Abstract
Subtype-selective agents for the dopamine D3 receptor (D3R) have been considered as potential medications for drug addiction and other neuropsychiatric disorders. Medicinal chemistry efforts have led to the discovery of 4-phenylpiperazine derivatives that are >100-fold selective for the dopamine D3 receptor over dopamine D2 receptor (D2R), despite high sequence identity (78% in the transmembrane domain). Based on the recent crystal structure of D3R, we demonstrated that the 4-phenylpiperazine moiety in this class of D3R-selective compounds binds to the conserved orthosteric binding site, whereas the extended aryl amide moiety is oriented toward a divergent secondary binding pocket (SBP). In an effort to further characterize molecular determinants of the selectivity of these compounds, we modeled their binding modes in D3R and D2R by comparative ligand docking and molecular dynamics simulations. We found that the aryl amide moiety in the SBP differentially induces conformational changes in transmembrane segment 2 and extracellular loop 1 (EL1), which amplify the divergence of the SBP in D3R and D2R. Receptor chimera and site-directed mutagenesis studies were used to validate these binding modes and to identify a divergent glycine in EL1 as critical to D3R over D2R subtype selectivity. A better understanding of drug-dependent receptor conformations such as these is key to the rational design of compounds targeting a specific receptor among closely related homologs, and may also lead to discovery of novel chemotypes that exploit subtle differences in protein conformations.
Introduction
Dopamine receptors, which belong to the class A rhodopsin-like G protein–coupled receptors (GPCRs), are implicated in cognition, motivation, and movement. The dopamine D3 receptor (D3R), a member of the dopamine D2-like receptor subfamily, is a potential therapeutic target for drug abuse and other neuropsychiatric disorders (Heidbreder and Newman, 2010; Newman et al., 2012b). Developing potent and selective D3R ligands is critical to understanding and dissecting their downstream signaling pathways and functional specificity (Holmes et al., 2004). However, the high degree of sequence identity within the transmembrane (TM) domain of D3R and dopamine D2 receptor (D2R) and the near-identity of the orthosteric binding site (OBS) residues, as revealed by the crystal structure of the human D3R (Chien et al., 2010), have made it challenging to create subtype-selective agents, especially with physicochemical properties suitable for in vivo characterization of the physiologic roles of these receptors.
Notwithstanding these challenges, extensive medicinal chemistry efforts have led to the discovery of a class of 4-phenylpiperazine derivatives that is highly selective for D3R over D2R (e.g., compound R-22 [(R)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-3-hydroxybutyl)-1H-indole-2-carboxamide] has >100-fold selectivity for D3R over D2R) (Boeckler and Gmeiner, 2006; Newman et al., 2009). These D3R-selective compounds are characterized by a 4-phenylpiperazine primary pharmacophore (PP) and an extended aryl amide secondary pharmacophore (SP) connected by a 4-carbon linking chain (Heidbreder and Newman, 2010). Previous structure-activity relationship studies have attributed the subtype selectivity for D3R over D2R to the substituents on the 4-phenylpiperazine, an extended aryl amide ring system, and the length and functionalization of the linking chain (Grundt et al., 2007; Newman et al., 2009; Banala et al., 2011). Based on the crystal structure of D3R, we recently demonstrated that D3R over D2R selectivity mainly arises from divergent interactions of the SP within a second binding pocket (SBP) lined by residues from TMs 1, 2, 3, and 7, and extracellular loops 1 and 2 (EL1 and EL2, respectively) (Newman et al., 2012a).
To date, very limited conformational differences have been observed within the OBS among crystal structures of the same receptor bound to a variety of ligands in the absence of G protein. For example, the side chain root-mean-square deviations (RMSDs) of the OBS residues are within 1.0 Å among the crystal structures of β1-adrenergic receptor (β1AR) (Katritch et al., 2012). In contrast to this similarity in the OBS, among the β1AR structures, greater conformational rearrangements are present in regions distal to the OBS when extended or bitopic ligands are bound (Warne et al., 2011, 2012). Interestingly, the SP in the 4-phenylpiperazine class of D3R-selective compounds, an extended aryl amide or bioisostere thereof, interacts with the divergent SBP, the size, shape, and plasticity of which are likely involved in D3R over D2R selectivity. In particular, the conserved flexible proline kinks (Prokinks) in TM2 and TM7 (Sansom and Weinstein, 2000; Ballesteros et al., 2001), together with the divergent EL1, EL2, and extracellular portion of TM1, may allow the SBP of D3R and D2R to adopt different configurations that differentially accommodate the SP of R-22 and other D3R-selective ligands.
To further dissect the structural basis of D3R over D2R selectivity, we carried out comparative computational docking and molecular dynamics (MD) simulations of D3R and D2R models complexed with a variety of 4-phenylpiperazine derivatives, focusing on their access to and binding within the SBP. Combining these data with those from receptor chimera and site-directed mutagenesis studies, we identified a critical receptor segment and then a specific residue in the SBP that are responsible for D3R over D2R selectivity.
Materials and Methods
Ligand Preparation and Molecular Docking.
Ligands were docked to an equilibrated model of the D3R crystal structure (PDB ID 3PBL), and an equilibrated homology model of D2R based on the D3R structure (Chien et al., 2010) by a core-restrained induced-fit docking (IFD) protocol (Sherman et al., 2006; Newman et al., 2012a). The ensemble of IFD poses was clustered by interaction fingerprint analysis with the SIFt module in Maestro software (version 9.3; Schrödinger, Inc., New York, NY), and several representative poses were selected from the largest low-energy clusters. The ligand-binding energy was calculated by the molecular mechanics-generalized Born surface area method using Prime software (version 3.1; Schrödinger, Inc.).
For the MD and replica exchange molecular dynamics (REMD) simulations, partial charges of the ligand atoms were calculated by the quantum mechanics-polarized ligand docking protocol using Schrödinger Suite 2012 (Cho et al., 2005).
MD.
Representative models obtained from the IFD docking trials were reinserted into the explicit water-membrane bilayer solvent environment, and isothermal-isobaric MD simulations were performed at 310 K for 18 nanoseconds or longer using the Desmond Molecular Dynamics System (D. E. Shaw Research, New York, NY) or Maestro-Desmond Interoperability Tools (Schrödinger, Inc.). The simulations were carried out until both the receptor and ligand conformations were equilibrated (RMSDs to within 1.0 Å for Cα atoms of ligand-interacting residues, and 0.5 Å for the ligand over the last 6 nanoseconds of the trajectory). Multiple trajectories were collected for each receptor-ligand complex.
REMD.
REMD enhances conformational sampling by simulating multiple copies (replicas) of the same system at different temperatures and allowing exchanges of replicas between neighboring temperatures according to the Metropolis criterion (Sugita and Okamoto, 1999). REMD has been used in sampling the folding landscape to study protein folding mechanisms (Zhou et al., 2001; Felts et al., 2004) and predicting protein-ligand interactions (Osguthorpe et al., 2012). REMD simulations in this study were started from initial configurations with poses different from the preferred pose as well as from the preferred pose, to demonstrate that a converged equilibrium distribution of poses can be obtained from different initial configurations. We used 14 or 18 replicas in the temperature range of 310–333 K. The temperature increments, with smaller spacing at lower temperatures (e.g., from ∆T1,2 = 1.2 to ∆T17,18 = 1.6 in the 18-replica runs), were chosen to ensure homogeneous acceptance ratio between all temperature pairs over the entire temperature range (Patriksson and van der Spoel, 2008). The acceptance ratio was approximately 0.15–0.25. The exchange between replicas was attempted every 1.2 picoseconds, and the simulations were run for at least 4.5 and up to approximately 13 nanoseconds. During the REMD simulation, most replicas visited temperatures broadly across the entire range, indicating that the temperature series was sufficiently optimized and the length of simulation was sufficiently long. Conformations sampled at each temperature in REMD, like an individual constant-temperature MD trajectory, follow a Boltzmann distribution (Sugita and Okamoto, 1999). Conformational ensembles from the same statistical distribution can be compared in terms of populations of low-energy conformational states.
The REMD was performed using Desmond, with all other parameters set to the same values as in the regular MD. The frames from the last 0.96 nanoseconds of the 310 K ensembles were clustered based on ligand heavy-atom RMSD with a fixed cluster radius of 1.5 Å, using the cluster.pl utility in the MMTSB Toolset (Feig et al., 2004).
WaterMap Calculations.
Representative frames were selected from MD ensembles of D3R and D2R stabilized by ligands R-22 and the C3 analog. For each receptor-ligand complex, a total of 750 frames were pooled from the last 6–9 nanoseconds of several trajectories, and clustered by Cα atoms of extracellular segments of TMs 2 and 3 (residues 2.58–2.66 and 3.22–3.31) at a fixed cluster radius of 1.5 Å RMSD, after aligning by Cα atoms of intracellular segments of TMs 2 and 3 (residues 2.38–2.55 and 3.34–3.55). For each cluster, the frame closest to the centroid was included in the representative set. WaterMap calculations (Abel et al., 2008) were performed on the representative set.
