcl-1 shRNA
Mcl-1 expression was silenced by infecting OCI-AML3 cells with short hairpin RNA (shRNA)-encoding oligonucleotide targeting Mcl-1 as described elsewhere[10]. The cells infected Mcl-1shRNA or scrambled control shRNA were monitored for GFP fluorescence, and treated with GDC-0941 and/or ABT-737.
Cell viability, apoptosis, and cell-cycle analysis
Cell viability was assessed by the trypan blue dye exclusion method. Apoptotic cell death was analyzed by annexin V staining. Briefly, fresh cells were washed twice with binding buffer (10 mM HEPES, 140 mM NaCl, and 5 mM CaCl2, pH 7.4) and then stained with fluorescein isothiocyanate–conjugated annexin V (Roche Diagnostics, Indianapolis, IN) and propidium iodide (PI). Annexin V fluorescence was quantified by a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems). To measure mitochondrial membrane potential (Δψm), cells were loaded with MitoTracker Red CMXRos (300 nM) and MitoTracker Green (100 μM, both from Molecular Probes, Eugene, OR) for 1hour at 37°C. The Δψm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence). The flow cytometric data were analyzed by CellQuest software.
Cell-cycle distribution was analyzed by flow cytometric analysis of PI-stained nuclei. Cells were fixed in ice-cold ethanol (70% vol/vol) and stained with PI solution (25μg/ml PI, 180 U/ml RNase, 0.1% Triton X-100, and 30 mg/ml polyethylene glycol in 4 mM citrate buffer, pH 7.8; Sigma-Aldrich, St. Louis, MO). DNA content was determined by a FACScan flow cytometer system, using CellQuest software for data acquisition and analysis. Gating was set to exclude cell debris, cell doublets, and cell clumps.
Western blot analysis
Cells were solubilized in lysis buffer (PBS, 1× cell lysis buffer [Cell Signaling Technology, Beverly, MA], 1× protease inhibitor [Roche], and 1× phosphatase inhibitor cocktails I and II [Calbiochem, San Diego, CA]) and incubated for 30 minutes on ice. The lysates were then subjected to centrifugation for 10 minutes at 13,000 rpm at 4°C, and the supernatants were further analyzed. Protein concentration was determined by the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's protocol. Total protein (40 μg) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad Laboratories) and transferred to polyvinylidene-fluoride membranes (0.45 μm, GE Healthcare, Buckinghamshire, UK), then probed with first and second antibodies according to the manufacturers’ protocols. For immunoblotting, the following antibodies were used: α-tubulin (Sigma-Aldrich), HIF-1α (Santa Cruz Biotechnology, Santa Cruz, CA), Mcl-1 (BD-Pharmingen, San Diego, CA), phospho-(p-)AktSer473, p-S6 ribosomal protein (S6K)Ser235/Ser236, Bim, Bcl-2, Bcl-XL, cleaved caspase-3, and horseradish peroxidase–linked anti-mouse and anti-rabbit IgG (all from Cell Signaling Technology).
Flow cytometric analysis of BAX activation
BAX conformational change was analyzed as previously described[21]. Cellular fixation, permeabilization, and staining with primary antibody or an isotypic control were performed using the Dako IntraStain kit (DakoCytomation, Carpinteria, CA), according to the manufacturer's instructions. Staining with conformation-specific monoclonal antibody against BAX (YTH-6A7; Trevigen, Gaithersburg, MD) and isotype-matched control antibody was performed with a 1:200 dilution in 100 μl of staining buffer. Then, cells were washed three times, resuspended in 100 μl of staining buffer containing 1 mg of Alexafluor 488–labeled chicken anti-rabbit IgG (Molecular Probes), and incubated on ice for 30minutes in the dark. After three washes, activation-specific BAX was immediately measured.
Statistical analysis
Synergism, additive effects, and antagonism were assessed by the Chou-Talalay method[22], utilizingCalcusyn software (Biosoft, Cambridge, UK). The effect on cellular proliferation was shown as a percentage reduction of cell viability when compared with dimethyl sulfoxide (DMSO)–treated controls. The average combination index (CI) value for the experimental combination was calculated from the 50%, 75%, and 90% effective doses (ED50, ED75, and ED90). By this method, CI values indicate the following: 0.3-0.7, strong synergism; 0.7-0.85, moderate synergism; 0.85-0.9, slight synergism; 0.9-1.1, nearly additive; 1.1-1.2, slight antagonism; 1.2-1.45, moderate antagonism; 1.45-3.3, antagonism; 3.3-10, strong antagonism[22].
