Abstract
Binding of the Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) to the NMDA-type glutamate receptor subunit GluN2B is an important control mechanism for the regulation of synaptic strength. CaMKII binding to GluN2B and CaMKII translocation to synapses are induced by an initial Ca2+/CaM stimulus, which also activates the kinase. Indeed, several mechanistically different CaMKII inhibitors [tatCN21 and KN-93 (N-[2-[[[3-(4-chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide)] and inactivating mutations (K42M, A302R, and T305/T306D) impair this interaction, suggesting that it requires CaMKII enzymatic activity. However, this study shows that two general kinase inhibitors, H7 [1-(5-isoquinolinylsulfonyl)-2-methylpiperazine] and staurosporine (Sta), which inhibit CaMKII activity by yet another mechanism, did not interfere with GluN2B binding in vitro or within cells. In contrast to a previous report, we found that Sta, like H7, inhibited CaMKII in an ATP-competitive manner. Nucleotide binding significantly enhances CaMKII/GluN2B binding in vitro, but the nucleotide competition by H7 or Sta did not prevent this effect and instead even mimicked it. H7 (700 µM) and Sta (2 µM) efficiently blocked enzymatic activity of CaMKII, both in vitro and within cells. However, neither H7 nor Sta prevented Ca2+-induced translocation of CaMKII to GluN2B in heterologous cells or to synapses in hippocampal neurons. Thus, activity of CaMKII (or of any other kinase inhibited by H7 or Sta) is not required for stimulation-induced GluN2B-binding or synaptic translocation of CaMKII, despite previous indication to the contrary. This shows that results with inhibitors and inhibiting mutants can be caused by structural effects independent from catalytic activity, and that detailed understanding of the mechanisms is required for their interpretation.
Introduction
Ca2+/Calmodulin (CaM)-dependent protein kinase II (CaMKII) and the N-methyl-d-aspartate–type glutamate receptor (NMDAR) are important mediators of long-term potentiation (LTP) (for review see Coultrap and Bayer, 2012a; Sanhueza and Lisman, 2013). During an LTP stimulus, CaMKII becomes activated, resulting in an increase of AMPA-type glutamate receptor (AMPAR) conductance (Derkach et al., 1999; Kristensen et al., 2011) and synaptic localization (Hayashi et al., 2000; Opazo et al., 2010). CaMKII has an important binding interaction with the GluN2B subunit of the NMDAR (Gardoni et al., 1998; Strack and Colbran, 1998; Bayer et al., 2001), which is required for normal LTP (Barria and Malinow, 2005; Sanhueza et al., 2011; Halt et al., 2012).
Stimulation of CaMKII by Ca2+/CaM induces both kinase activation and GluN2B binding by displacing a regulatory domain, which blocks the substrate-binding “S site” and the neighboring GluN2B-binding “T site” in the inactive state (Bayer et al., 2001; Coultrap and Bayer, 2012a). Additionally, Ca2+/CaM-binding can induce autophosphorylation at T286, preventing reassociation of the regulatory region even after Ca2+/CaM dissociation (Lou et al., 1986; Miller and Kennedy, 1986; Schworer et al., 1988; Thiel et al., 1988). Consequently, either Ca2+/CaM-binding or T286 phosphorylation alone are sufficient to induce GluN2B binding (Bayer et al., 2001). Because T286 phosphorylation and GluN2B binding both keep the regulatory domain partially displaced, both enable “autonomous” CaMKII activity that persists after an initial Ca2+ stimulus has subsided. In contrast to T286 phosphorylation (Giese et al., 1998; Buard et al., 2010), the GluN2B-mediated autonomy is phosphatase resistant and may be important not only for LTP induction (Bayer et al., 2001; Barria and Malinow, 2005; Halt et al., 2012) but also for LTP maintenance (Sanhueza et al., 2011). However, regulation of CaMKII localization may be an even more relevant function of CaMKII/GluN2B binding in LTP (Colbran and Brown, 2004; Merrill et al., 2005; Coultrap and Bayer, 2012a). Upon Ca2+/CaM stimulation, CaMKII translocates to the synapse (Shen and Meyer, 1999; Bayer et al., 2001), a process mediated at least in part by direct GluN2B binding (Bayer et al., 2001; Halt et al., 2012; She et al., 2012). Once CaMKII is at the synapse, it is localized in close proximity to important substrates and to its source of stimulation. Indeed, the CaMKII-dependent increase in AMPAR conductance in LTP requires CaMKII/GluN2B binding (Halt et al., 2012). Thus, CaMKII/GluN2B binding induces autonomous kinase activity, maintains the presence of CaMKII at the synapse, and regulates LTP.
While CaMKII/GluN2B binding is widely accepted as the molecular mechanism underlying synaptic CaMKII translocation, the regulation of this process still poses at least one major conundrum: while enzymatic activity of CaMKII negatively regulates binding [by phosphorylating S1303, which lies within the CaMKII binding site on the GluN2B C-tail (Strack et al., 2000)], it also appears to be essential for synaptic translocation, as previously tested manipulations that block CaMKII activity inhibit translocation (detailed below). Due to the inhibitory effect of GluN2B phosphorylation at S1303, CaMKII/GluN2B binding is greater in the presence of ADP relative to ATP in vitro (O'Leary et al., 2011). However, even ATP significantly enhances GluN2B binding compared with conditions without nucleotides, due to a direct positive regulation by nucleotide binding to CaMKII (O'Leary et al., 2011). Consistent with this nucleotide effect, both CaMKII/GluN2B binding in heterologous cells and CaMKII translocation to synapses in neurons is significantly impaired by the CaMKII K42M mutation, which prevents nucleotide binding (Hanson et al., 1994). However, K42M also blocks enzymatic activity of CaMKII, which may provide an alternate explanation. Indeed, synaptic CaMKII translocation is also impaired by other inactivating mutations, A302R (Shen and Meyer, 1999) or T305/306D (O'Leary et al., 2011), and by the CaMKII inhibitors KN-93 (N-[2-[[[3-(4-chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide) (Vest et al., 2010) and tatCN21 (Vest et al., 2007). These effects together provide strong evidence for the requirement of CaMKII activity in its translocation, as the manipulations used various inhibitory mechanisms.
To further test the activity dependence of CaMKII/GluN2B binding, we used ATP-competitive inhibitors. Importantly, these inhibitors mimicked the positive regulation by nucleotides on CaMKII/GluN2B binding. While the inhibitors effectively blocked enzymatic activity of CaMKII, they did not block CaMKII translocation within cells. Thus, enzymatic activity of CaMKII (or of other kinases blocked by these inhibitors) is not required for CaMKII translocation, and, as discussed here, the effects of previous CaMKII inhibitors were due to structural effects independent of enzymatic activity. These findings highlight that detailed understanding of inhibitory mechanisms is important in the interpretation of results with both drugs and mutations.
Materials and Methods
CaMKIIα and CaM were isolated and purified as described previously (Bayer et al., 2001, 2006; O'Leary et al., 2011). Syntide-2 peptide was obtained from Genscript (Piscataway, NJ). Unless otherwise indicated, reagents were obtained from Sigma-Aldrich (St. Louis, MO). 1-(5-IsoquinolinylsuIfonyl)-2-methylpiperazine (H7) and staurosporine (Sta) were from Tocris (Bristol, UK).
Data Analysis and Statistics.
All statistical tests and inhibitory mechanism calculations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA). Unless otherwise indicated, methods of post-hoc analysis were Tukey’s test for one-way analysis of variance (ANOVA) and the Bonferroni test for two-way ANOVA/repeated measures ANOVA. Error bars indicate mean ± S.E.M.
CaMKII In Vitro Activity Assay.