Conformational Analyses.
We computed the helix angles of Prokinks at Pro2.59 (Pro84 in D3R, Pro89 in D2R) using the Prokink program in Simulaid (Visiers et al., 2000), which quantifies the kink angle in terms of three aspects: bend angle, wobble angle, and face shift. We use seven residues before and after the proline residue to define the helical segments preceding and following the kink.
We counted the number of water molecules in the SBP to approximate the volume not occupied by ligand in the SBP. We arbitrarily define a water molecule to be in the SBP if its oxygen atom is within 5 Å of residues in TMs 1, 2, 3, and 7, and EL2 that face the SBP, but not within 6 Å of lipid atoms, and its z-coordinate is within 12 Å and above that of the Asp3.32 Cα atom (the z-axis is perpendicular to the membrane and points toward the extracellular side).
The flexibility of the receptor-bound ligand conformations was quantified by measuring the angle formed between the PP and the SP moieties. The angle was obtained using the fit_angle.py utility in the Visual Molecular Dynamics program, which measures the angle between the “best-fit” lines through two selections. The best-fit line was estimated by a least-squares linear regression model of the coordinates of the selected atoms.
Receptor Chimera Creation and Site-Directed Mutagenesis.
Wild-type human D3R and D2R were tagged with a signal peptide (Guo et al., 2003) followed by a hemagglutinin epitope and SNAP tag, and cloned into pcDNA5/FRT/TO (Invitrogen, Carlsbad, CA) using standard molecular biology procedures. Chimeric receptors were generated starting with constructs provided by Robert Luedtke (University of North Texas Health Science Center, Fort Worth, TX) (Banala et al., 2011). The N terminus-TM1-intracellular loop 1 (NT-TM1-IL1) (residues 1–67), EL1 (residues 98–102), and EL2 (residues 173–185) of D2R alone or in combination were replaced with that of D3R (residues 1–62, 93–98, and 171–184, respectively). Site-directed mutants of D3R and D2R were generated using a polymerase chain reaction–based based strategy (QuikChange site-directed mutagenesis; Agilent Technologies, Inc., Santa Clara, CA). All of the constructs were confirmed by sequencing analysis.
Radioligand and Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer Competition Binding.
Radioligand binding and homogeneous time-resolved fluorescence resonance energy transfer (HTRF) experiments were performed in HEK293 cells stably transfected with SNAP-tagged wild-type or chimeric and mutant receptors using the Flp-in T-Rex system (Invitrogen) and maintained in growth medium (Dulbecco’s modified Eagle’s medium; 10% fetal bovine serum; 2 mM l-glutamine). For radioligand competition binding, studies were carried out with 100 pM [3H]N-methylspiperone (PerkinElmer Life Sciences, Alameda, CA) using 1 μM haloperidol to define nonspecific binding. Cells were induced for 24 hours prior to competition binding assay with 1 μg/ml tetracycline. Binding was performed on membrane or whole cell preparations as previously described (Javitch et al., 1995).
For HTRF experiments, 50,000 cells per well were seeded into black 96-well plates (Greiner 655086; Greiner Bio-One, Monroe, NC) pretreated with 50 μg/ml poly(d-lysine). Cells were induced with 1 μg/ml tetracycline in growth medium 24 hours after seeding. Forty-eight hours after seeding, cells were incubated with 100 nM Tag-Lite Lumi4 (CisBio, Codolet, France) in growth medium for 1 hour and washed three times with Tris-Krebs buffer (20 mM Tris, pH 7.4; 118 mM NaCl; 1.2 mM KH2PO4; 1.2 mM MgSO4; 4.7 mM KCl; 1.8 mM CaCl2). Ten nanomoles of NAPS (N-azidophenethylspiperone)–DY-647 (Cisbio, Codolet, France), along with various concentrations of test ligand in a total volume of 100 µl Tris-Krebs/0.1% bovine serum albumin buffer, were added to each well and plates were incubated overnight at 4°C. Preparations were excited at 337 nm (excitation of Tag-Lite Lumi4) and emission was measured at 620 nm (emission for Tag-Lite Lumi4) and 665 nm (emission for DY-647) on a Pherastar FS plate reader (BMG LabTech, Ortenberg, Germany). We measured 400-microsecond readings after a 50-microsecond delay to avoid short-life fluorescence background from the signal. Of note, the conditions we used here led to ligand depletion. Additional analysis suggests that lowering the seeded cell number to 10,000 and increasing the volume to 200 µl prevents ligand depletion in the competition assay.
Disintegrations per minute from radioligand binding or fluorescence resonance energy transfer ratios (665 nm/620 nm) from HTRF competition binding experiments were modeled using nonlinear regression analysis with GraphPad Prism 5 software (GraphPad Software, La Jolla, CA) to determine IC50 values for each ligand, which were converted to equilibrium dissociation constants (Ki) using the Cheng−Prusoff correction (Cheng and Prusoff, 1973) for radioligand binding data and, to account for ligand depletion, the Munson–Rodbard correction (Munson and Rodbard, 1988) for HTRF binding data. Mean Ki values ± S.E.M. are reported for at least three independent experiments. One-way analysis of variance and Bonferroni post hoc statistical tests were applied using GraphPad Prism 5 software.
Results
The Impact of the Linker on D3R over D2R Selectivity.
Based on molecular docking and dynamics simulations, we previously characterized the binding mode of compound R-22 in D3R and showed that the SP interacts with a subpocket of the SBP at the interface of TM2, TM3, EL1, and EL2 (Ptm23), formed by residues Val862.61, Leu892.64, Gly94EL1, Phe1063.28, and Cys181EL2. In contrast, in D2R, the SP is positioned closer to the interface of TMs 1, 2, and 7 (Ptm27) and the binding pose is less well defined (Newman et al., 2012a).
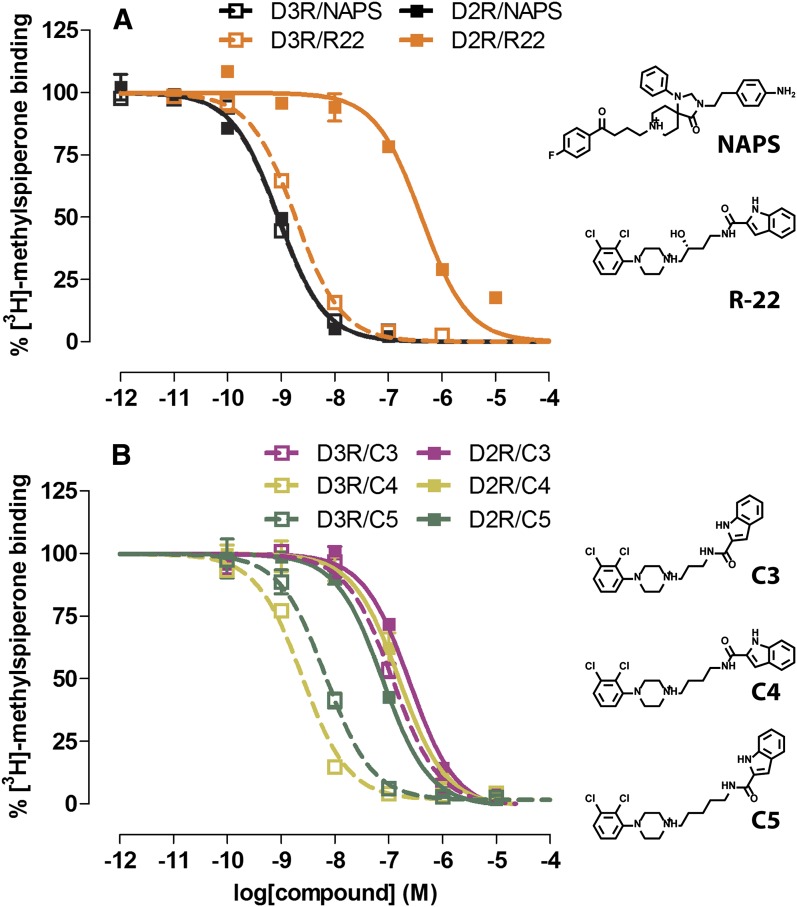
To further characterize the propensity of the SP indole moiety of R-22 to interact with either the Ptm23 or Ptm27 subpocket of D3R and D2R, we first measured the binding affinities of a series of R-22 analogs without the 3-OH group and with either 3, 4, or 5 carbon atoms in the linking chain (C3, C4, and C5, respectively; the C4 analog was previously described by Newman et al.. 2012a; synthesis of the C3 and C5 analogs is described in the Supplemental Material, Synthesis). As seen with a previously reported set of 2,3-diCl-phenylpiperazine analogs (Robarge et al., 2001), the C4 analog is the most D3R selective (approximately 46-fold), whereas the C3 analog has significantly lower affinity for D3R compared with R-22 and the C4 analog, resulting in a nearly complete loss of selectivity for D3R over D2R (Fig. 1; Table 1). The C5 analog retains high affinity for D3R, albeit approximately 5-fold lower than the C4 analog, and has slightly improved affinity for D2R, resulting in only approximately 9-fold selectivity for D3R. We hypothesized that the shorter linker in the C3 analog prevents the optimal placement of the SP in the Ptm23 subpocket of D3R, leading to the observed changes in binding affinity.