Results
Antiproliferative effects of the GDC-0941 and ABT-737 combination in AML cells
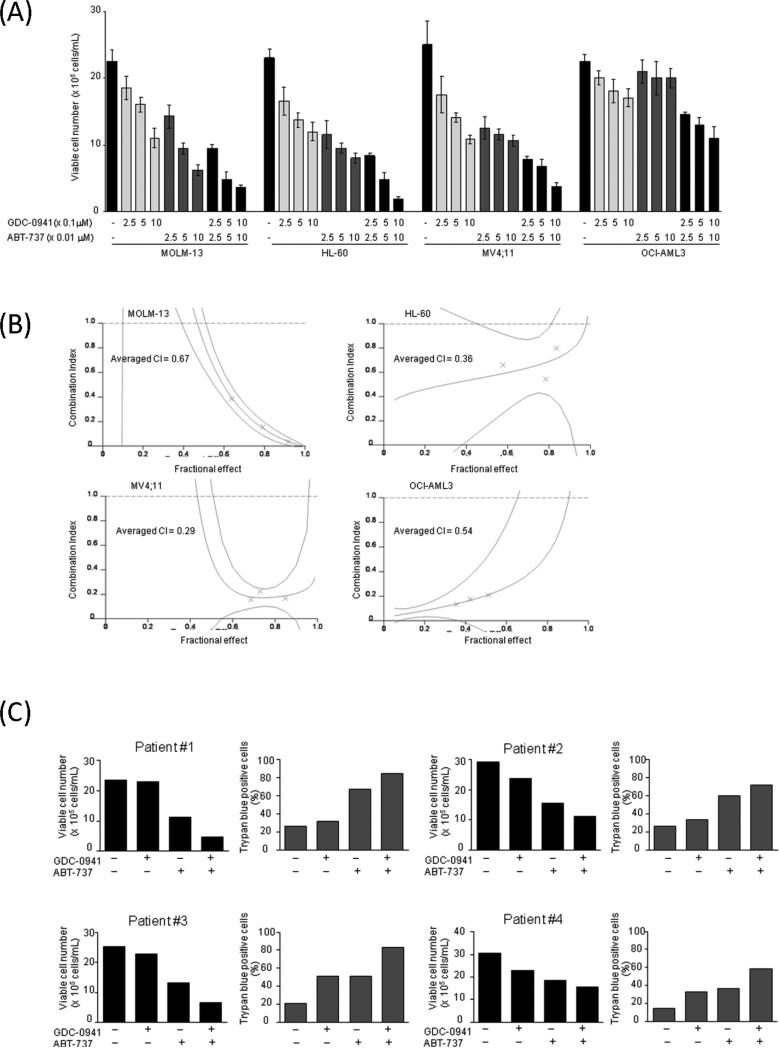
We first examined the effect of the class I PI3K isoforms inhibitor GDC-0941 and the BH-3 mimetic agent ABT-737 on the proliferation of cultured AML cell lines. AML cells were treated with GDC-0941, ABT-737, or their combination for 48 hours, and the numbers of viable cells were measured. The GDC-0941 concentrations used were 0, 0.25, 0.5, or 1.0 μM and the ABT-737 concentrations were 0, 0.025, 0.05, or 0.1 μM; the concentration ratio of GDC-0941 to ABT-737 was kept constant at 10:1. As shown in Figure 2A, GDC-0941 treatment resulted in dose-dependent cell growth inhibition in AML cells, with OCI-AML3 cells being relatively resistant to the compound (IC50: 1.0 μM for MOLM-13, 1.1 μM for HL-60, 0.7 μM for MV4;11, 4.7 μM for OCI-AML3). No significant difference in ABT-737 sensitivity was noted between wild-type p53 MOLM-13, MV4;11 cells (IC50 0.04 μM and 0.03 μM) and p53-deleted HL-60 cells (IC50 0.02 μM), concordant with a previous report[9]. However, OCI-AML3 cells, known to express high levels of Mcl-1[23] were resistant to ABT-737 (IC50 15.7 μM). Isobologram analysis demonstrated synergistic nature of pharmacological interactions between GDC-0941 and ABT-737 causing profound cell growth inhibition in HL-60, MOLM-13 and MV4;11 cells (Figure 2B). Although combined use of ABT-737 and GDC-0941 caused significant reduction of viable cell numbers in ABT-737-resistant OCI-AML3 cells (CI = 0.54), the effects remained modest even at highest concentrations of both agents used (Figure 2A).
Figure 2. Synergistic interaction between GDC-0941 and ABT-737 in AML cells.
(A) MOLM-13, HL-60, MV4;11and OCI-AML3 cells were cultured in the presence of escalating doses of GDC-0941 and ABT-737 using fixed ratios. After 48 hours, the effects on cell growth were evaluated by viable cell counts using the trypanblue exclusion method. Graphs show the mean ± SEM of results of three independent experiments. (B) Combination index (CI) plots were generated using CalcuSyn software (crosses represent actual data points for the GDC-0941 and ABT-737 combination). The averaged CI values calculated from the values for ED50, ED75, and ED90. The values less than 1.0 indicate synergism. (C) Primary AML cells (n=4) were incubated for 48 h with 0.5 μM GDC-0941, 0.05μM ABT-737 or both. Clinical characteristics of patients are summarized in Supplementary Table 1.
We next tested effects of GDC-0941 and ABT-737 in primary AML samples with high blast count (Supplementary Table 1). GDC-0941 induced moderate cell growth inhibition in 3/4 primary AML samples (Pt #2,3,4), and ABT-737 caused more profound reduction in viability (Figure 2C). Combination of GDC-0941 and ABT-737 resulted in further decrease in viable cell numbers with corresponding increase in fraction of trypan-blue (+) blast cells in all primary AML samples tested. In turn, no significant cell growth suppression or apoptosis induction was observed in normal bone marrow-derived CD34+ cells treated by 0.5 μM GDC-0941, 0.05 μM ABT-737 or both under normal MSCs co-culture conditions (Supplementary Figure 1).
We previously reported that MEK/ERK pathway inhibition potently synergizes with ABT-737 in inducing apoptosis in AML cells [24], at least in part through Mcl-1 modulation. Because combined use of GDC-0941 and ABT-737 caused a reduction of cell viability in Mcl-1-overexpressing ABT-737-resistant OCI-AML3 cells, we investigated whether the inhibition of MEK/ERK pathway could further enhance the effects of GDC-0941/ABT-737. The MEK inhibitor U2016, however, showed no further increase in growth-inhibitory or pro-apoptotic effects of GDC-0941/ABT-737 combination (data not shown), indicating that PI3K/Akt and MAPK pathways confer resistance to ABT-737 through redundant downstream pathways in AML cells with high levels of Mcl-1.