Kinase activity was tested as previously described (Coultrap and Bayer, 2011). Standard reactions (1 minute at 30°C) were started by adding CaMKIIα (2.5 nM subunits) to a mix of 50 mM PIPES (1,4-piperazinediethanesulfonic acid) pH 7.2, 0.1% bovine serum albumin (BSA), 1 μM CaM, 1 mM CaCl2, 10 mM MgCl2, 100 μM [γ-32P]ATP (∼1 Ci/mmole), and 75 μM syntide-2 substrate peptide. Kinase inhibitors H7 or Sta were added at concentrations indicated. Reactions were stopped by spotting on P81 (Whatman; Maidstone, Kent, UK) paper, and phosphorylation of the substrate peptide was measured in a scintillation counter by the Cherenkov method (Coultrap and Bayer, 2012b). To determine the Sta inhibitory mechanism of CaMKII, concentrations of ATP were varied (4–256 μM) in the absence and presence of four Sta concentrations. Data were fit by nonlinear regression to models for either competitive, noncompetitive, mixed model, or uncompetitive inhibition; the best-fit model was determined by an extra sum-of-squares F-test. The data were also analyzed using the Morrison Ki model for tight-binding inhibitors (Morrison, 1969). For this analysis the initial reaction velocity was plotted as a function of inhibitor (Sta) concentration for the different ATP concentrations. In GraphPad Prism, the Morrison Ki model is designed specifically for competitive inhibitors; therefore a modified version of the Morrison Ki was created in the program to model a tight binding mixed noncompetitive inhibitor (equations shown below). The mixed noncompetitive model incorporates both the competitive (where α = ∞) and noncompetitive (where α = 1) models. From this equation, the mode of inhibition was determined, as were the Ki and the α values (ratio of Ki for enzyme-substrate complex to the Ki for enzyme alone). As with the previous analysis, the two models were compared by an extra sum-of-squares F-test to determine the better fit.
Inhibitory properties were determined with the following equations: vi = reaction velocity with inhibitor, v0 = reaction velocity without inhibitor, [E] = enzyme concentration, [I] = inhibitor concentration, [S] = substrate concentration, Km = Michaelis-Menten constant, Ki = inhibitory constant, Kiapp = apparent inhibitory constant, α = cooperativity parameter. The cooperativity parameter, α, represents the degree to which the binding of inhibitor changes the affinity of the enzyme for substrate. If α = 1, then binding does not change the affinity, therefore the mechanism is noncompetitive; if α > 1, then binding of inhibitor prevents substrate binding, therefore binding is competitive; if 0 < α < 1, then binding of inhibitor enhances substrate binding, therefore binding is uncompetitive.
Morrison equation (Morrison, 1969):
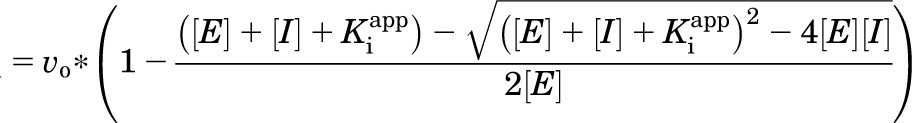 |
(1) |
For competitive inhibitors:
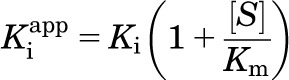 |
(2) |
For noncompetitive (and mixed) inhibitors:
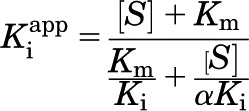 |
(3) |
CaMKII Activity within Cells.
Human embryonic kidney (HEK) 293 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand Island, NY) supplemented with fetal bovine serum (Invitrogen) and penicillin/streptomycin. Cells were transfected with green fluorescent protein (GFP)-CaMKIIα and hemagglutinin-GluA1 vectors at 1:10 by the Ca2+-phosphate method. At 24–48 hours after transfection, medium was replaced with imaging solution (0.87× Hanks’ buffered saline solution (Invitrogen), 25 mM HEPES (Invitrogen) pH 7.4, 2 mM glucose, 2 mM CaCl2, and 1 mM MgCl2) containing H7 (700 μM), Sta (2 μM), or control, and incubated for 5 minutes at 37°C. Following this treatment, the cells were either stimulated with 10 μM ionomycin (Tocris) or mock and then incubated at room temperature for 5 minutes. Proteins were extracted in a homogenization buffer (50 mM PIPES pH 7.2, 1 mM EGTA, 150 mM NaCl, 1% Triton X-100, protease inhibitor cocktail (Roche, Indianapolis, IN), plus 1 μM microcystin-LR (CalBiochem, San Diego, CA), 10 mM sodium fluoride, 50 mM β-glycerophosphate, and 0.1% dithiothreitol). Protein levels were assessed with Western blot analysis as described (Vest et al., 2007) using antibodies against GluA1 (1:2000; Millipore, Billerica, MA) and phospho-S831 GluA1 (1:1000; PhosphoSolutions, Aurora, CO). Using SuperSignal West Femto Substrate (Pierce, Rockford, IL), chemiluminescence was detected using a ChemiImager system (Alpha Innotech, San Leandro, CA). Immunodetection values were measured with AlphaEase ChemiImager software.
In Vitro Binding Assay.
Binding was assessed as previously described (Bayer et al., 2001; O'Leary et al., 2011; Coultrap and Bayer, 2012b). The cytoplasmic C-tail of GluN2B (amino acids 1120–1482) was expressed in bacteria as a glutathione S-transferase (GST)-fusion protein and then purified with glutathione-sepharose, eluted with 100 mM reduced glutathione in 200 mM Tris pH 9, and dialyzed twice into an excess volume of 50 mM Tris pH 7.6 with 300 mM NaCl. GST-GluN2B (300 nM) was bound to anti–GST-coated plates (Pierce) for 1 hour. This concentration of GST-GluN2B represents an excess for the immobilization reaction to saturate the binding sites and ensure equal loading of GST-GluN2B. After blocking with 5% BSA, purified CaMKIIα (40 nM in 1× PIPES-buffered saline + 0.05% Tween, 0.1% BSA, 1 mM CaCl2, 1 μM CaM, and 10 mM MgCl2) was added to the wells and incubated for 15 minutes. The kinase solution contained various conditions of ATP or ADP (100 μM), H7 (700 μM), and Sta (2 μM), as indicated. After incubation, wells were washed with 1 mM EGTA in 1× PIPES-buffered saline + 0.05% Tween, and bound kinase was eluted at 95–100°C in sodium dodecyl sulfate loading buffer. Bound protein levels were assessed with Western blot analysis using a CaMKIIα-selective antibody (1:4000; made in-house).
HEK293 Cell Imaging.
Cells were transfected by the Ca2+-phosphate method with GFP-CaMKIIα and GluN2B (wild-type or S1303A) vectors at 1:10 (O'Leary et al., 2011). For live imaging, ionomycin-induced translocation of CaMKII was monitored in imaging solution (0.87× Hanks’ buffered saline solution, 25 mM HEPES pH 7.4, 2 mM glucose, 2 mM CaCl2, and 1mM MgCl2) containing H7 (700 μM), Sta (2 μM), or control at 32°C, collecting images over 12 minutes (10 μM ionomycin stimulation at 2 minutes into recording). Cells in the images were blindly scored as “translocated” or “not translocated” at each minute of the capture, resulting in a “Percent Translocation” score at each minute. For fixed imaging, cells were incubated in imaging solution for 5 minutes at 37°C and then received stimulation (10 μM ionomycin or mock) for 5 minutes at room temperature. After this stimulation the cells were immediately fixed and immunostained for GluN2B (anti-GluN2B 1:200; NeuroMab, Davis, CA; TexasRed anti-rabbit secondary antibody 1:500; Invitrogen). Coverslips were mounted with ProLong Gold (Invitrogen) containing 4′,6-diamidino-2-phenylindole (DAPI) stain. The resulting images were used to determine the Pearson correlation of GFP-CaMKIIα expression and GluN2B staining. All images were collected on a Zeiss Axiovert 200M with climate control chamber (Carl Zeiss GmbH, Oberkochen, Germany) (Vest et al., 2007) using z-stacks with a step size of 0.2 μm over 2.4 μm and were analyzed using SlideBook5 software.
Neuron Imaging.