Fig. 1.
Binding affinity of R-22 and its analogs to D3R and D2R. Radioligand competition binding curves for R-22 and nonselective D2R-like family antagonist NAPS (A) and R-22 analogs C3, C4, and C5 (B). Binding curves are representatives of at least three independent experiments, for which data are summarized in Table 1.
TABLE 1.
Radioligand binding assay in D2R and D3R
Statistical significance was determined by one-way analysis of variance with the Bonferroni post-hoc test.
| Compound | D2R | D3R | D2R/D3R |
||
|---|---|---|---|---|---|
| pKi | S.E.M. | pKi | S.E.M. | Ki/Ki Ratio | |
| NAPS | 9.47 | 0.04 | 9.26 | 0.06 | 0.6 |
| R-22a | 6.82 | 0.01 | 9.02 | 0.07 | 157.2 |
| C3 | 7.07 | 0.04 | 7.31 | 0.06 | 1.7 |
| C4a | 7.24 | 0.01 | 8.90 | 0.06 | 45.7 |
| C5a | 7.48 | 0.09 | 8.42 | 0.06 | 8.8 |
pKi values at D2R and D3R for a given compound are significantly different (P < 0.05).
To explore this hypothesis, we carried out MD simulations of models of D3R or D2R complexed with R-22, as well as the C3, C4, and C5 analogs (Fig. 2). To reduce bias due to the choice of starting conformation, multiple MD simulations (approximately 20 ns each) were started from representative docked poses that positioned the SP in different subpockets within the SBP (Supplemental Table 1). The SP behaved differently in D3R and D2R: in five of the six D3R/R-22 trajectories, the SP interacted with the Ptm23 subpocket, whereas in all D2R/R-22 trajectories, the SP interacted with the Ptm27 subpocket (Supplemental Fig. 1). When we forced the SP into Ptm23 of D2R by docking, this resulted in binding poses characterized by a highly strained linker region (data not shown). Similar to the results for R-22, the analysis of C4 analog trajectories showed a tendency for its SP to interact with the Ptm23 subpocket in D3R and with the Ptm27 subpocket in D2R (Supplemental Table 1). Of note, the SP poses for C4 were more broadly distributed compared with that of R-22 in D2R (larger standard deviations for the angle between the axes of the PP and the SP moieties, 124.9° ± 8.7° for R-22, and 121.3° ± 11.2° for C4). A similar trend was also observed in D3R but to a lesser extent (132.5° ± 5.4° for R-22 and 129.4° ± 7.4° for C4). The narrower distribution of SP poses for R-22 may result from the H-bond formed between the 3-OH in the linker and Tyr7.43.
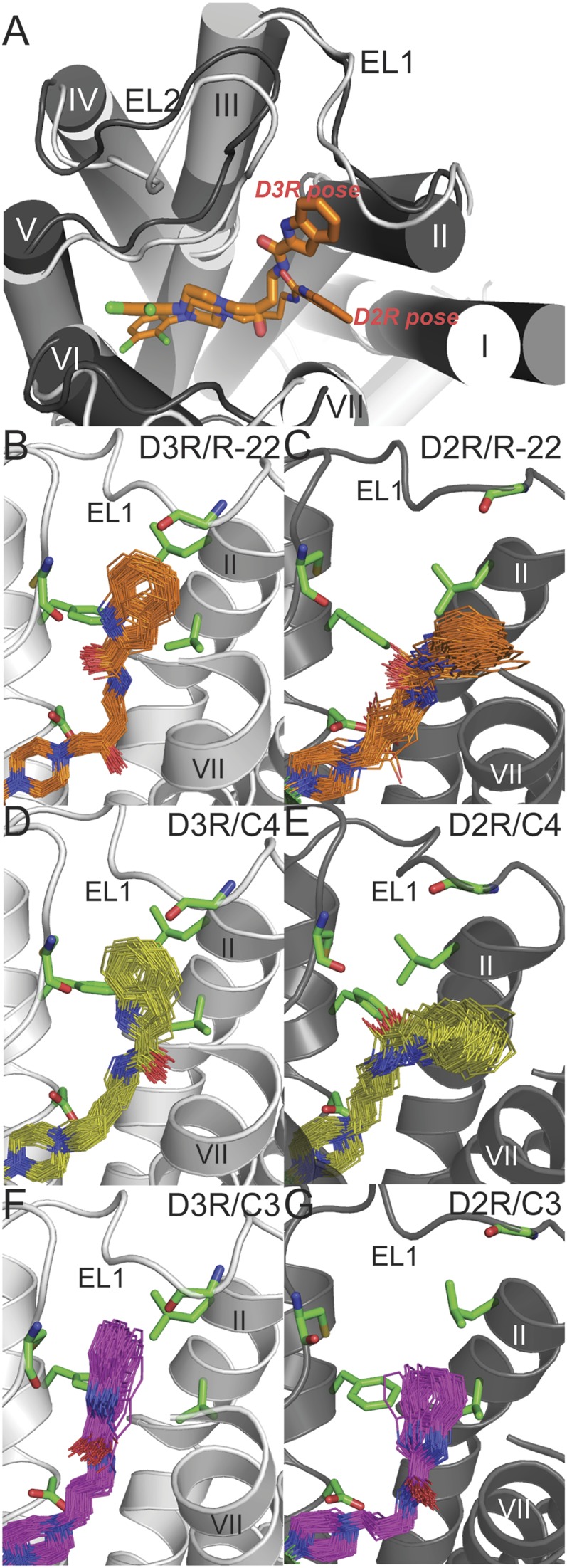
Fig. 2.
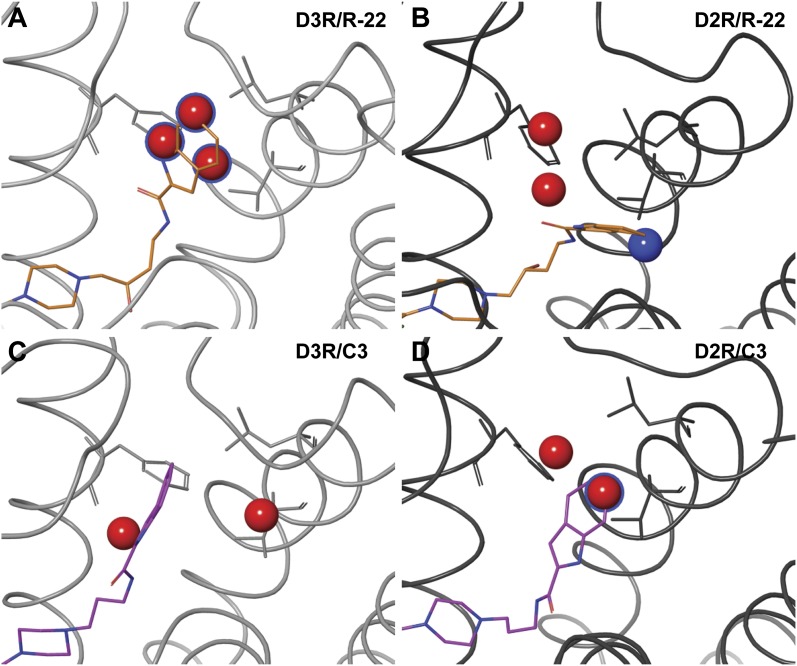
Binding mode of R-22 and the C4 and C3 analogs in D3R and D2R. (A) The superimposed binding modes of R-22 in D3R (light gray) and D2R (dark gray). The SP of R-22 (thick and thin sticks in D3R and D2R, respectively) interacts with the Ptm23 subpocket in D3R, and with the Ptm27 subpocket in D2R. For receptor-ligand complexes, R-22 (orange lines in B and C), C4 (yellow lines in D and E), or C3 (magenta lines in F and G) bound in D3R (left) or D2R (right), poses from the last 2.4 ns of representative MD trajectories are shown. The side chains of the Ptm23 residues (Val862.61, Leu892.64, Gly94EL1, Phe1063.28, and Cys181EL2) are shown in green sticks in B–G.
In the D3R/C3 analog simulations, we found that the indole ring of the SP tended to be perpendicular to the Ptm23 subpocket (defined as Ptm23′ in Supplemental Table 1; see Supplemental Fig. 2), where it is not as tightly packed against the Ptm23 residues as is the case for R-22 (Supplemental Fig. 1). In D2R/C3 analog simulations, the SP was either in the Ptm23′ pose or extensively exposed to the water phase (Supplemental Table 1).