GDC-0941 increases ABT-737–induced apoptosis in AML cells co-cultured with MSC under hypoxic conditions
BM-derived stromal cells are known to activate pro-survival Akt signaling in co-cultured leukemic cells[19], and hypoxia is reported to increase activation of the PI3K/Akt/mTOR pathway[25]. In turn, both may contribute to leukemic cell adaptation and survival in the BM microenvironment[17]. The antiapoptotic Bcl-2 family members, such as Mcl-1 and Bcl-XL, are the downstream targets of Akt and mTOR signaling[26]. Hypoxia-induced mTOR activation is modulated in a PI3K/Akt–dependent[27] and –independent manner[28], while mTOR itself mediates the downstream signaling of PI3K/Akt through TORK2-dependent phosphorylation of Akt[29]. GDC-0941 has high potency against class I PI3Ks, but less against mTOR[4].
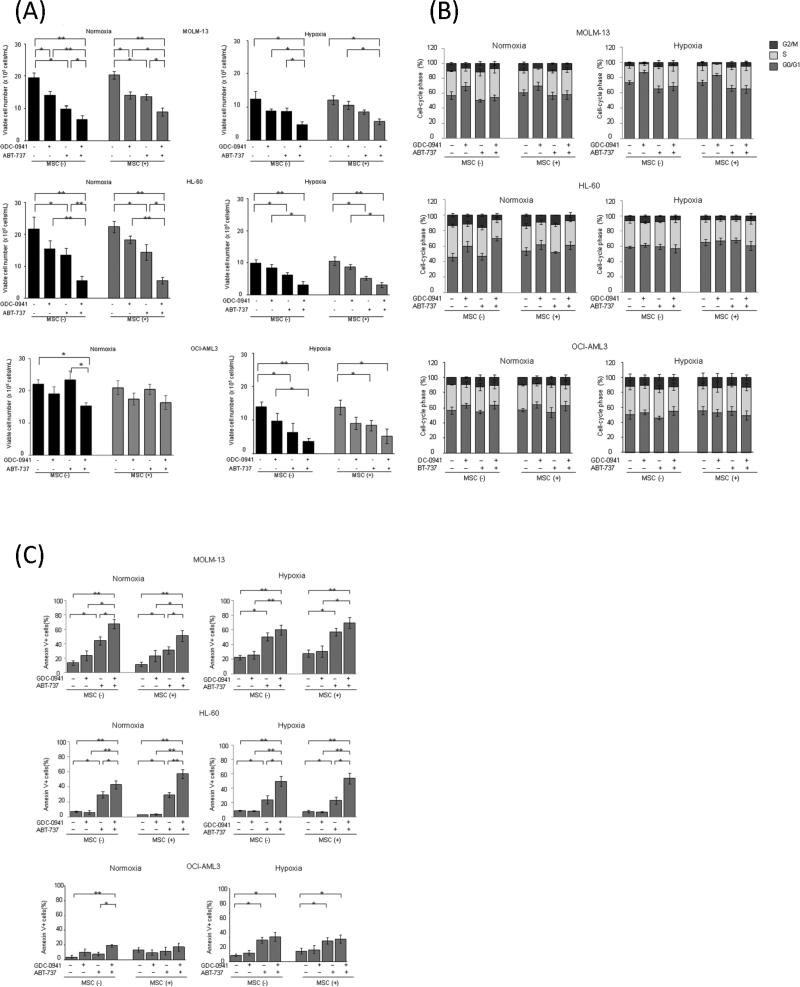
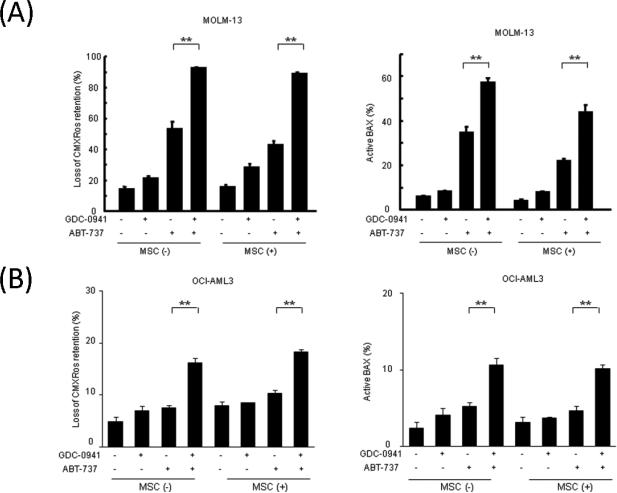
We, therefore, investigated whether MSC-leukemia cell interaction or hypoxia affects GDC-0941– and ABT-737–induced inhibition of AML cell growth. MOLM-13, HL-60 and OCI-AML3 cells co-cultured with normal MSCs under normoxic and hypoxic conditions were exposed to 0.5 μM GDC-0941, 0.05 μM ABT-737, or both for 48 hours. As shown in Figure 3A, in MOLM-13 and HL-60 cells, MSC had no significant protective effect against GDC-0941– or ABT-737–induced cell growth inhibition. Hypoxia on its own decreased cell growth of MOLM-13 and HL-60 cells, which translated in less potent growth suppression by GDC-0941 or ABT-737. Curiously, hypoxia rendered resistant OCI-AML3 cells sensitive to ABT-737 (Figure 3A). The combination of GDC-0941 and ABT-737 significantly inhibited cell proliferation irrespective of oxygen concentration in the tested cells except OCI-AML3 in normoxia. GDC-0941 (0.5 μM) treatment resulted in G1-phase cell-cycle arrest without significant apoptosis induction under normoxic and hypoxic conditions in MOLM-13 cells, and under normoxic conditions in HL-60 and OCI-AML3 cells (Figures 3B and 3C, Supplementary Figures 1A and 1B). Consistent with published reports in solid tumors[27], hypoxia promoted accumulation in quiescent state of MOLM-13 cells and less so of HL-60 cells. On the other hand, ABT-737 caused significant increases in annexin V–positive fractions in MOLM-13 and HL-60 cells (Figure 3C, p<0.05). The combination of GDC-0941 and ABT-737 resulted in further increases in apoptosis and was equally potent in normoxic and hypoxic conditions with or without MSC co-culture (Figure 3C, Supplemental Figure 1B). These findings suggest that blockade of PI3K combined with ABT-737 in the hypoxic condition exerts apoptotic efficacy against AML cells. Consistent with viability results, in OCI-AML3 cells that are resistant to ABT-737 in normoxic condition, hypoxic condition reversed the resistance to ABT-737 in both suspension culture and MSC co-culture. In these Mcl-1 expressing cells, the combination with GDC-0941 further inhibited cell proliferation with increased apoptotic cell death under hypoxic condition.