Primary dissociated neuronal cultures were isolated from mixed-gender neonatal Sprague-Dawley rats at medium density on poly(d-lysine) (0.1 mg/ml) and laminin (0.01 mg/ml; Invitrogen) coated coverslips (Hudmon et al., 2005; O'Leary et al., 2011). Pregnant Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) for preparation of primary neuron cultures; all animal treatments and housing were in accordance with the University of Colorado Denver Institutional Animal Care and Use Committee. Neurons were maintained at 37°C and 5% CO2 in Neurobasal A media with B27 supplements (both Invitrogen) and glutamine. At 4 to 5 days in vitro (DIV), glial growth was inhibited with 70 μM 5-fluoro-2′-deoxyuridine plus 140 μM uridine. For live imaging, neurons were transfected with GFP-CaMKIIα vector at 12 DIV, using lipofectamine 2000 (Invitrogen) (Vest et al., 2010). Transfected neurons in imaging solution (see above) containing 700 μM H7, 2 μM Sta, or control were observed at 32°C over 5 minutes with glutamate/glycine (100/10 μM) stimulation at minute 1. Images were analyzed blindly by measuring the spine-to-shaft ratio (mean intensity at 10 synaptic sites divided by mean intensity at 10 respective shafts). For each neuron the spine-to-shaft ratio was normalized to the ratio at −1 minutes, where 0 minutes represents the time of stimulation. For fixed imaging, neurons at 12 DIV were treated with either H7 (700 μM), Sta (0.02, 0.2, or 2 μM), or control (5 minutes, 37°C) and then stimulated with glutamate/glycine at room temperature for “0” (mock), 30, 60, or 120 seconds. Neurons were immediately fixed and immunostained for CaMKIIα (1:5000; 1:500 Alexa 488 anti-mouse secondary antibody; Invitrogen) and the synaptic marker Shank (1:500; Invitrogen; 1:500 TexasRed anti-rabbit secondary antibody). Coverslips were mounted with ProLong Gold. Images were analyzed by determining the sum intensity of GFP-CaMKII in synaptically localized CaMKII puncta as a percent of the sum intensity of all CaMKII puncta, relative to the sum Shank intensity at those synapses; this measure was compared between inhibitor conditions.
Results
Sta Inhibits CaMKII by an ATP-Competitive Mechanism.
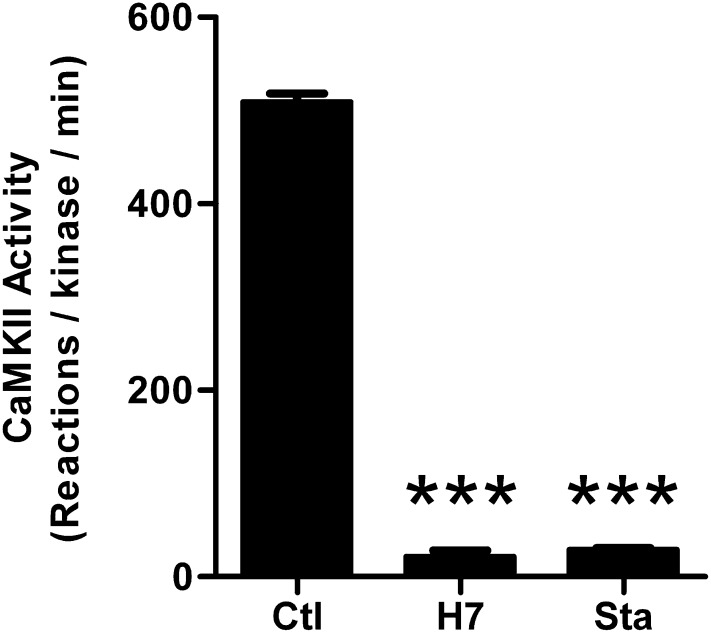
To study the dependence of the CaMKII/GluN2B interaction on kinase activity, the nucleotide competitive kinase inhibitors H7 (Hidaka et al., 1984) and Sta (Tamaoki et al., 1986) were used. Both H7 and Sta have been previously described to inhibit CaMKII activity, with Ki of 7 μM and IC50 of 20 nM, respectively (Malinow et al., 1989; Yanagihara et al., 1991). Indeed, as expected, complete inhibition of CaMKII activity was seen here in in vitro phosphorylation assays with purified protein, using the inhibitors at 100-fold their respective Ki/IC50 values (Fig. 1).
Fig. 1.
H7 and Sta inhibit CaMKII enzymatic activity in vitro. To test kinase activity, recombinant, purified CaMKIIα (2.5 nM) was used to phosphorylate syntide-2 peptide (75 μM) in the presence of control (Ctl), 700 μM H7, or 2 μM Sta. Results were measured in phosphorylation reactions per minute. Both H7 and Sta significantly reduced the activity from control. One-way ANOVA; ***P < 0.001
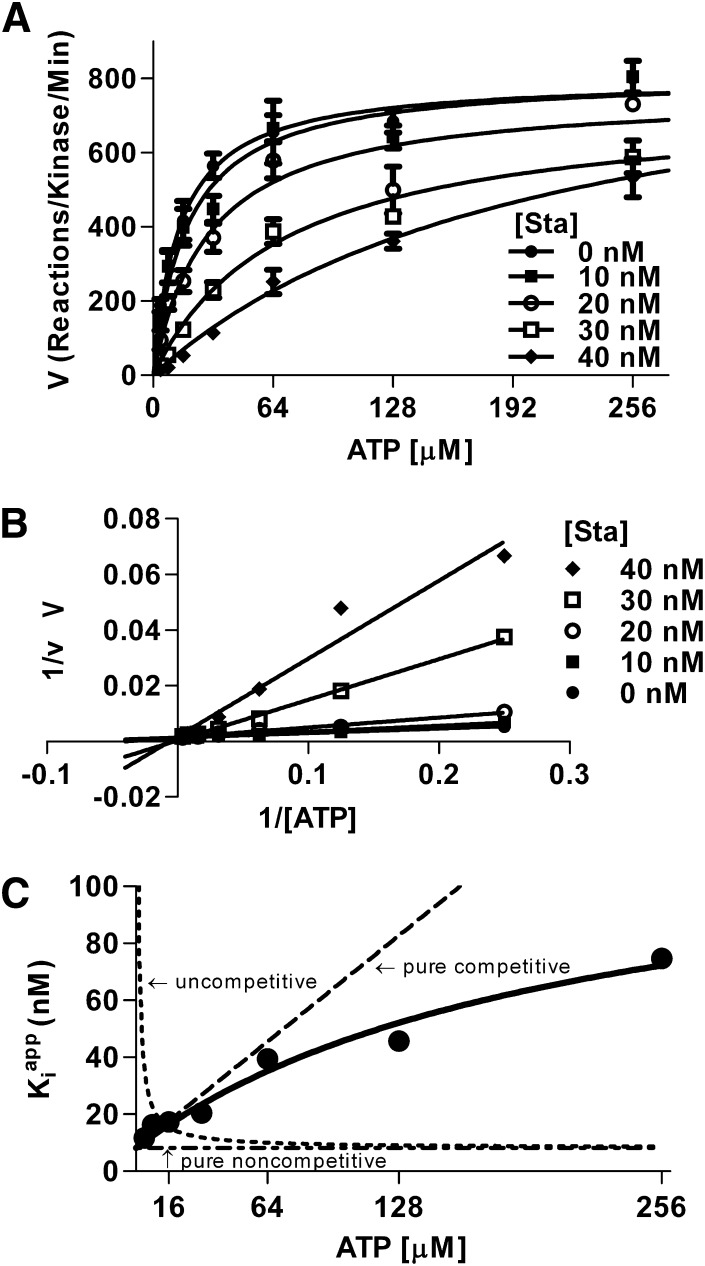
Notably, while Sta is shown to be ATP-competitive for most kinases (Omura et al., 1995; Prade et al., 1997), it is described as noncompetitive with ATP for CaMKII (Yanagihara et al., 1991). However, this determination was based on experiments likely using tight binding conditions. Tight binding conditions occur when the concentration of enzyme is close to the IC50 of a potent inhibitor. Under such conditions, the formation of enzyme substrate complex significantly alters the amount of free inhibitor. The Michaelis-Menten model of enzyme kinetics is based on the assumption that the free inhibitor concentration is well represented by the total concentration of inhibitor added (i.e., binding of inhibitor to enzyme has a negligible effect on the concentration of free inhibitor) (Copeland, 2000). Tight binding conditions thus render Michaelis-Menten kinetics invalid, and results from such experiments will appear to be noncompetitive on a double-reciprocal plot regardless of the actual underlying mechanism (Morrison, 1969; Copeland, 2000). To determine if Sta indeed inhibits CaMKII by competing with ATP, kinase activity was measured using varying concentrations of ATP in the absence and presence of multiple concentrations of Sta. The results were analyzed by nonlinear regression fitting to both traditional and tight-binding models of inhibition. This analysis found that the increasing concentrations of Sta caused an increase in the apparent Km for ATP without an effect on the Vmax (Fig. 2A). A double-reciprocal plot of these data displayed relationships of substrate concentration to reaction rate that had the same y-axis (1/V) intercept and varying x-axis (1/[ATP]) intercepts, consistent with a competitive interaction. However, this determination of a competitive mechanism was not apparent in the graphical representation of the data, due to the high affinity of Sta for CaMKII (Fig. 2B). Nonlinear regression analysis found that the curves fit best to a mixed model inhibition, with a much higher affinity of Sta for CaMKII not bound to ATP than bound, Ki = 8.8 nM and α = 15.4 (i.e., Ki for CaMKII-ATP complex is 15.4-fold higher; F[1, 145] = 5.36, P = 0.022). As the Ki for Sta was on the same order as the enzyme concentration in the assay (2.5 nM), the tight-binding inhibitor model may be more appropriate for analysis of the data. Thus, we also fit the data to the Morrison equation for tight-binding inhibitors (Morrison, 1969; Copeland, 2000) (Fig. 2C). Again, the data fit best to a mixed model inhibition with Ki = 8.6 nM and α = 13.9 (F[1, 140] = 4.2, P < 0.05). Therefore, Sta inhibits CaMKII activity primarily by an ATP-competitive mechanism.