We sought to improve sampling further by carrying out REMD simulations. REMD is an advanced sampling technique that enhances conformational sampling compared with regular constant-temperature MD (Sugita and Okamoto, 1999) (see Materials and Methods), and has been used previously to optimize sampling of protein-ligand binding modes (Osguthorpe et al., 2012). As expected, the 310 K REMD ensembles (enhanced by the exchange of states sampled at higher temperatures) show better convergence of ligand binding poses compared with the corresponding regular MD ensembles generated at 310 K (Supplemental Fig. 3; Supplemental Tables 1 and 2). Specifically, in the REMD simulations of the D3R and D2R in complex with R-22 starting with the SP of R-22 interacting with Ptm27 (Supplemental Table 2), the analysis of the 310 K ensembles showed that the R-22 in D3R gradually became more populated with poses having the SP in the Ptm23 subpocket, whereas the SP in the D2R simulations remained in the Ptm27 subpocket. Thus, our REMD results are consistent with the results from regular MD, suggesting distinct binding modes of R-22 in D3R and D2R (Fig. 2, A–C). In the REMD simulations of the D3R/C3 analog complex starting from binding poses in which the SP was positioned close to Ptm23, we found that the 310 K ensembles tend to be populated with the SP moiety adopting the alternate pose Ptm23′, consistent with the MD results. In this pose, the SP moiety loses contacts with two residues of Ptm23, Gly94, and Val862.61 (Fig. 2F; Supplemental Fig. 1C). Thus the shorter linker results in the SP being positioned further from Gly94 in EL1 than is the case for R-22 (Fig. 3; Supplemental Table 3). When the REMD simulations of the D2R/C3 analog complex were started from poses in which the SP interacted with either Ptm23 or Ptm27, the resulting 310 K ensembles consisted of a spectrum of poses that were not in tight association with either of the Ptm23 and Ptm27 subpockets (Fig. 2G). These simulations show that the SP moiety of the C3 analog does not associate tightly with the Ptm23 subpocket in either D3R or D2R, consistent with the lower binding affinity for D3R compared with R-22 and the C4 analog. By contrast, MD and REMD simulations of the C5 analog with D3R and D2R show that the SP moiety interacts preferably with Ptm23 in D3R but is positioned further away from the pocket in D2R, consistent with its higher affinity for D3R.
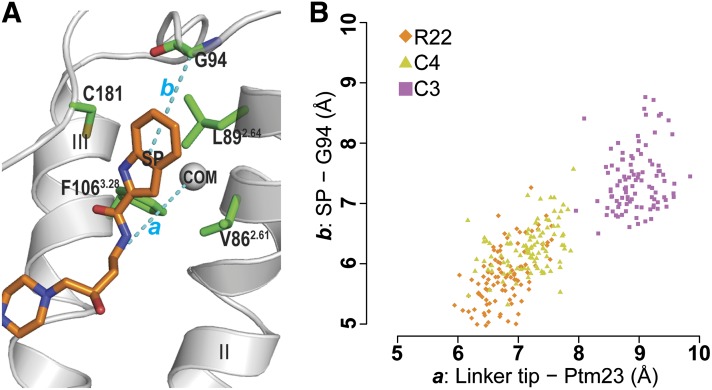
Fig. 3.
Receptor-ligand distances for D3R/R-22, D3R/C4, and D3R/C3. (A) To compare the orientations of the bound ligands in Ptm23 in the MD simulations, distances (cyan dotted lines) were measured between the last carbon atom of the ligand linker region and the center of mass of the Cα atoms of three Ptm23 residues Val2.61, Leu2.64, and Phe3.28 (the gray sphere labeled with “COM”) (a), and between the center of mass of the SP indole ring moiety and the Cα atom of Gly94 (b). The receptor backbone is in gray ribbons, side chains of the Ptm23 residues are in green sticks, and R-22 is in orange sticks. (B) A scatterplot of the receptor-ligand distances, a and b, from the last 2.4 nanoseconds of representative MD trajectories of D3R/R-22 (orange diamonds), D3R/C4 (yellow triangles), and D3R/C3 (magenta squares) shows that the SP moiety of the C3 analog is positioned farther away from the Ptm23 subpocket than those of R-22 and the C4 analog.
Divergence in the Size and Shape of the SBP in D3R and D2R.
Using comparative MD simulations with the D3R and D2R models complexed with the nonselective ligand eticlopride, we showed previously that the orientation of the SBP with respect to the highly conserved OBS is divergent in D3R and D2R (Chien et al., 2010). The difference in distance between two conserved residues, Glu2.65 (in the SBP) and Tyr7.43 (at the border of the SBP and OBS), can be as large as 2 Å between D3R and D2R despite the fact that eticlopride, unlike R-22, does not substantially occupy the SBP (Chien et al., 2010). This suggests that the volume of the SBP in the D3R and D2R is likely to be different. To characterize this volume, we counted the number of water molecules in the SBP during MD simulations (as the number of waters correlates with volume under constant temperature and constant pressure MD simulation conditions). We found a significantly greater number of waters in D3R than in D2R in the eticlopride-bound MD simulations, supporting an intrinsic divergence of this region in the two receptors. In addition, water counts in frames collected from MD trajectories of D3R and D2R complexed with R-22 were also significantly greater in D3R compared with D2R (P < 0.0005), suggesting that the volume of the SBP remains larger in D3R than in D2R even when a subpocket of SBP is occupied (Supplemental Fig. 4).
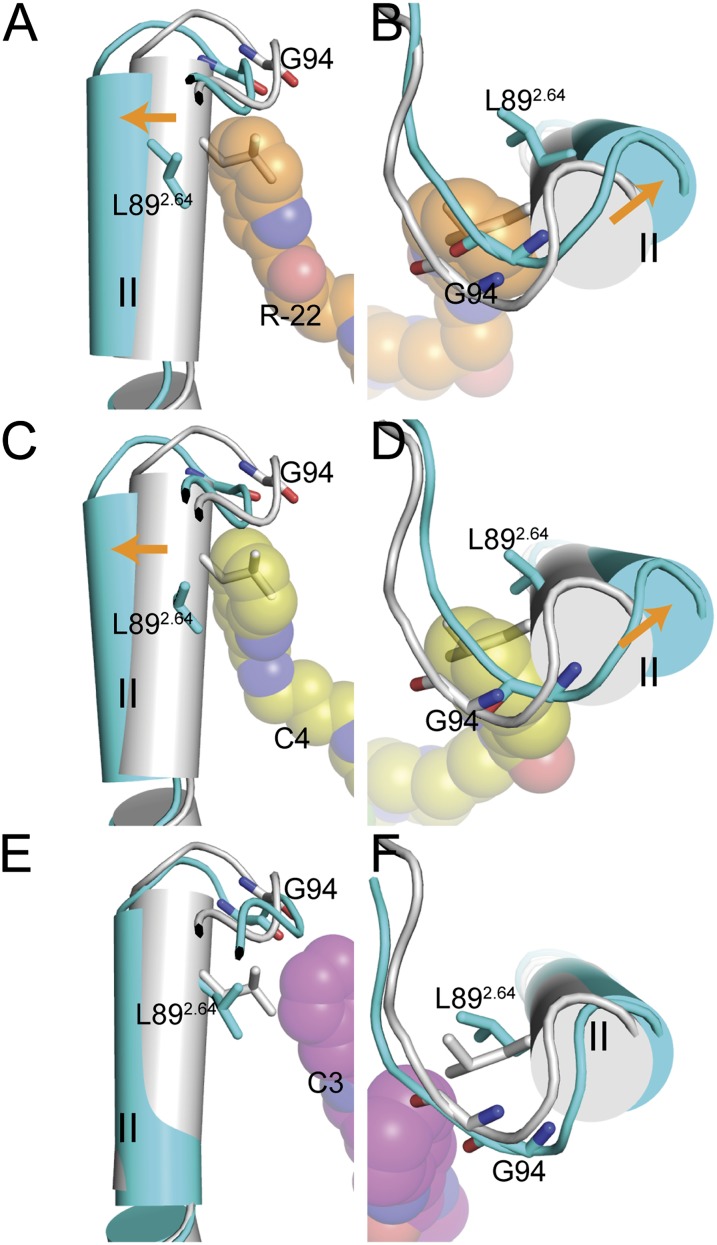
To identify differences between D3R and D2R not only in size but also in the shape of the SBP, we analyzed and compared the receptor conformations around the SBP from MD trajectories of D3R and D2R complexed with R-22 or its C4 analog. For both compounds, we observed conformational rearrangements of the SBP with respect to the eticlopride-bound state in D3R but not in D2R. We quantified these differences with Prokink angle measurements (Visiers et al., 2000; Shi et al., 2001) at the conserved Pro2.59. The results showed that wobble angles in the D3R models complexed with these two compounds are approximately 15° greater than in the D3R models complexed with eticlopride, corresponding to an outward rearrangement of the extracellular segment of TM2 (Fig. 4). In contrast, no significant changes were observed in D2R bound to the two ligands. This trend correlates with the SP binding to the Ptm23 in D3R and the Ptm27 in D2R (Fig. 2, A–E; Supplemental Table 4). Interestingly, the models bound to 4-phenylpiperazine synthons BEN 01-30 and BAK 02-43 that do not contain a SP (Newman et al., 2012a) and do not reach the Ptm23 subpocket have Prokink angles similar to those in the eticlopride-bound conformations, suggesting that the conformational change is dependent upon the presence of the SP in the Ptm23 subpocket (Supplemental Table 4). In the case of the C3 analog, the Prokink angles do not differ significantly from the eticlopride-bound conformations in either D3R or D2R, which is again consistent with relatively weak interactions between the SP and Ptm23, as discussed above.