Figure 3. Combination of GDC-0941 and ABT-737 induces apoptosis in AML cells under normoxic and hypoxic and MSC co-culture conditions.
MOLM-13, HL-60 and OCI-AML3 cells were incubated for 48 hours with 0.5μM GDC-0941, 0.05μM ABT-737, or both, in the presence of absence of MSCs from 3 normal subjects under normoxic or hypoxic conditions. The viable cell numbers were measured by trypan blue exclusion assay (A). The DNA content (B) and annexin V–positive fractions (C) were measured by flow cytometry. Graphs show the means ± SEM of results of three independent experiments. *p<0.05, ** p<0.01.
Since these studies were conducted using normal BM-derived MSCs, we next investigated whether MSCs derived from AML patients are more efficient in protecting AML cells against GDC-0941– or ABT-737–induced cell death. While no significant difference was seen in response to PI3K inhibitor, AML patient BM-derived MSCs conferred improved protective effects against GDC-0941/ABT-737 combination in MOLM-13 and HL-60 cells (Supplementary Figure 3).
GDC-0941 and/or ABT-737 induce Mcl-1 downregulation under hypoxic conditions in AML cells
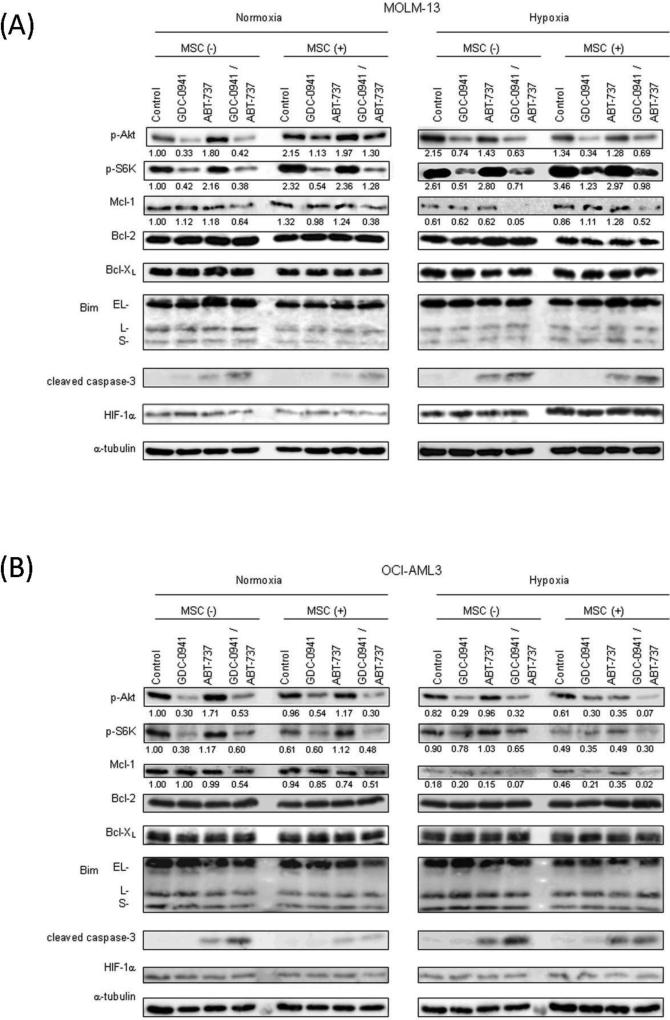
To investigate the molecular events that contribute to apoptosis induced by the combination of GDC-0941 and ABT-737, we treated ABT-737-sensitive MOLM-13 and ABT-resistant OCI-AML3 cells with GDC-0941, ABT-737, or both, with or without MSC co-culture, under normoxic or hypoxic conditions; PI3K/Akt/mTOR signaling factors and pro- and anti-apoptotic protein levels were investigated (Figure 4). Hypoxia increased phosphorylation of Akt in MOLM-13 cells but not in OCI-AML3 cells. However, in both cells, the Akt phosphorylation was effectively blocked by GDC-0941 both in normoxic and hypoxic conditions. Whereas GDC-0941 inhibited phosphorylation of mTOR downstream target S6K in normoxic condition, hypoxia itself or MSC co-culture under hypoxia interfered with the ability of GDC-0941 to block mTOR signaling recognized by p-S6K downregulation in OCI-AML3 or MOLM-13 cells, respectively. ABT-737 did not significantly affect phosphorylation of Akt or S6K. Neither GDC-0941 nor ABT-737 affected HIF-1α expression.
Figure 4. Combination of GDC-0941 and ABT-737 downregulates expression of antiapoptotic Mcl-1 in AML cells under hypoxic condition.
Expression of various proteins in MOLM-13(A) and OCI-AML3(B)that had been treated for 24 hours with GDC-0941 and/or ABT-737 was measured by immunoblotting. MOLM-13 and OCI-AML3 were treated by 0.5μM GDC-0941 and/or 0.05μM ABT-737with or without MSC co-culture under normoxic or hypoxic conditions. α–tubulin was used to confirm equal loading of proteins. Results are representative of three independent experiments. Intensity of the immunoblot signals after background subtraction was quantified using ImageJ software, and the relative intensity compared to that of α–tubulin was calculated.