Fig. 2.
Sta is competitive with ATP. To determine the inhibitory mechanism of Sta, concentration-response curves of kinase activity were measured over a range of ATP concentrations. (A) Increasing concentrations of Sta increase the apparent Km for ATP without an effect on Vmax, suggesting a competitive model by Michaelis-Menten kinetics. (B) Using the Lineweaver-Burk model, the lines all intersect at the same y-intercept with varying x-intercepts, mathematically suggesting a competitive model. (C) Testing the data under tight-binding equations shows a mixed model of inhibition that is predominantly competitive with ATP.
H7 and Sta Mimic the Positive Regulation of CaMKII/GluN2B Binding by Nucleotides In Vitro.
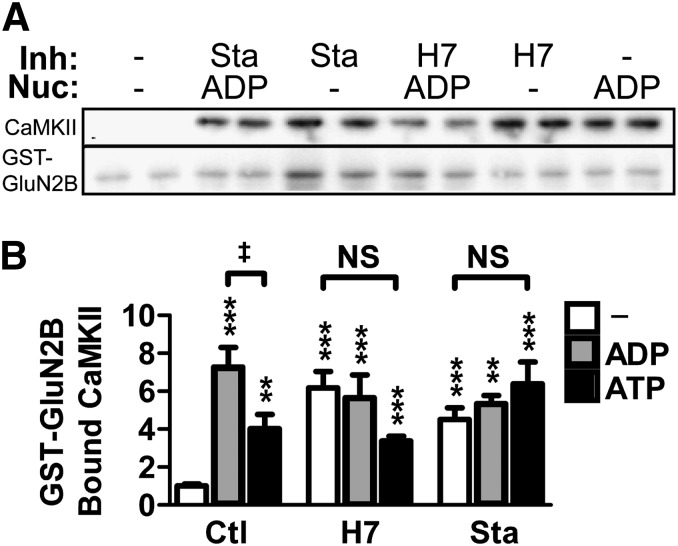
Maximal CaMKII/GluN2B binding requires the presence of a nucleotide in the ATP-binding pocket of CaMKII (O'Leary et al., 2011). Thus, we decided to test the effect of the ATP-competitive inhibitors H7 and Sta on CaMKII/GluN2B binding in vitro, using purified protein. GST-tagged GluN2B cytoplasmic C terminus was immobilized to anti–GST-coated plates, and CaMKII was bound in the presence of Ca2+/CaM; the amount of bound CaMKII was then measured by Western blot analysis. Because H7 and Sta are competitive with nucleotides, we determined their effect on CaMKII/GluN2B binding both in the presence and the absence of two different nucleotides: ADP or ATP (Fig. 3). A one-way ANOVA revealed a significant effect of inhibitor/nucleotide condition on CaMKII/GluN2B binding (F[8, 63] = 10.59, P < 0.001). Post-hoc Newman-Keul’s analysis showed that all inhibitors, nucleotides, and their combinations significantly enhanced binding above the control condition (Fig. 3; P < 0.01 for all conditions). CaMKII/GluN2B binding was stronger in the presence of ADP compared with ATP, as described previously (O'Leary et al., 2011), but only in the absence of the inhibitors. This is consistent with prevention of the negative-regulatory phosphorylation of GluN2B at S1303 by CaMKII in the presence of the inhibitors. Most importantly, the inhibitors directly enhance CaMKII/GluN2B binding to the same extent as nucleotides, and addition of nucleotide had no further effects. These results demonstrate that H7 and Sta do not inhibit but rather mimic the nucleotide-enhancement of CaMKII/GluN2B binding. Thus, while maximal CaMKII/GluN2B binding requires occupation of the ATP binding pocket, this effect can be elicited by either nucleotide or by competitive inhibitors that bind to the same site. Notably, the demonstration that ATP-competitive inhibitors do not directly interfere with CaMKII/GluN2B binding in vitro makes them useful tools to assess dependence of the CaMKII/GluN2B interaction on enzymatic kinase activity within cells.
Fig. 3.
H7 and Sta mimic the nucleotide enhancement of CaMKII/GluN2B binding in vitro. CaMKII-binding to GluN2B was assessed by immobilizing GST-tagged GluN2B C-tail to anti–GST-coated plates and adding CaMKII in the presence of Ca2+ and calmodulin. Bound protein was measured by Western blot. (A) Representative Western blot of bound fractions from an in vitro binding assay run in biologic replicates. The membranes were probed for CaMKII (above) and GST-GluN2B (below). Binding reactions contained varied combinations of inhibitors (Inh)—700 μM H7 or 2 μM Sta—and nucleotide (Nuc)—100 μM ADP or ATP. These conditions are indicated above the lanes. (B) Quantification of the Western blot results normalized to no-nucleotide control showing enhancement of binding under all conditions relative to no-nucleotide control (one-way ANOVA; **P < 0.01; ***P < 0.001). No differences existed within H7 or Sta treatments, though binding in the presence of ADP was greater than ATP (one-way ANOVA; ‡P < 0.05). (n = 6–17 per group)
H7 and Sta Efficiently Inhibit CaMKII Activity within Cells.