Fig. 4.
Conformational changes induced by R-22 and the C4 and C3 analogs in D3R. Side and top views (left and right, respectively) of the overlaid R-22–, C4-, or C3-bound D3R models (all in cyan) and the eticlopride-bound D3R model (gray) are shown. Conformational changes induced by R-22 (orange spheres in A and B) and C4 (yellow spheres in C and D) in D3R involve the extracellular segment of TM2 (C-terminal to Pro2.59) shifting outward (orange arrows) relative to the eticlopride-bound model, resulting in an approximately 15° larger Prokink wobble angle. In contrast, for the C3 analog (magenta spheres in E and F) the Prokink angles do not differ significantly from the eticlopride-bound model.
To assess whether the structural divergence between D2R and D3R in the induced conformations of the SBP results in a general difference in the capacity for binding small molecules, we characterized the SBP using WaterMap calculations. WaterMap describes the thermodynamic properties of hydration sites in ligand binding pockets. The displacement of hydration sites with high free energy contributes favorably to the affinity of a ligand. Recent benchmark studies have documented the ability of this method to distinguish binding sites of low and high affinities (Beuming et al., 2012; Mason et al., 2012). When the D3R-Ptm23 subpocket is occupied by R-22, a cluster of three high-energy hydration sites is displaced by the indole ring, consistent with the high affinity of Ptm23-bound R-22 in D3R (Fig. 5A). This displacement of high-energy sites is not observed in the C3 analog–bound D3R conformation (Fig. 5C). In the R-22–bound D2R conformation, the Ptm27 subpocket contains hydration sites of significantly lower energy, thereby limiting the contribution of solvent displacement on the affinity of the ligand (Fig. 5B). For the C3-bound D2R conformation, not only is the pose less tightly associated with Ptm23 (as revealed by a large distance (approximately >8 Å) between the SP and Gly98 in EL1) but the subpocket also contains fewer high-energy sites than Ptm23 in the R-22–bound D3R conformation (Fig. 5D). In summary, only the R-22–bound D3R model is consistent with a substantial displacement of high-energy hydration sites from the receptor by the bound ligand, in agreement with the observed high affinity of this ligand/receptor complex.
Fig. 5.
Predicted hydration sites in the SBP of D3R (left) and D2R (right) bound to R-22 (orange, top) and C3 (magenta, bottom). High-energy hydration sites with a ΔG >2.5 kcal/mol are also shown as large spheres (Beuming et al., 2009). Hydration sites that are in the Ptm23 subpocket (within 6 Å to the center of mass of Ptm23 residues Val2.61, Leu2.64, and Phe3.28) are colored in red. Sites that are displaced by the ligand (within 2 Å of the indole group) are colored in blue, or have a blue silhouette if they are also in the Ptm23. Multiple high-energy sites are displaced by the aryl amide in the R-22–bound (orange) D3R model (A), but not in the C3 analog–bound D3R model (C), nor the R-22–bound (B) and the C3 analog–bound (D) D2R models.
EL1 Is Critical for D3R over D2R Selectivity.
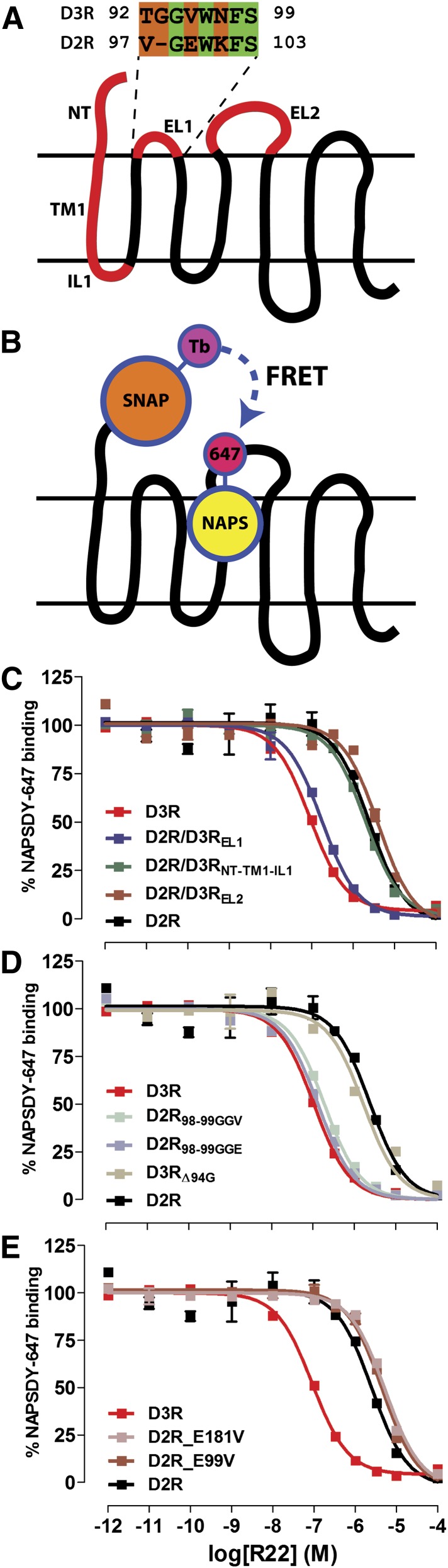
Based on these computational results, we hypothesized that the ability of the SP to adopt a favorable binding pose in Ptm23 is dependent on local conformational flexibility. In particular, the flexibility of TM2 is likely determined both by the conserved Pro2.59, which acts as a hinge, and by the length and configuration of EL1, which modulates the degree of bending at the Prokink (Fig. 4A). EL1 in D3R is one residue longer than in D2R: D3R has two Gly residues in EL1 (93GGV95), whereas D2R has only one Gly residue (98GE99). Whereas Gly94 in D3R faces Ptm23, in D2R the structurally aligned Gly98 does not directly face Ptm23 (Supplemental Fig. 5). Differences in the electrostatic properties near Ptm23 may also contribute to the D3R over D2R selectivity of R-22 and related compounds, and there are two negatively charged residues, Glu99 and Glu181, in EL1 and EL2, respectively, that are present in D2R, but not D3R (Chien et al., 2010) (Supplemental Fig. 5).
To test our hypothesis that the Prokink in TM2 and EL1 play an important role in selectivity, we carried out binding experiments on mutants of D3R and D2R lacking the TM2 Prokink, as well as D3R/D2R chimeras in which the extracellular segments of D2R, including the NT-TM1-IL1, EL1, EL2 alone or in combination were swapped with that of D3R (Fig. 6A; Table 2). To screen a large number of mutants and chimeras efficiently, we used a HTRF-based binding assay (Albizu et al., 2010) to determine affinities of the nonselective D2R-like subfamily ligand NAPS as well as R-22. Receptors were N-terminally tagged with a SNAP tag, which can be covalently labeled using a benzyl guanine terbium–based donor chromophore. When a NAPS-DY-647 acceptor chromophore is bound to the OBS of the receptor, there is a detectable fluorescence resonance energy transfer signal that can be used to quantitate competition by an unlabeled ligand (Fig. 6B).
Fig. 6.
The role of EL1 in determining R-22 binding affinity to D3R and D2R. (A) NT-TM1-IL1, EL1, and EL2 of D2R, alone or in combination, were exchanged with that of D3R. (B) Schematic of the HTRF-based binding assay. Receptors were fused with SNAP at their NT, which covalently binds a terbium (Tb)-based donor chromophore that fluorescence resonance energy transfers with an DY-647–conjugated NAPS molecule bound to the OBS. (C) D3R EL1, but not NT-TM1-IL1 or EL2, enhances R-22 binding affinity to D2R similarly to that at wild-type D3R. (D) Addition of a single Gly residue after position 98 of D2R EL1 is sufficient to enhance R-22 affinity similarly to that at D3R. Deletion of Gly94 of D3R reduces R-22 affinity similarly to that at D2R. (E) Mutation of either D2R E99 or E181 to Val has no significant effect on R-22 binding affinity. Binding curves are representative of at least three independent experiments, for which data are summarized in Table 2.
TABLE 2.