Given the role of Bcl-2 family members in regulation of apoptosis, we examined effects of GDC-0941 on the expression levels of Bcl-2 family proteins. It was shown recently that Mcl-1 levels in cancer cells are consistently lower in hypoxic conditions than in normoxic conditions[18], and our observations were consistent with that both in MOLM-13 and OCI-AML3 cells. Especially, in OCI-AML3 cells that were resistant to ABT-737 in normoxia but displayed increased sensitivities under hypoxia, hypoxia reduced Mcl-1 expression to barely detectable levels (Figure 4B).
In MOLM-13 cells, GDC-0941 or ABT-737 alone did not reduce Mcl-1 levels, but the GDC-0941/ABT-737 combination markedly downregulated Mcl-1 levels in normoxic and hypoxic conditions (Figure 4A). Mcl-1 is a substrate for caspase-mediated degradation, and the GDC-0941/ABT-737 combination increased cleavage of pro-caspase-3, suggesting an activation of the mitochondrial apoptotic pathway by combined use of these two agents. Expression levels of antiapoptotic Bcl-2 and Bcl-XL and proapoptotic Bim remained unchanged. We next investigated whether GDC-0941 facilitates BAX activation by ABT-737 in MOLM-13 and OCI-AML3 cells. GDC-0941 increased ABT-737–induced loss of the mitochondrial membrane potential (Δψm) and BAX conformational change in MOLM-13 cells (Figure 5A, Supplementary Figure 3). These findings suggest that inhibition of PI3K/Akt signaling stimulates ABT-737–induced mitochondrial apoptosis, resulting in caspase activation and caspase-dependent Mcl-1 cleavage. Similar albeit less pronounced effects were seen in OCI-AML3 cells co-treated with GDC-0941/ABT-737 (Figure 5B).
Figure 5. Combination of GDC-0941 and ABT-737induced mitochondrial apoptosis in AML cells.
MOLM-13 (A) and OCI-AML3 (B) cells were treated with GDC-0941 (0.5μM for MOLM-13, 1.0μM for OCI-AML3), ABT-737 (0.05μM for MOLM-13, 0.1μM for OCI-AML3), or both; Δϕm was assessed after 18 hours of treatment and Bax conformational change after 16 hours of treatment, both by flow cytometry. Graphs show the means ± SD of results of three independent experiments. ** p<0.01.
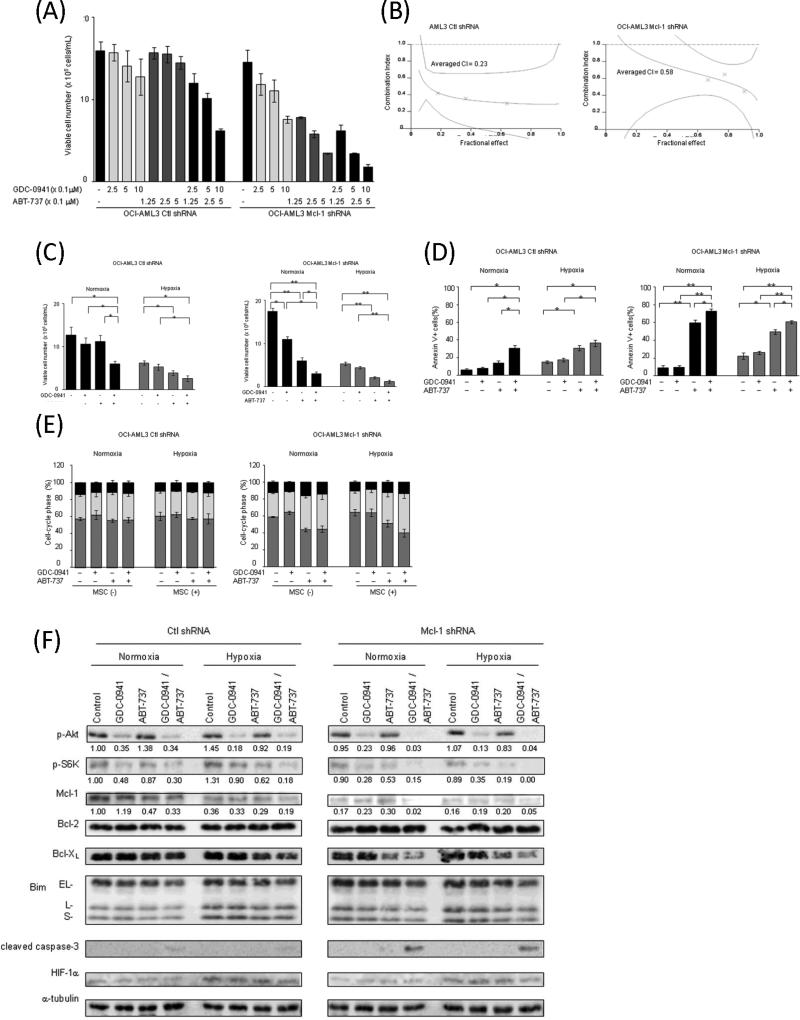
To confirm whether the Mcl-1 expression levels correlate with sensitivity to GDC-0941 and ABT-737, we utilized OCI-AML3 cells in which Mcl-1 was silenced using lentiviral shRNA construct. Stable infection of OCI-AML3 with Mcl-1-targeting shRNA resulted in 83% lower Mcl-1 protein expression compared with scrambled shRNA-infected cells (Figure 6F). As shown in Figure 6A, silencing of Mcl-1 dramatically increased sensitivity of OCI-AML3 cells to ABT-737-induced growth inhibition (IC50: 5.0 μM for control shRNA, 0.1 μM for Mcl-1 shRNA). In contrast, downregulation of Mcl-1 only modestly promoted growth inhibition by GDC-0941(IC50: 2.7 μM for control shRNA, 1.1 μM for Mcl-1 shRNA-infected cells). Combination of ABT-737 and GDC-0941 caused synergistic cell growth inhibition in both scrambled shRNA- and Mcl-1 shRNA- transfected OCI-AML3 cells as demonstrated by isobologram analyses (Figure 6B). However, the overall effect was more evident in cells with low Mcl-1 levels. Consistent with our published data[10], downregulation of Mcl-1 increased cell sensitivity to ABT-737 through induction of apoptosis but did not induce cell cycle arrest (Figures 6C-E, Supplementary Figures 4A,B). Similar to the findings in parental OCI-AML3 cells, hypoxia sensitized scrambled shRNA-infected OCI-AML3 to ABT-737-induced growth inhibition and apoptosis induction (Figures 6C and 6D, Supplementary Figure 4A). In turn, hypoxia, which by itself decreased Mcl-1 levels to the extent of knock-down in transfected cells (Fig. 6F), did not enhance ABT-induced apoptosis in Mcl-1 knockdown OCI-AML3 cells (Fig. 6D), indicating that Mcl-1 downregulation is the main mechanism of increased sensitivity of leukemic cells to ABT-737 under low oxygen conditions. The GDC-0941/ ABT-737 combination further increased in apoptosis under normoxic and hypoxic conditions in both scrambled shRNA- and Mcl-1 shRNA-transfected OCI-AML3 cells, however cell death was significantly higher in cells with low Mcl-1 levels.