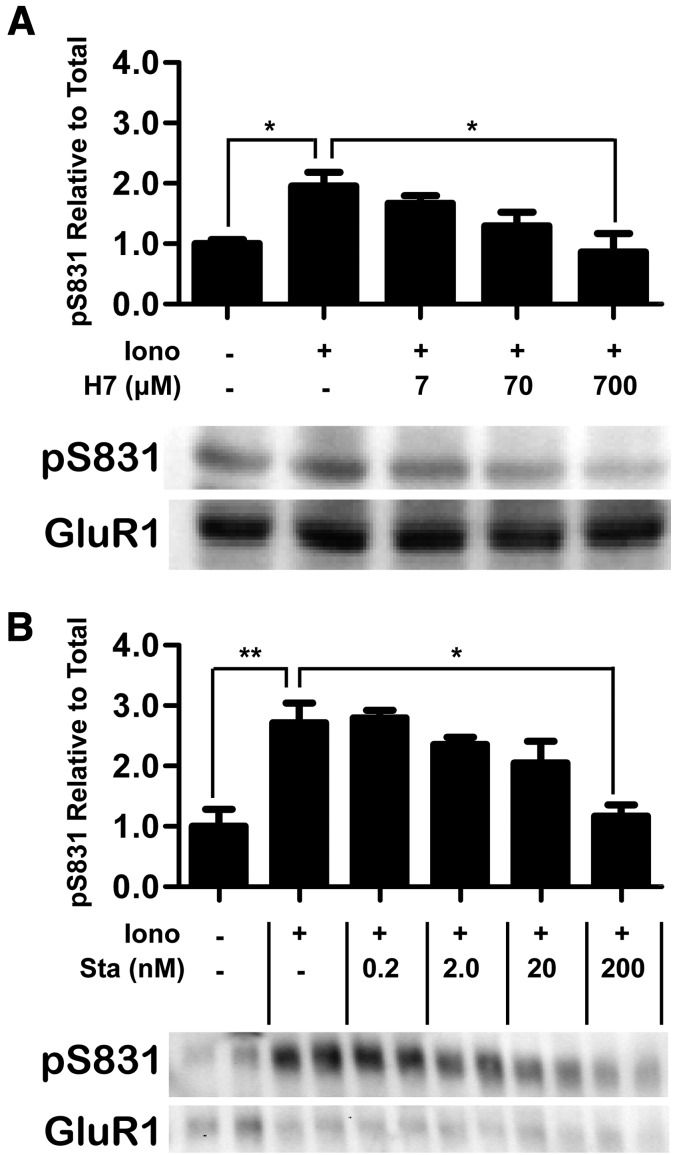
After showing efficient inhibition of CaMKII activity by H7 and Sta in vitro (Figs. 1 and 2), their effect on CaMKII activity was also assessed within cells. These experiments were performed in HEK293 cells transfected with both CaMKII and its substrate protein GluA1. Cells were incubated with inhibitor and then stimulated with the Ca2+-ionophore ionomycin. To assess inhibition of CaMKII, the cells were harvested in the presence of phosphatase inhibitors, and the extracts were analyzed by Western blot using an antibody against the CaMKII phosphorylation site on GluA1, S831 (Fig. 4). A one-way ANOVA determined a significant concentration-dependent reduction of S831 phosphorylation by both H7 (Fig. 4, A and B; F[4, 15] = 4.79, P < 0.02) and Sta (Fig. 4, C and D; F[5, 11] = 8.08, P < 0.003). Post-hoc analysis showed that phosphorylation was significantly increased by ionomycin stimulation (P < 0.05) and that the stimulation-induced increase was significantly attenuated by 700 μM H7 (P < 0.05) or 200 nM Sta (P < 0.05). Importantly, at these concentrations, S831 phosphorylation was not different from the nonstimulated control, indicating that that both H7 and Sta completely blocked CaMKII activity when administered at 100-fold IC50. Thus, H7 and Sta effectively inhibit CaMKII activity, both in vitro and within cells.
Fig. 4.
H7 and Sta inhibit CaMKII enzymatic activity within cells. To assess inhibition of CaMKII enzymatic activity within cells, HEK293 cells were cotransfected with CaMKII and GluA1. These cells were incubated with inhibitor and then stimulated with ionomycin (Iono). After these treatments, the cells were harvested with phosphatase inhibitors, and the resultant extracts were analyzed by Western blotting. (A) H7 reduced S831-phosphorylation in a dose-dependent manner, seen comparing stimulated cells in the absence of inhibitor to stimulated cells incubated with 700 μM H7 (one way ANOVA; *P < 0.05) in the graph of Western blot quantification. Below the graph is a representative Western blot of phospho-S831 GluA1 (top) and total GluA1 (bottom). H7 treatment was from 7 to 700 μM; H7 IC50 = 7 μM (n = 4 per group). (B) Sta also reduced S831-phosphorylation in a dose-dependent manner, seen comparing stimulated cells in the absence of inhibitor to stimulated cells incubated with 200 nM H7 (one-way ANOVA; *P < 0.05; **P < 0.01) in the graph of Western blot quantification. Below the graph is a Western blot of phospho-S831 GluA1 (top) and total GluA1 (bottom), run in biologic replicates. Sta treatment was from 2 to 200 nM; Sta IC50 = 20 nM (n = 3 per group).
CaMKII Binding to GluN2B within Cells Is Not Inhibited by H7 or Sta.
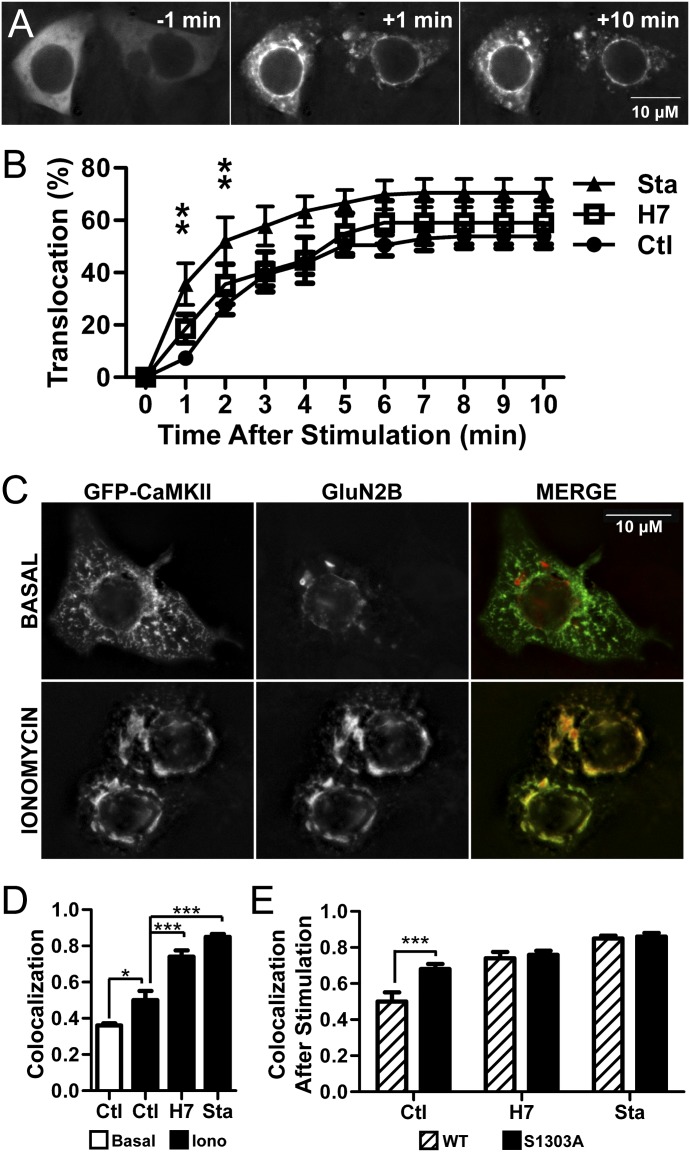
Stimulation-induced CaMKII/GluN2B binding within cells can be assessed by ionomycin-stimulated CaMKII translocation in heterologous cells transfected to coexpress CaMKII and GluN2B (Strack et al., 2000; O'Leary et al., 2011). This process can be visually observed in real time by using fluorescently-tagged CaMKII (Bayer et al., 2001). To test the effect of H7 and Sta on translocation, HEK293 cells were cotransfected with GFP-CaMKII and GluN2B, and translocation was induced by ionomycin stimulation (Fig. 5). The time courses in Fig. 5, A and B show that GFP-CaMKII successfully translocated in the presence of either 700 μM H7 or 2 μM Sta. Translocation was quantified by determining the percent of cells that displayed translocation at each time point. The data were analyzed with a two-way repeated measures ANOVA, which indicated that the degree of translocation depended both on the duration of stimulation (F[10, 280] = 124.60, P < 0.001) and inhibitor type (F[2, 280] = 4.13, P < 0.05), in addition to the interaction between those two factors (F[20, 280] = 1.69, P < 0.05). Specifically, the percent of cells showing translocation of CaMKII was greater under Sta treatment than under control conditions at both 1 and 2 minutes into the stimulation (P < 0.01). There was no reduction by H7 or Sta compared with control at any time points. Thus, H7 and Sta do not inhibit translocation of CaMKII to GluN2B in the heterologous expression system used here, despite blocking the enzymatic activity of CaMKII.