HTRF whole cell binding affinities of NAPS and R-22 at chimera and mutant constructs
Statistical significance was determined by one-way analysis of variance with the Bonferroni post hoc test. None of the mutations significantly altered the pKi values for NAPS (P > 0.1).
| Construct | NAPS |
R-22 |
||||||
|---|---|---|---|---|---|---|---|---|
| pKi | S.E.M. | Fold Affinity Increasea | Fold Affinity Decreaseb | pKi | S.E.M. | Fold Affinity Increasea | Fold Affinity Decreaseb | |
| D3R | 9.21 | 0.14 | 1.2 | 1.0 | 8.37 | 0.13 | 48.0 | 1.0 |
| D3R(Δ94G) | 9.41 | 0.19 | 0.6 | 6.99c | 0.23 | 24.1 | ||
| D2R_D3R(EL1) | 9.60 | 0.15 | 2.9 | 7.78d | 0.02 | 12.4 | ||
| D2R_D3R(EL2) | 9.21 | 0.12 | 1.2 | 6.41d | 0.04 | 0.5 | ||
| D2R_D3R(EL1_EL2) | 9.44 | 0.08 | 2.0 | 8.10e | 0.02 | 25.9 | ||
| D2R_D3R(NT-TM1-IL1) | 9.51 | 0.10 | 2.4 | 6.62c | 0.01 | 0.9 | ||
| D2R_D3R(NT-TM1-IL1_EL1) | 9.47 | 0.15 | 2.2 | 7.83d | 0.05 | 13.7 | ||
| D2R_D3R(NT-TM1-IL1_EL2) | 9.28 | 0.08 | 1.4 | 6.57c | 0.03 | 0.8 | ||
| D2R_D3R(NT-TM1-IL1_EL1_EL2) | 9.04 | 0.08 | 0.8 | 7.89e | 0.01 | 15.9 | ||
| D2R(98-99GGV) | 9.57 | 0.18 | 2.8 | 7.76d | 0.00 | 11.7 | ||
| D2R(98-99GGE) | 9.56 | 0.13 | 2.7 | 7.89e | 0.03 | 15.9 | ||
| D2R(E99V) | 9.53 | 0.11 | 2.5 | 6.33d | 0.02 | 0.4 | ||
| D2R(E181V) | 9.49 | 0.17 | 2.3 | 6.28d | 0.02 | 0.4 | ||
| D2R | 9.13 | 0.24 | 1.0 | 1.2 | 6.69 | 0.08 | 1.0 | 48.0 |
Relative to D2R wild-type.
Relative to D3R wild-type.
pKi is significantly different from that at D3R (P < 0.001) but not at D2R (P > 0.1).
pKi is significantly different from that at D2R (P < 0.001) but also at D3R (P < 0.05).
pKi is significantly different from that at D2R (P < 0.001) but not at D3R (P > 0.1).
To probe the role of the TM2 Prokink in selectivity, Pro2.59 was mutated to alanine in both the D2R and D3R. Unfortunately, specific binding to these mutants was too low to determine binding affinities of NAPS or R-22 in the intact cell HTRF assay as well as in the radioligand binding assay with membrane preparations. Efforts to enhance expression or folding using possible chaperones, including the membrane permeant receptor antagonist raclopride and dimethylsulfoxide as well as prolonged incubation of cells at temperatures shown to overcome folding abnormalities in other GPCRs (Segaloff, 2012), failed to enhance binding (data not shown), highlighting the importance of the conserved Pro2.59 residue for proper receptor folding.
In contrast, chimeric receptors used to probe the role of NT-TM1-IL1, EL1, and EL2 in R-22 selectivity were properly folded based on the finding that NAPS affinity was not significantly affected by exchange of any receptor segment tested (Table 2). However, swapping the EL1 of D3R but not the NT-TM1-IL1 or EL2 segment alone into D2R resulted in an approximately 12-fold increase in the affinity of R-22, consistent with our prediction that EL1 is the critical structural determinant of D3R selectivity (Fig. 6C). Consistent with this finding, R-22 affinity was enhanced significantly in all D2R chimeras containing EL1 from D3R (Table 2).
A Critical Role for an EL1 Glycine in Determining D3R over D2R Selectivity.
Strikingly, upon further dissection of EL1, we found that the impact of swapping EL1 can be attributed to a single Gly residue. Remarkably, the insertion of an extra Gly residue at position 98 in D2R (D2RGGE) increased R-22’s affinity approximately 16-fold to the same level as swapping EL1, not significantly different from that of D3R, whereas deletion of Gly94 in D3R (D3RΔ94G) lowered affinity approximately 24-fold, essentially to that of D2R (Fig. 6D). In contrast, removal of the negative charges in EL1 and EL2 (E99V or E181V) had no impact on R-22 affinity in D2R (Fig. 6E).
Curiously, the extent of R-22 selectivity for D3R over D2R was slightly less when measured in the HTRF assay (approximately 48-fold) than in the radioligand binding assay (approximately 157-fold). To confirm that the determinants of selectivity were similar in both assays we reassayed the selectivity of NAPS and R-22 for selected chimeras and point mutants in the radioligand membrane binding assay and validated our findings regarding their critical contribution to the pharmacological specificity of R-22 (Supplemental Table 5).
Of note, our HTRF assay used intact cells, whereas radioligand binding used a membrane preparation. Surprisingly, when we carried out radioligand binding assays with intact cells, the selectivity measurements were more similar to that of the HTRF results (Supplemental Table 6). In both intact cell assays, the affinity of R-22 for the D3R is several-fold lower than in membranes, which leads to several-fold lower selectivity ratios. Thus, it is the receptor preparation and not the probe or assay format that contributes to the observed selectivity differences. The explanation for this difference is unclear and merits further study and attention in comparing results from different assays.
Discussion
Our simulations of D3R and D2R in complex with D3R-selective 4-phenylpiperazine compounds suggest that the extra Gly94 in EL1 modulates specificity for D3R in multiple ways: first, it interacts directly with the SP of R-22; and second, it gives EL1 sufficient flexibility to allow the outward conformational rearrangement of TM2 necessary for the Ptm23 subpocket to optimally accommodate the SP of R-22. This is consistent with the dramatic loss of affinity of R-22 for D3R when this residue is deleted, and the large increase of affinity of R-22 for D2R when a Gly is inserted into EL1. In addition, computational hydration site predictions suggest that the Ptm23 subpocket has higher binding affinity in D3R than in D2R, which is optimally exploited by the R-22 binding mode in the SBP. Thus, our combined computational and experimental results point to the difference in the conformations of the SBP between D3R and D2R and identify TM2 and EL1 as the critical determinants for D3R over D2R selectivity. Interestingly, TM2 plays a critical role in selectivity despite being completely conserved between the two receptors. This is due to an alteration of its positioning as a result of the additional Gly, which lengthens EL1 and allows TM2 to move.
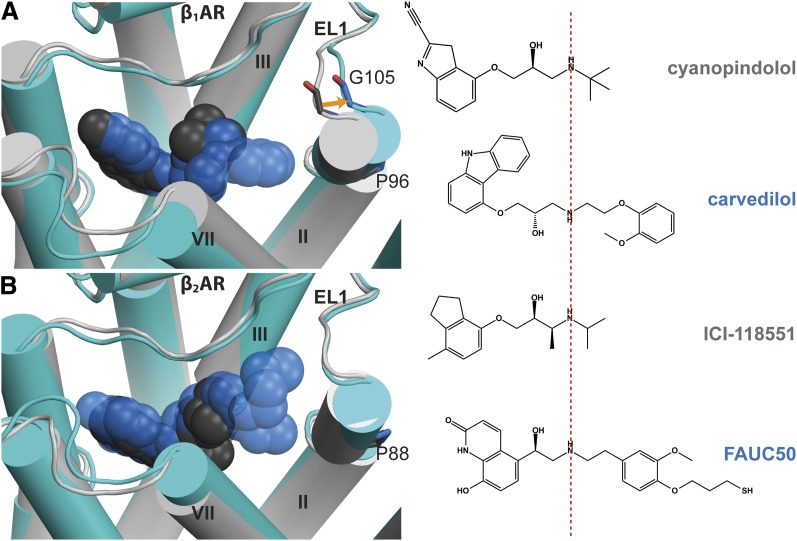
Comparison of the available inactive structures of the β1AR (11 structures, PDB IDs 2VT4, 2Y00, 2Y01, 2Y04, 2Y02, 2Y03, 2YCW, 2YCX, 2YCZ, 4AMI, 4AMJ) versus the β2-adrenergic receptor (β2AR) (6 structures, PDB IDs 2RH1, 3D4S, 3NY8, 3NY9, 3NYA, 3PDS) shows a remarkable parallel to our findings for the specificity determinants of R-22 for D3R over D2R. Thus, the extent of structural rearrangements of EL1 and TM2 induced by bulky ligands is correlated with the presence of a Gly residue in EL1 of β1AR but not β2AR. In β1AR, the bulky ligand carvedilol induces an outward shift of the extracellular segment of TM2 (PDB ID 4AMJ; Warne et al., 2012), resulting in 2.3 Å outward movement of the Cα atom of Gly105 compared with the receptor complexed with the smaller ligand cyanopindolol (PDB ID 2VT4; Warne et al., 2008) (Fig. 7A). In contrast, in β2AR, the bulky ligand, FAUC50 (PDB ID 3PDS; Rosenbaum et al., 2011), does not induce any outward shift of TM2 (Fig. 7B). Thus, the presence of Gly105 in EL1 of β1AR and the lack of a Gly residue at the aligned position in EL1 of β2AR likely contribute to the difference in the flexibility of the extracellular portion of the TM2 Prokink between β1AR and β2AR, in a manner highly analogous to that observed in D3R and D2R. Similarly, modeling studies of the A3 adenosine receptor in complex with an agonist containing rigid C2 extensions have suggested that an outward displacement of TM2 is required to accommodate such a bulky ligand (Tosh et al., 2012).