Figure 6. Suppression of Mcl-1 expression restores sensitivity to ABT-737 in OCI-AML cells.
OCI-AML3 cells were infected with lentiviral Mcl-1 shRNA or scrambled shRNA as described in the Materials and Methods.
(A) Cells were cultured in the presence of escalating doses of GDC-0941 and ABT-737 using fixed ratios. After 48 hours, the effects on cell growth were evaluated by viable cell counts using the trypan blue exclusion method. Graphs show the mean ± SEM of results of three independent experiments. (B) Combination index (CI) plots were generated using CalcuSyn software. (C) The viable cells number was measured by trypan blue exclusion assay. (D,E) Annexin V–positive fractions (D) and DNA content (E) were measured by flow cytometry. Graphs show the means ± SEM of results of three independent experiments. *p<0.05, ** p<0.01.
(F) Expression of various proteins in OCI-AML cells transfected Mcl-1 shRNA or scrambled control shRNA that had been treated for 24 hours with 0.5μM GDC-0941 and/or 0.25μM ABT-737 under normoxic or hypoxic conditions. α–tubulin was used to confirm equal loading of proteins. Results are representative of three independent experiments. Intensity of the immunoblot signals after background subtraction was quantified using ImageJ software, and the relative intensity compared to that of α–tubulin was calculated.
Discussion
Our results suggest that the class I PI3K inhibitor GDC-0941 enhances ABT-737–mediated mitochondrial apoptosis in AML cells. Many small-molecule inhibitors have been developed to target the PI3K/Akt/mTOR pathway, an attractive candidate for targeted therapeutics; some are in clinical trials, and some have been approved for cancer therapy[3]. GDC-0941, which as a single agent predominantly induces G1 cell-cycle arrest with minimal apoptosis, promoted ABT-737–induced apoptosis with BAX activation, suggesting that blockade of PI3K/Akt signaling enhances mitochondrial outer membrane permeabilization by ABT-737 in AML cells. The efficacy of BH3 mimetic ABT-737 as a single agent is known to be limited because it binds tightly to Bcl-2, Bcl-xL, and Bcl-w but not to Mcl-1[10]. Previous reports suggest that high expression of Mcl-1 contributes to resistance to ABT-737 and that decreased expression of Mcl-1 confers sensitivity to ABT-737[10, 11, 30]. Mcl-1 blocks cytochrome c release from mitochondria in part by heterodimerizing with proapoptotic member of the Bcl-2 family BAX, thereby preventing BAX/BAK activation and mitochondrial outer membrane permeabilization and finally maintaining mitochondrial homeostasis. Therefore, a strategy that targets Mcl-1 is critical to sensitize tumor cells to ABT-737, including AML[10, 11]. The association of decreased Mcl-1 levels with increases in cleaved caspase-3 or in percentages of cells with loss of Δψm and Bax activation supports the conclusion that reduction of Mcl-1 is closely related to apoptosis induction. Glycogen synthase kinase-3 (GSK-3), a downstream target of PI3K/Akt signaling that is inactivated by Akt, is associated with the destabilization of Mcl-1[31], and mTOR is known to positively control Mcl-1 translation[32]. GDC-0941 and ABT-737 have synergistically inhibited growth of breast cancer cells, downregulating Mcl-1 expression[33]. These results indicate the possibility that alternative apoptotic pathways other than Mcl-1 have contributed to apoptosis induced by GDC-0941 and ABT-737. In this context, recent report showed efficient Mcl-1 decrease in AML cells upon treatment with dual PI3K/mTOR inhibitor[34], suggesting significant contribution of mTOR signaling in controlling Mcl-1 stability.
Very recently, Spender et al. reported that the combined inhibition of PI3K/Akt/mTOR and ABT-737 induced synergistic caspase activation and increase in Bim/Mcl-1 expression ratios, which was associated with a loss of c-Myc expression in Burkitt's lymphoma cells[7]. The synergistic apoptosis induction by combination of c-Myc inhibitor with ABT-737 suggests that c-Myc, the downstream target of PI3K/Akt/mTOR, promotes resistance to ABT-737[7]. c-Myc is one of the direct target gene products of Stat-5 which cross-talks with Akt/mTOR and directly targets Bcl-2 and Bcl-xL[6], and the combined targeting of Akt/mTOR using rapamycin and of Bcl-2 and Bcl-xL using ABT-737 has been shown to suppress the survival of Stat-5-dependent myeloproliferative neoplasms[35]. Given the proposed importance of c-Myc in AML biology[36], further studies dissecting the role of c-Myc in ABT-737 resistance may be informative.