Fig. 5.
H7 and Sta permit translocation of GFP-CaMKII in heterologous expression systems. (A) Representative time-course of stimulation-induced GFP-CaMKII translocation to GluN2B in HEK293 cells. Ionomycin-stimulation induces distinctive puncta and increased signal intensity in the perinuclear region. (B) Quantitation of the translocation data shows an increase in ionomycin-induced translocation of CaMKII. There were no differences in time courses between control (Ctl) and H7; Sta induced greater translocation at minutes 1 and 2 (two-way repeated measures ANOVA; **P < 0.01). (n = 7–14 per group). (C) Representative images of HEK293 cells fixed without (top) and with (below) ionomycin stimulation. The latter condition displays increased colocalization of GFP-CaMKII (left) and immunostained GluN2B (center), as seen in the merged image (right; GFP-CaMKII shown in green, GluN2B shown in red). (D) Quantitation of colocalization between GFP-CaMKII signal and GluN2B staining shows that stimulation increased colocalization in the control condition (indicating translocation), which was further increased when cells were pretreated with 700 μM H7 or 2 μM Sta (one-way ANOVA; *P < 0.05; ***P < 0.001), (n = 12–100 per group; note that ionomycin stimulation decreased cellular adherence, decreasing the number of cells able to be visualized). (E) Comparison of colocalization after stimulation of CaMKII with either wild-type (WT) or S1303A mutant GluN2B. S1303A displayed increased colocalization with CaMKII relative to WT in the control condition but not after pretreatment with H7 or Sta, which likely prevent S1303 phosphorylation (one-way ANOVA; ***P < 0.001), (n = 12–47 per group). Scale bar = 10 μM.
Next, CaMKII translocation was assessed in cells by immunostaining after fixation. While this technique does not afford the temporal resolution of live imaging, it allows for a more direct colocalization analysis of CaMKII and GluN2B after immunostaining. Transfected cells were treated with inhibitor and then stimulated with ionomycin, followed by fixation and immunostaining of GluN2B (Fig. 5C). Colocalization of GFP-CaMKII and GluN2B from the resulting images was determined (Fig. 5D), and data were analyzed by a one-way ANOVA comparing across treatments; a significant effect of treatment was found (F[5, 351] = 77.79, P < 0.0001). As expected, a post-hoc analysis found that ionomycin stimulation significantly enhanced colocalization of CaMKII and GluN2B as compared with the nonstimulated control (P < 0.05). The stimulation also enhanced colocalization in cells pretreated with H7 (P < 0.001) or Sta (P < 0.001) as compared with their respective nonstimulated controls, indicating translocation under all pretreatment conditions (Fig. 5D). Interestingly, stimulation-induced colocalization was even greater in cells pretreated with either H7 (P < 0.001) or Sta (P < 0.001) compared with the stimulated control. Therefore, these inhibitors not only allow translocation but also appear to enhance it. This enhancing effect is likely due to a decrease in GluN2B phosphorylation by CaMKII, as described in the next section.
Inhibition of GluN2B S1303 Phosphorylation Does Not Mask Any Positive Regulatory Effects of CaMKII Activity on GluN2B Binding.
In addition to having a binding interaction with GluN2B, CaMKII also has an enzymatic interaction with it. Specifically, CaMKII phosphorylates GluN2B S1303, which significantly reduces the binding interaction (Strack et al., 2000; O'Leary et al., 2011). Thus, the increase in stimulation-induced CaMKII/GluN2B colocalization by H7 and Sta (Fig. 5D) may be explained by a decrease in this negative-regulatory phosphorylation. However, inhibition of this negative-regulatory effect could also mask another, positive-regulatory effect of enzymatic activity. To eliminate potentially confounding effects of the S1303 phosphorylation state on other effects of CaMKII activity on translocation, colocalization of CaMKII with GluN2B was additionally tested for a nonphosphorylatable GluN2B S1303A mutant (Fig. 5E). A two-way ANOVA found a significant interaction between GluN2B mutation status and treatment (F[2, 157] = 4.09, P < 0.001). CaMKII/GluN2B colocalization after stimulation was greater for the S1303A mutant compared with wild-type (P < 0.01), but in contrast to the findings with GluN2B wild-type, H7 or Sta pretreatment did not further increase this colocalization with the S1303A mutant. Taken together, these results show that inhibiting activity does not prevent the binding of CaMKII to GluN2B within cells. The inhibitors may even enhance the interaction by mimicking the nucleotide effect on binding while also preventing the negative-regulatory S1303 phosphorylation.
H7 and Sta Do Not Prevent Glutamate-Induced CaMKII Translocation to Synaptic Sites in Neurons.
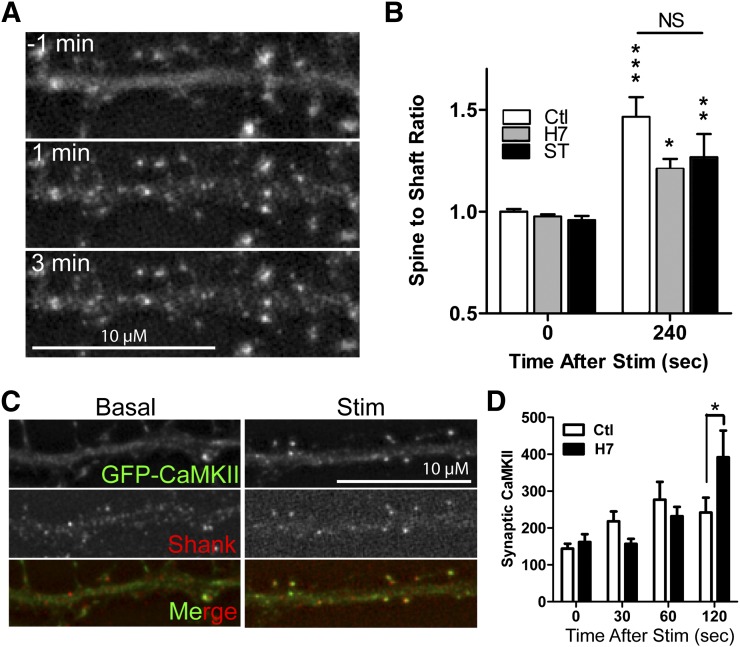
Translocation of CaMKII to synaptic sites within neurons is triggered by the excitatory neurotransmitter glutamate and is thought to be mediated through the stimulation-induced CaMKII interaction with GluN2B (Strack and Colbran, 1998; Bayer et al., 2001; She et al., 2012). Thus, the enzymatic activity dependence of translocation was also tested in neurons, the endogenous environment of the proteins. Primary hippocampal cultures were transfected with GFP-CaMKII at 12 DIV and, after 24 hours of expression, live images were captured during induction of a Ca2+-stimulus with glutamate/glycine (100/10 μM, in the presence of 1 mM Mg2+). Under these conditions, costimulation of AMPARs helps to alleviate the Mg2+ block of NMDARs to trigger a sufficiently strong NMDAR-mediated Ca2+-stimulus (Bayer et al., 2006; Marsden et al., 2010). CaMKII is known to translocate to synaptic sites under this stimulation protocol (Shen and Meyer, 1999), a process that is dependent on GluN2B (She et al., 2012). The movement of CaMKII to dendritic spines (Fig. 6A) can be quantified by an increase in the spine-to-shaft ratio of GFP-CaMKII signal intensity (Fig. 6B). A two-way ANOVA comparing the spine-to-shaft ratio between two time points (before and after stimulation) and between treatment groups found an effect of time (F[1,58] = 41.81, P < 0.001) but no significant interactions, indicating that translocation was induced by stimulation irrespective of inhibitor treatment. Post-hoc analysis further confirmed that the spine-to-shaft ratio increased equally after stimulation both under control conditions (P < 0.001) and in the presence of H7 (P < 0.05), or Sta (P < 0.01). Thus in neurons, as in heterologous expression systems, H7 and Sta do not prevent CaMKII translocation despite inhibiting enzymatic activity.