Fig. 7.
Comparison of the structural rearrangement in TM2 and EL1 induced by binding of bulky ligands in β1AR and β2AR. (A) Superposition of the cyanopindolol-bound structure (PDB ID 2VT4, cyanopindolol in dark gray, receptor in light gray) and the carvedilol-bound structure (PDB ID 4AMJ, carvedilol in blue, receptor in cyan) of β1AR shows an outward movement of the extracellular segment of TM2 in the presence of bulky ligand carvedilol, resulting in a 2.3 Å shift of the Cα atom of Gly105 in EL1, and thereby an outward tilting of the extracellular portion of TM2, compared with the cyanopindolol-bound structure. (B) Superposition of the ICI-118551–bound structure (PDB ID 3NY8, ICI-118551 in dark gray, receptor in light gray) and the FAUC50-bound structure (PDB ID 3PDS, FAUC50 in blue, receptor in cyan) of β2AR shows similar conformations of TM2 and EL1 in the presence of these two ligands. The structures were superimposed by the Cα atoms. ICI-118551, (2S,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol.
Prior chimeric receptor and mutagenesis studies were consistent with a contribution of a region containing EL1 and, to a much lesser degree, EL2, in D3R over D2R selectivity for R-22 (Newman et al., 2009) and another highly D3R-selective F-analog of R-22 (Banala et al., 2011). The current results detail the specific contribution of EL1 to the D3R over D2R selectivity of R-22 and C4 analog, and identify Gly94 as the critical residue in D3R for this selectivity, through its ability to modulate the size and shape of the SBP and allow the SP to bind in Ptm23. The combination of EL1 and EL2 led to a slightly greater D3R selectivity (Table 2), consistent with the higher affinity seen for the F-analog of R-22 in the D2R chimera that included both EL1 and EL2 of D3R (Banala et al., 2011). EL2 may act synergistically with EL1, given the extensive interactions in our models between EL1 and EL2 that involve divergent residues in D3R and D2R. In D3R, Val95 and Asn97 of EL1 interact with Val180 and Thr179 of EL2, respectively, whereas in D2R, the corresponding interactions are formed between charged Glu99 and Lys101 of EL1 and Glu181 and Asn180 of EL2 (Supplemental Fig. 5). Interestingly, the Ptm23 subpocket overlaps with the aromatic microdomain in TMs 2, 3, and 7 of D2R that had been identified by mutagenesis experiments to contribute to D4R over D2R selectivity, in this case through steric clash of the bulky substituents on the D4R-selective antagonists with this domain (Simpson et al., 1999).
Among β-adrenergic and muscarinic receptor subtypes, poorly conserved regions in the extracellular vestibule of the receptor have been proposed to act as a potential “selectivity filter,” influencing association and dissociation kinetics of the ligands as they enter the binding pocket, and it has been suggested that different interactions with the filter between the subtypes might provide opportunities for design of subtype-specific ligands (Dror et al., 2011; Kruse et al., 2012). Similarly, we propose a strategy for designing subtype-selective ligands for highly homologous GPCRs by targeting nonconserved binding pockets adjacent to the OBS by designing bivalent ligands in which the second pharmacophore can optimally access subpockets in the SBP. It is important to note that the divergence of these subpockets can result either from lack of conservation of component residues or from differences in structural plasticity that stem from differences elsewhere, or both. Indeed, targeting the differential conformational plasticity of a binding site has been proposed as an approach to designing selective ligands (Huggins et al., 2012).
Consistent with our previous results (Newman et al., 2012a), our analysis of the angle between the axes of the PP and the SP moieties quantitatively demonstrated that the SP poses of R-22 and C4 are more widely distributed in D2R compared with their poses in D3R due to the less tight binding in Ptm27 than Ptm23. Such differences in flexibility also provide interesting clues about the entropy contribution to the binding of these ligands. Thus, although the 3- to 4-fold affinity difference between R-22 and C4 at D2R is likely beyond the predictive power of computational modeling, it is possible that configurational entropy differences (Gilson and Zhou, 2007) between the binding poses of R-22 and C4 account for the small differences in their affinities for the D2R—the entropy penalty due to the loss of the flexibility by the constraining effect of the 3-OH on R-22 is larger than the favorable enthalpic contribution to the binding affinity by the H-bond. In comparison, the enthalpy (i.e., the optimal interactions between the SP and Ptm23) is dominant in the R-22 and C4 binding in D3R, which results in similar affinities of these two compounds in D3R. Interestingly, it has been noted that as the size of a compound increases, especially starting around the size of C4 or R-22 (30 and 31 heavy atoms, respectively), entropic contributions become more and more important to binding affinity (Hann and Keserü, 2012).
In the compounds studied here, the length of the linker determines to a large extent whether the SP can optimally access Ptm23. Thus, the C3 analog with one fewer carbon in the linker region loses high affinity for D3R compared with R-22, whereas its affinity for D2R remains weak. Our simulations show that the SP of this analog is less tightly associated with the Ptm23 subpocket in D3R (Fig. 2F) than is R-22. We have previously reported that the 3-carbon linked analog of the prototypic D3R selective antagonist, NGB 2904 (N-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butyl]-9H-fluorene-2-carboxamide), showed an approximately 100-fold decrease in D3R affinity, but remained D3R selective, as did another 3-carbon linked analog with the 2,3-diCl-substituted 4-phenylpiperazine (Robarge et al., 2001). In contrast, a 2-OCH3–substituted 4-phenylpiperazine derivative with a 3-carbon linker has previously been reported to be approximately 14-fold selective for D2R over D3R (Ehrlich et al., 2009). The decrease in D3R affinity in this case was also accompanied by an increase in D2R affinity and could be due to the 2-OCH3 substitution of the 4-phenylpiperazine bound in the OBS and/or the azaindole as the SP. Collectively, these findings underscore the nature and influence of the PP in the OBS and the SP in the SBP to D3R selectivity, as we previously described (Newman et al., 2012a).
Being able to computationally simulate and predict locally induced conformational changes by different ligands based on crystal structures is of great interest not only because of the implications for the design of subtype-selective ligands, but also because these ligand-specific conformations may represent distinct functionally selective states. Rosenkilde et al. (2010) previously suggested the important role of a so-called “minor binding pocket” surrounding the conserved TM2 Prokink in receptor activation and functional selectivity (e.g., in angiotensin AT1 receptor). This pocket is very similar to the SBP described here for D2R and D3R. Furthermore, Chen et al. (2012) recently showed that the bicyclic aromatic moiety of the aripiprazole scaffold plays a critical role in modulating the functional selectivity of these analogs at the D2R. The binding mode of the bicyclic aromatic moiety in the SBP and the correspondingly induced receptor conformational changes could well be the structural basis for functional selectivity of these aripiprazole derivatives.
Supplementary Material
Acknowledgments
Computations were performed on the Ranger at the Texas Advanced Computing Center [Project Number TG-MCB090022].
Abbreviations
- β1AR
β1-adrenergic receptor
- β2AR
β2-adrenergic receptor
- D2R
dopamine D2 receptor
- D3R
dopamine D3 receptor
- EL1
extracellular loop 1
- EL2
extracellular loop 2
- GPCR
G protein–coupled receptor
- HTRF
homogeneous time-resolved fluorescence resonance energy transfer
- ICI-118551
(2S,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol
- IFD
induced-fit docking
- IL1
intracellular loop 1
- MD
molecular dynamics
- NAPS
N-azidophenethylspiperone
- NGB 2904
N-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butyl]-9H-fluorene-2-carboxamide
- NT
N terminus
- OBS
orthosteric binding site
- PP
primary pharmacophore
- Prokink
proline kink
- R-22
(R)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)-3-hydroxybutyl)-1H-indole-2-carboxamide
- REMD
replica exchange molecular dynamics
- RMSD
root-mean-square deviation
- SBP
secondary binding pocket
- SP
secondary pharmacophore
- TM
transmembrane
Authorship Contributions
Participated in research design: Michino, Donthamsetti, Duan, Newman, Javitch, Shi.