In this study, we further investigated efficacy of this combination in AML cells co-cultured with MSCs in hypoxic conditions mimicking pathologic conditions of the leukemic BM microenvironment. It has been recently reported that matrix-attached tumor cells initiate an adaptive response involving upregulation of Bcl-2 antiapoptotic family proteins Bcl-2 and Bcl-xL through cap-independent translation and FOXO-mediated transcription[37]. In turn, combined inhibition of Bcl-2 and PI3K/mTOR was capable of effectively abrogating matrix-associated resistance of cancer cells[37]. Co-culture with MSC promoted phosphorylation of AKT and of mTOR downstream target pS6K in AML cells, consistent with our previous findings[19]. Although both, Bcl-2 and Mcl-1 have been reportedly induced in leukemic cells in co-culture with stromal cells[38], we did not observe change in the expression levels of these proteins. These discrepancies are likely related to the type of the stromal cells used (stromal cell lines vs. human BM-derived stromal cells in our study). Nevertheless, our published data are consistent with findings in other studies and confirm observation that human BM-derived MSC confer protection against traditionally used chemotherapeutic agents[39]. It is conceivable that stroma-induced activation of PI3K/AKT/mTOR pathway mediates resistance through post-translational modulation of Bcl-2 family proteins, or transcriptional modification of other apoptotic players through FOXO transcriptional factor. Of note, we observed better protective effects of AML patient BM-derived MSCs against GDC-0941/ABT-737 compared to normal BM MSCs, and this finding warrants further systematic studies utilizing leukemia-derived stromal cells. Overall, our data demonstrate the potential of PI3K and Bcl-2/Bcl-xL inhibitors to cause impressive inhibition of AML cell growth even under protective conditions of stromal co-cultures.
We have further explored the effect of hypoxia on GDC-0941/ABT-737 efficacy. Recent studies demonstrated that hypoxia downregulated Mcl-1 through HIF-1α–dependent or –independent mechanisms, in a cell type-dependent fashion[18]. These findings indicate that hypoxia, a known resistance factor, may paradoxically mediate enhanced sensitivity of hypoxic tumor cells to BH3 mimetic ABT-737. Consistent with the published data, we found that hypoxia, a condition prevalent in leukemic BM, reduced expression levels of antiapoptotic Mcl-1 in MOLM-13 and OCI-AML3 cells, and reversed the resistance to ABT-737 in Mcl-1-dependent OCI-AML3 cells. While knock-down of Mcl-1 sharply increased sensitivity of OCI-AML3 cells to ABT-737 under normoxic conditions, no further sensitization was conferred by culturing cell in hypoxic environment, indicating that reduction of Mcl-1 is a main mediator of increased sensitivity of AML cells under hypoxia. Although we did not observe enhanced sensitivity to ABT-737 or ABT-737/GDC-0941 in HL-60 and MOLM-13 under hypoxic conditions, pharmacological interactions between two targeted agents remained synergistic. This synergism was maintained in shMcl-1-infected OCI-AML3, pointing out to the additional mechanisms of sensitization to cell death upon combined blockade of both pathways. Taken together, our results showed that albeit hypoxia reversed Mcl-1-dependent resistance to ABT-737, in normoxia GDC-0941/ABT-737 combination was synergistic regardless of Mcl-1 silencing. These findings suggest the contribution of other potential mechanisms regulating mitochondrial apoptosis upon GDC-0941/ABT-737 treatment. Rahmani et al. [34] has recently demonstrated that PI3K/Akt inhibition increased Bim binding to Bcl-2/Bcl-xL which was abrogated by ABT-737, while combined treatment of PI3K/Akt inhibitor with ABT-737 diminished Bax/Bak binding to Mcl-1, Bcl-2, Bcl-xL. These findings suggest that apoptosis induction induced by GDC-0941/ABT-737 combination is a multifactorial process, involving release of Bim from Bcl-2/Bcl-xL or Bak/Bax from Mcl-1/Bcl-2/Bcl-xL complexes in addition to Mcl-1 downregulation, which ultimately results in apoptosis induction through Bax/Bak activation.
Overall, these findings indicate that 1) high expression of Mcl-1 does not necessarily translate to the resistance to ABT-737 in AML blasts residing within hypoxic conditions of BM microenvironment; 2) dual inhibition of Bcl-2 and PI3K pathways inhibits AML cell survival in a synergistic manner and may be particularly applicable under the pathologic conditions of leukemic BM microenvironment shown by us to promote resistance to cytotoxic chemotherapeutic agents.
In summary, the concomitant inhibition of PI3K and antiapoptotic Bcl-2 family proteins warrants further exploration as attractive therapeutic modality in AML, capable of overcoming specific mechanisms of matrix-associated and hypoxia-induced AML cell survival in the BM microenvironment.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan and Juntendo Institutional Research Grant of Environmental and Gender Specific Medicine (to Y.T.), Juntendo Institutional Project Research Program (to L.J.); Leukemia and Lymphoma Society Scholar Grant (to M.K.); NIH/NCI 5 P50 CA100632-08 (to M.K.); NIH/NCI 5 R01 CA155056-03 (to M.K.)