Fig. 6.
H7 and Sta permit synaptic translocation of CaMKII in primary hippocampal neurons. (A) Representative time-course of stimulation-induced synaptic translocation of GFP-CaMKII. The GFP-CaMKII signal moves from a diffuse pattern throughout the dendrite to concentrated puncta at dendritic spines. (B) The spine-to-shaft ratio of GFP-CaMKII signal at 0 and 240 seconds after glut/gly stimulation shows an increase in signal from 0 to 240 seconds in all conditions and no differences between control (Ctl), H7, and Sta (n = 10 and 11). Statistics relative to the nonstimulated condition within each treatment (two-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001). (C) Representative images of neurons fixed with (top) or without (bottom) glut/gly stimulation and stained for CaMKII (left) and the synaptic marker Shank (center); merge (right) shows overlay of CaMKII (green) and Shank (red). (D) Synaptic enrichment of CaMKII increases after stimulation of increasing duration in both control and H7-pretreated neurons. Synaptic CaMKII was greater for H7 than control at 120 seconds; no other differences existed between treatments (one-way ANOVA; *P < 0.05), (n = 23–62 per condition). Scale bar = 10 μM.
Translocation of endogenous CaMKII was also assessed by fixing and immunostaining neurons at different time points after glutamate/glycine stimulation. The neurons were pretreated with control, H7, or Sta. Surprisingly, Sta pretreatment interfered with the fixation and/or immunostaining process. Cells fixed after Sta pretreatment stained significantly dimmer than cells that had been treated with H7 or control (Supplemental Fig. 1). This phenomenon held true for both stimulated and nonstimulated cells, and over a range of Sta concentrations (0.02–2.0 μM). To determine if this reduction in CaMKII signal intensity was due to a Sta-induced degradation of CaMKII, GFP-CaMKII-transfected neurons were imaged live over 10 minutes in the presence of control, H7, or Sta. Neurons in all conditions had only a small reduction in signal intensity over 10 minutes (15 ± 3%) and no differences existed between the groups (Supplemental Fig. 1). Because the Sta effect on fixation did not appear to occur in HEK293 cells, it seems that it uniquely interferes with the fixation/immunostaining process in neurons. However, neurons were successfully fixed and immunostained after control or H7 pretreatment, as shown in Fig. 6C. A two-way ANOVA comparing synaptic CaMKII enrichment over time between those two treatment groups found a main effect of time (F[3,285] = 8.11, P < 0.001) and a significant interaction between time and treatment (F[3,285] = 3.52, P < 0.05). Post hoc analysis found that H7 treatment caused significantly greater synaptic enrichment than control after 2 minutes of stimulation (P < 0.01), but no other differences existed between the two groups (Fig. 6D). Taken together, our data demonstrate that enzymatic activity of CaMKII is not required for its interaction with GluN2B or for its translocation to synapses.
Discussion
Binding of CaMKII to GluN2B is essential for normal LTP induction (Barria and Malinow, 2005; Halt et al., 2012) and may also mediate LTP maintenance (Sanhueza et al., 2011). Previously, a requirement for CaMKII activity in CaMKII/GluN2B binding and in CaMKII synaptic translocation was indicated by various pharmacological or genetic manipulations that inhibit CaMKII (Shen and Meyer, 1999; Vest et al., 2007; O'Leary et al., 2011). However, the current study demonstrates that kinase activity is not required for CaMKII/GluN2B binding: the ATP-competitive inhibitors H7 and Sta efficiently inhibited CaMKII, but permitted both CaMKII/GluN2B binding in vitro and stimulation-induced CaMKII translocation in heterologous cells and neurons. As discussed below, the previous manipulations inhibited CaMKII activity by several mechanisms, but in all cases exerted their inhibition of CaMKII/GluN2B binding by structural effects that are independent of catalytic activity. Thus, apparently obvious conclusions from results with enzyme inhibition can be misleading, and structural effects of the underlying inhibitory mechanisms need to be taken into account.
While H7 and Sta compete with nucleotides, they were found to mimic, rather than inhibit, the nucleotide mediated enhancement of CaMKII/GluN2B binding. This effect was not entirely surprising as crystal structures of H7 and Sta bound to protein kinase A (PKA) demonstrate that they induce similar conformational changes in PKA as nucleotides do (Engh et al., 1996; Prade et al., 1997). Importantly, this effect allows H7 and Sta to inhibit activity without interfering with known mechanisms regulating CaMKII/GluN2B binding. By contrast, previous manipulations used to inhibit CaMKII activity disrupted CaMKII/GluN2B binding by interfering with one of three distinct regulatory mechanisms. The K42M mutant inhibits CaMKII by preventing nucleotide binding, but unlike H7 and Sta, it prevents rather than mimics the positive-regulatory nucleotide effect (O'Leary et al., 2011). The A302R and T305/306D mutants (Rich and Schulman, 1998; Shen and Meyer, 1999), as well as the inhibitor KN-93 (Sumi et al., 1991), block CaMKII stimulation by preventing Ca2+/CaM binding, which is required not only to induce catalytic activity but also to induce binding to GluN2B (Bayer et al., 2001). Finally, the inhibitor tatCN21 directly binds to the CaMKII T site (Vest et al., 2007), which is also the GluN2B binding site (Bayer et al., 2001). Thus, the structural interaction between CaMKII and GluN2B requires coenzyme-induced conformational changes in the ATP-binding pocket, dissociation of the CaMKII regulatory domain, and access to the T site, all of which were maintained by H7 and Sta but not other inhibitory manipulations. For assessing activity-dependence of other protein–protein interactions of CaMKII (or other enzymes), similar structural considerations should be taken into account. Thus, it is essential to analyze the biochemical effects of inhibitors and mutations when using them to assess the function of enzymatic activity.
Careful examination of inhibitory mechanisms is especially vital in the study of CaMKII. Because of the regulatory conditions affecting the three activity states of CaMKII: inactive, stimulated, and autonomous, determining an appropriate inhibitor/mutation is not trivial. For example, a structural role of CaMKII/GluN2B has been suggested in LTP maintenance (Sanhueza et al., 2011; Gouet et al., 2012). All CaMKII inhibitors block LTP induction, but most do not alter maintenance (Malinow et al., 1989; Otmakhov et al., 1997; Chen et al., 2001; Buard et al., 2010). However, most CaMKII inhibitors interfere with the formation of CaMKII/GluN2B complexes but do not disrupt these complexes after they form. An exception is tatCN21: at high concentrations, it disrupts both CaMKII/NMDAR complexes and LTP maintenance (Sanhueza et al., 2011); however, lower concentrations that are sufficient to inhibit CaMKII but not disrupt CaMKII/NMDAR complexes block only LTP induction (Buard et al., 2010). This appears to indicate that LTP maintenance requires only the structural aspect of CaMKII/GluN2B binding, and not enzymatic activity. However, as tatCN21 is competitive with GluN2B binding, tatCN21 should not inhibit GluN2B-bound CaMKII unless it also disrupts the interaction. Thus, it is unclear if LTP maintenance requires enzymatic activity of GluN2B-bound CaMKII. Both H7 and Sta should inhibit activity of GluN2B-bound CaMKII without interfering with the binding, and both are described to inhibit LTP induction and maintenance (Malinow et al., 1989; Colley et al., 1990; Matthies et al., 1991; Hanse and Gustafsson, 1994), although results on the Sta effect on maintenance have been conflicting (Denny et al., 1990; Ling et al., 2002; Pastalkova et al., 2006). However, as H7 and Sta inhibit multiple kinases involved in LTP, these findings do not provide evidence for a role of the activity of GluN2B-bound CaMKII in LTP maintenance.