Conducted experiments: Michino, Donthamsetti, Beuming, Banala, Duan, Han, Shi.
Contributed new reagents or analytic tools: Michino, Banala, Roux, Trinquet, Newman, Shi.
Performed data analysis: Michino, Donthamsetti, Beuming, Duan, Han, Shi.
Wrote or contributed to the writing of the manuscript: Michino, Donthamsetti, Beuming, Newman, Javitch, Shi.
Footnotes
This work was supported in part by the Intramural Research Program of the National Institutes of Health [National Institute on Drug Abuse (to A.H.N.)]; the National Institutes of Health National Institute on Drug Abuse [Grants K05-DA022413 and R00-DA023694]; and the National Institutes of Health National Institute of Mental Health [Grant R01-MH54137].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abel R, Young T, Farid R, Berne BJ, Friesner RA. (2008) Role of the active-site solvent in the thermodynamics of factor Xa ligand binding. J Am Chem Soc 130:2817–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L, Cottet M, Kralikova M, Stoev S, Seyer R, Brabet I, Roux T, Bazin H, Bourrier E, Lamarque L, et al. (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol 6:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JA, Shi L, Javitch JA. (2001) Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol 60:1–19 [PubMed] [Google Scholar]
- Banala AK, Levy BA, Khatri SS, Furman CA, Roof RA, Mishra Y, Griffin SA, Sibley DR, Luedtke RR, Newman AH. (2011) N-(3-fluoro-4-(4-(2-methoxy or 2,3-dichlorophenyl)piperazine-1-yl)butyl)arylcarboxamides as selective dopamine D3 receptor ligands: critical role of the carboxamide linker for D3 receptor selectivity. J Med Chem 54:3581–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Che Y, Abel R, Kim B, Shanmugasundaram V, Sherman W. (2012) Thermodynamic analysis of water molecules at the surface of proteins and applications to binding site prediction and characterization. Proteins 80:871–883 [DOI] [PubMed] [Google Scholar]
- Beuming T, Farid R, Sherman W. (2009) High-energy water sites determine peptide binding affinity and specificity of PDZ domains. Protein Sci 18:1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckler F, Gmeiner P. (2006) The structural evolution of dopamine D3 receptor ligands: structure-activity relationships and selected neuropharmacological aspects. Pharmacol Ther 112:281–333 [DOI] [PubMed] [Google Scholar]
- Chen X, Sassano MF, Zheng L, Setola V, Chen M, Bai X, Frye SV, Wetsel WC, Roth BL, Jin J. (2012) Structure-functional selectivity relationship studies of β-arrestin-biased dopamine D₂ receptor agonists. J Med Chem 55:7141–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, et al. (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330:1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AE, Guallar V, Berne BJ, Friesner R. (2005) Importance of accurate charges in molecular docking: quantum mechanical/molecular mechanical (QM/MM) approach. J Comput Chem 26:915–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror RO, Pan AC, Arlow DH, Borhani DW, Maragakis P, Shan Y, Xu H, Shaw DE. (2011) Pathway and mechanism of drug binding to G-protein-coupled receptors. Proc Natl Acad Sci USA 108:13118–13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K, Götz A, Bollinger S, Tschammer N, Bettinetti L, Härterich S, Hübner H, Lanig H, Gmeiner P. (2009) Dopamine D2, D3, and D4 selective phenylpiperazines as molecular probes to explore the origins of subtype specific receptor binding. J Med Chem 52:4923–4935 [DOI] [PubMed] [Google Scholar]
- Feig M, Karanicolas J, Brooks CL., 3rd (2004) MMTSB Tool Set: enhanced sampling and multiscale modeling methods for applications in structural biology. J Mol Graph Model 22:377–395 [DOI] [PubMed] [Google Scholar]
- Felts AK, Harano Y, Gallicchio E, Levy RM. (2004) Free energy surfaces of beta-hairpin and alpha-helical peptides generated by replica exchange molecular dynamics with the AGBNP implicit solvent model. Proteins 56:310–321 [DOI] [PubMed] [Google Scholar]
- Gilson MK, Zhou HX. (2007) Calculation of protein-ligand binding affinities. Annu Rev Biophys Biomol Struct 36:21–42 [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH. (2007) Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem 50:4135–4146 [DOI] [PubMed] [Google Scholar]
- Guo W, Shi L, Javitch JA. (2003) The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem 278:4385–4388 [DOI] [PubMed] [Google Scholar]
- Hann MM, Keserü GM. (2012) Finding the sweet spot: the role of nature and nurture in medicinal chemistry. Nat Rev Drug Discov 11:355–365 [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. (2010) Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187:4–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Lachowicz JE, Sibley DR. (2004) Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology 47:1117–1134 [DOI] [PubMed] [Google Scholar]
- Huggins DJ, Sherman W, Tidor B. (2012) Rational approaches to improving selectivity in drug design. J Med Chem 55:1424–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitch JA, Fu D, Chen J. (1995) Residues in the fifth membrane-spanning segment of the dopamine D2 receptor exposed in the binding-site crevice. Biochemistry 34:16433–16439 [DOI] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC. (2012) Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci 33:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, et al. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482:552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JS, Bortolato A, Congreve M, Marshall FH. (2012) New insights from structural biology into the druggability of G protein-coupled receptors. Trends Pharmacol Sci 33:249–260 [DOI] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. (1988) An exact correction to the “Cheng-Prusoff” correction. J Recept Res 8:533–546 [DOI] [PubMed] [Google Scholar]
- Newman AH, Beuming T, Banala AK, Donthamsetti P, Pongetti K, LaBounty A, Levy B, Cao J, Michino M, Luedtke RR, et al. (2012a) Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. J Med Chem 55:6689–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Blaylock BL, Nader MA, Bergman J, Sibley DR, Skolnick P. (2012b) Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol 84:882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, Ho D, Luedtke RR. (2009) N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. J Med Chem 52:2559–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osguthorpe DJ, Sherman W, Hagler AT. (2012) Exploring protein flexibility: incorporating structural ensembles from crystal structures and simulation into virtual screening protocols. J Phys Chem B 116:6952–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriksson A, van der Spoel D. (2008) A temperature predictor for parallel tempering simulations. Phys Chem Chem Phys 10:2073–2077 [DOI] [PubMed] [Google Scholar]
- Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. (2001) Design and synthesis of [(2,3-dichlorophenyl)piperazin-1-yl]alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor subtype. J Med Chem 44:3175–3186 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, et al. (2011) Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469:236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde MM, Benned-Jensen T, Frimurer TM, Schwartz TW. (2010) The minor binding pocket: a major player in 7TM receptor activation. Trends Pharmacol Sci 31:567–574 [DOI] [PubMed] [Google Scholar]
- Sansom MS, Weinstein H. (2000) Hinges, swivels and switches: the role of prolines in signalling via transmembrane alpha-helices. Trends Pharmacol Sci 21:445–451 [DOI] [PubMed] [Google Scholar]
- Segaloff DL. (2012) Regulatory processes governing the cell surface expression of LH and FSH receptors. Subcell Biochem 63:113–129 [DOI] [PubMed] [Google Scholar]
- Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. (2006) Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 49:534–553 [DOI] [PubMed] [Google Scholar]
- Shi L, Simpson MM, Ballesteros JA, Javitch JA. (2001) The first transmembrane segment of the dopamine D2 receptor: accessibility in the binding-site crevice and position in the transmembrane bundle. Biochemistry 40:12339–12348 [DOI] [PubMed] [Google Scholar]
- Simpson MM, Ballesteros JA, Chiappa V, Chen J, Suehiro M, Hartman DS, Godel T, Snyder LA, Sakmar TP, Javitch JA. (1999) Dopamine D4/D2 receptor selectivity is determined by A divergent aromatic microdomain contained within the second, third, and seventh membrane-spanning segments. Mol Pharmacol 56:1116–1126 [DOI] [PubMed] [Google Scholar]
- Sugita Y, Okamoto Y. (1999) Replica-exchange molecular dynamics method for protein folding. Chem Phys Lett 314:141–151 [Google Scholar]
- Tosh DK, Deflorian F, Phan K, Gao ZG, Wan TC, Gizewski E, Auchampach JA, Jacobson KA. (2012) Structure-guided design of A(3) adenosine receptor-selective nucleosides: combination of 2-arylethynyl and bicyclo[3.1.0]hexane substitutions. J Med Chem 55:4847–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visiers I, Braunheim BB, Weinstein H. (2000) Prokink: a protocol for numerical evaluation of helix distortions by proline. Protein Eng 13:603–606 [DOI] [PubMed] [Google Scholar]
- Warne T, Edwards PC, Leslie AG, Tate CG. (2012) Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure 20:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. (2011) The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature 469:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Berne BJ, Germain R. (2001) The free energy landscape for beta hairpin folding in explicit water. Proc Natl Acad Sci USA 98:14931–14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.