The authors wish to thank Hiroko Iwanami, Yasuhito Hatanaka, Tomomi Ikeda, Takako Ikegami and Akemi Koyanagi for technical assistance. We thank Laboratories of Molecular and Biochemical Research and Cell Biology, Research Support Center, Juntendo University Graduate School of Medicine for use of facilities. We thank Kathryn Hale for manuscript review, and Melodie England for help in the preparation of the manuscript.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. DOI 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleva M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. DOI 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juric D, Baselga J. Tumor genetic testing for patient selection in phase I clinical trials: the case of PI3K inhibitors. J Clin Oncol. 2012;30:765–766. doi: 10.1200/JCO.2011.39.6390. DOI 10.1200/JCO.2011.39.6390. [DOI] [PubMed] [Google Scholar]
- 4.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. DOI 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 5.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. DOI 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Miskimen KL, Wang Z, Xie XY, Brenzovich J, Ryan JJ, Tse W, Moriggl R, Bunting KD. STAT5 requires the N-domain for suppression of miR15/16, induction of bcl-2, and survival signaling in myeloproliferative disease. Blood. 2010;115:1416–1424. doi: 10.1182/blood-2009-07-234963. DOI 10.1182/blood-2009-07-234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spender LC, Inman GJ. Phosphoinositide 3-kinase/AKT/mTORC1/2 signaling determines sensitivity of Burkitt's lymphoma cells to BH3 mimetics. Mol Cancer Res. 2012;10:347–359. doi: 10.1158/1541-7786.MCR-11-0394. DOI 10.1158/1541-7786.MCR-11-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–367. doi: 10.1038/cdd.2008.137. DOI 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 9.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. DOI 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 10.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. DOI 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. DOI 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 12.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. DOI 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, Konoplev S, Fang W, Zweidler-McKay PA, Campana D, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6:e23108. doi: 10.1371/journal.pone.0023108. DOI 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frolova O, Samudio I, Benito JM, Jacamo R, Kornblau SM, Markovic A, Schober W, Lu H, Qiu YH, Buglio D, et al. Regulation of HIF-1alpha signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther. 2012;13:858–870. doi: 10.4161/cbt.20838. DOI 10.4161/cbt.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. DOI 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegeman H, Kaanders JH, Wheeler DL, van der Kogel AJ, Verheijen MM, Waaijer SJ, Iida M, Grenman R, Span PN, Bussink J. Activation of AKT by hypoxia: a potential target for hypoxic tumors of the head and neck. BMC Cancer. 2012;12:463. doi: 10.1186/1471-2407-12-463. DOI 10.1186/1471-2407-12-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. DOI 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison LR, Micha D, Brandenburg M, Simpson KL, Morrow CJ, Denneny O, Hodgkinson C, Yunus Z, Dempsey C, Roberts D, et al. Hypoxic human cancer cells are sensitized to BH-3 mimetic-induced apoptosis via downregulation of the Bcl-2 protein Mcl-1. J Clin Invest. 2011;121:1075–1087. doi: 10.1172/JCI43505. DOI 10.1172/JCI43505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabe Y, Jin L, Tsutsumi-Ishii Y, Xu Y, McQueen T, Priebe W, Mills GB, Ohsaka A, Nagaoka I, Andreeff M, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67:684–694. doi: 10.1158/0008-5472.CAN-06-3166. DOI 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 20.Rahmani M, Nguyen TK, Dent P, Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol Pharmacol. 2007;72:788–795. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- 21.Kojima K, Konopleva M, Tsao T, Andreeff M, Ishida H, Shiotsu Y, Jin L, Tabe Y, Nakakuma H. Selective FLT3 inhibitor FI-700 neutralizes Mcl-1 and enhances p53-mediated apoptosis in AML cells with activating mutations of FLT3 through Mcl-1/Noxa axis. Leukemia. 2010;24:33–43. doi: 10.1038/leu.2009.212. DOI 10.1038/leu.2009.212. [DOI] [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. DOI 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 24.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, McQueen T, Bornmann W, Tsao T, Bergamo P, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26:778–787. doi: 10.1038/leu.2011.287. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Lee SM, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett. 2006;242:231–238. doi: 10.1016/j.canlet.2005.11.001. DOI 10.1016/j.canlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. [PubMed] [Google Scholar]
- 27.Kunz M, Ibrahim SM. Molecular responses to hypoxia in tumor cells. Mol Cancer. 2003;2:23. doi: 10.1186/1476-4598-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci U S A. 2009;106:1111–1116. doi: 10.1073/pnas.0812745106. DOI 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun HL, Liu YN, Huang YT, Pan SL, Huang DY, Guh JH, Lee FY, Kuo SC, Teng CM. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-kappaB signaling to HIF-1alpha accumulation during hypoxia. Oncogene. 2007;26:3941–3951. doi: 10.1038/sj.onc.1210169. DOI 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- 30.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. DOI 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. DOI 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, Trojahn U, Wendel HG, Charest A, Bronson RT, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. DOI 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng L, Yang W, Zhang C, Ding WJ, Zhu H, Lin NM, Wu HH, He QJ, Yang B. GDC-0941 sensitizes breast cancer to ABT-737 in vitro and in vivo through promoting the degradation of Mcl-1. Cancer Lett. 2011;309:27–36. doi: 10.1016/j.canlet.2011.05.011. DOI 10.1016/j.canlet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Rahmani M, Aust MM, Attkisson E, Williams DC, Jr., Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-1365. DOI 10.1158/0008-5472.CAN-12-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Miskimen KL, Wang Z, Xie XY, Tse W, Gouilleux F, Moriggl R, Bunting KD. Effective targeting of STAT5-mediated survival in myeloproliferative neoplasms using ABT-737 combined with rapamycin. Leukemia. 2010;24:1397–1405. doi: 10.1038/leu.2010.131. DOI 10.1038/leu.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogstrand K, Hejll E, Sander B, Rozell B, Larsson LG, Grandien A. Inhibition of the intrinsic but not the extrinsic apoptosis pathway accelerates and drives MYC-driven tumorigenesis towards acute myeloid leukemia. PLoS One. 2012;7:e31366. doi: 10.1371/journal.pone.0031366. DOI 10.1371/journal.pone.0031366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, Gao S, Mills GB, Brugge JS. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–239. doi: 10.1016/j.ccr.2011.12.024. DOI 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi V, Balakrishnan K, Chen LS. Mcl-1: the 1 in CLL. Blood. 2008;112:3538–3540. doi: 10.1182/blood-2008-07-170241. DOI 10.1182/blood-2008-07-170241. [DOI] [PubMed] [Google Scholar]
- 39.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. DOI 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.