While H7 and Sta are not selective for CaMKII, the results of our experiments allow a clear and specific interpretation: CaMKII binding to GluN2B or translocation to synapses does not require catalytic activity of CaMKII or any other kinase inhibited by H7 or Sta. Some additional kinases inhibited by both H7 and Sta that are important in LTP include PKA and protein kinase C (PKC) (Soderling and Derkach, 2000). H7 has similar affinity for CaMKII (Ki = 7 μM) (Malinow et al., 1989), PKA (Ki = 3 μM (Hidaka et al., 1984), and PKC (Ki = 6 μM (Hidaka et al., 1984). Sta has slightly higher potency for PKC (IC50 = 5 nM (Meggio et al., 1995) than PKA (IC50 = 15 nM) (Meggio et al., 1995) and CaMKII (IC50 = 20 nM) (Yanagihara et al., 1991). In addition to serine/threonine kinases, Sta inhibits tyrosine kinases including the Src-family (Meggio et al., 1995), which is implicated in LTP (Soderling and Derkach, 2000). The potency of Sta for these kinases is similar to the others: v-Src IC50 = 6 nM (Nakano et al., 1987), Lyn IC50 = 20 nM (Meggio et al., 1995). Thus, the activities of these kinases—CaMKII, PKA, PKC, and Src-family—are not required for the CaMKII/GluN2B interaction or activity-dependent CaMKII synaptic translocation.
Many ATP-competitive inhibitors, like H7 and Sta, are not kinase selective, owing to well-conserved ATP-binding pockets (Hanks et al., 1988; Omura et al., 1995). Because of this homology, it may be expected that their mechanism of inhibition for kinases would be consistent. However, Sta was described as noncompetitive with ATP for CaMKII (Yanagihara et al., 1991; Meggio et al., 1995; Omura et al., 1995), even though it is ATP-competitive for other kinases (Meggio et al., 1995; Omura et al., 1995; Prade et al., 1997). This classification was reexamined here after finding that tight-binding conditions—CaMKII concentration approximated the Sta IC50—may have been used to determine the mechanism. Under these conditions, the Michaelis-Menten equation no longer holds true, and regardless of the mechanism it will appear noncompetitive (Morrison, 1969; Copeland, 2000). In our experiments, the concentration of CaMKII (2.5 nM) was much lower than that used previously (50 nM) (Yanagihara et al., 1991), but was still only ∼10-fold lower than the Sta IC50, conditions that may require analysis by a tight-binding inhibitor model (Morrison, 1969). Nevertheless, both the traditional nonlinear regression model and the Morrison tight-binding inhibitor model showed that Sta inhibits CaMKII through a mixed mode of inhibition. In both models, the affinity of Sta for CaMKII was approximately 15-fold greater for CaMKII without ATP bound compared to ATP-bound CaMKII. Interestingly, PKC inhibition by Sta was also described as noncompetitive with ATP (Tamaoki et al., 1986; Nakano et al., 1987), though it is now accepted as ATP-competitive (Meggio et al., 1995; Omura et al., 1995).
Multiple studies have suggested that CaMKII translocation to the synapse is at least in part mediated by binding to GluN2B (Colbran and Brown, 2004; Merrill et al., 2005; Coultrap and Bayer, 2012a). Direct evidence was provided by a mouse mutant with CaMKII-binding-incompetent GluN2B (Halt et al., 2012). Additionally, neurons cultured from GluN2B−/− mice are deficient for synaptic translocation induced by chemical LTP, which can be rescued by re-expression of wild-type but not CaMKII-binding incompetent GluN2B (She et al., 2012). Furthermore, CaMKII translocation can be reconstituted in heterologous cells by coexpressing GluN2B (Bayer et al., 2001). Here, we show that neither synaptic translocation nor CaMKII/GluN2B binding requires kinase activity. These shared characteristics strengthen the idea that GluN2B is both necessary and sufficient for CaMKII synaptic translocation, although additional mediating factors may contribute (Colbran and Brown, 2004; Jalan-Sakrikar et al., 2012).
In conclusion, the CaMKII/GluN2B interaction within cells is independent of enzymatic activity, although maximal binding requires occupation of the ATP-binding pocket. This finding highlights the importance of understanding all biochemical effects of inhibitors and mutations, as five different approaches to testing CaMKII/GluN2B binding using inactive mutants or inhibitors provided misleading results. Using detailed understanding of CaMKII activity and interactions, along with careful selection of inhibitory mechanisms, may lead to the development of better and more specific drugs targeting neuronal plasticity. For example, having the ability to reverse the LTP maintenance phase, without secondary effects on other functions of CaMKII activity, may be therapeutic in disorders of maladaptive plasticity, such as addiction (Hyman et al., 2006) or posttraumatic stress disorder (Nathan et al., 2011). Indeed, persistent disruption of CaMKII/GluN2B complexes by a single transient treatment (Sanhueza et al., 2011) appears to have the potential to persistently reverse addiction behavior (Loweth et al., 2013).
Supplementary Material
Abbreviations
- AMPAR
AMPA-type glutamate receptor
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CaM
calmodulin
- CaMKII
Ca2+/CaM-dependent protein kinase II
- DAPI
4′,6-diamidino-2-phenylindole
- DIV
days in vitro
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- H7
1-(5-isoquinolinylsulfonyl)-2-methylpiperazine
- HEK
human embryonic kidney
- KN-93
N-[2-[[[3-(4-chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulphonamide
- LTP
long-term potentiation
- NMDAR
N-methyl-d-aspartate type glutamate receptor
- PIPES
1,4-piperazinediethanesulfonic acid
- PKA
protein kinase A
- PKC
protein kinase C
- Sta
staurosporine
Authorship Contributions
Participated in research design: Barcomb, Coultrap, Bayer.
Conducted experiments: Barcomb, Coultrap.
Performed data analysis: Barcomb, Coultrap, Bayer.
Wrote or contributed to the writing of the manuscript: Barcomb, Coultrap, Bayer.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01NS081248 and P30NS048154]; the National Institutes of Health National Institute on Drug Abuse [Grant R21DA36300]; and the National Institutes of Health National Institute of General Medical Sciences [Grant T32GM007635].
This work is part of the PhD thesis for K.B. and was previously presented as follows: Barcomb K, Buard I, Coultrap SJ, and Bayer KU (2013) CaMKII activity and GluN2B binding in regulation of synaptic strength. Gordon Research Conference on Excitatory Synapses & Brain Function; 2013 Jun 9–14; Les Diablerets, Switzerland (poster and lecture); and Barcomb K, Coultrap S, and Bayer KU (2012) CaMKII activity is not required for GluN2B binding. Rocky Mountain Regional Neuroscience Group Annual Meeting; 2012 May 10; Aurora, CO; and Barcomb K, Buard I, Coultrap SJ, and Bayer KU (2013) CaMKII activity and GluN2B binding in the regulation of synaptic strength. Rocky Mountain Regional Neuroscience Group Annual Meeting; 2013 May 16; Aurora, CO.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Barria A, Malinow R. (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48:289–301 [DOI] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411:801–805 [DOI] [PubMed] [Google Scholar]
- Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H, De Koninck P. (2006) Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci 26:1164–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard I, Coultrap SJ, Freund RK, Lee Y-S, Dell’Acqua ML, Silva AJ, Bayer KU. (2010) CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci 30:8214–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Strack S, Colbran RJ, Lisman JE. (2001) Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol 85:1368–1376 [DOI] [PubMed] [Google Scholar]
- Colbran RJ, Brown AM. (2004) Calcium/calmodulin-dependent protein kinase II and synaptic plasticity. Curr Opin Neurobiol 14:318–327 [DOI] [PubMed] [Google Scholar]
- Colley PA, Sheu FS, Routtenberg A. (1990) Inhibition of protein kinase C blocks two components of LTP persistence, leaving initial potentiation intact. J Neurosci 10:3353–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RA. (2000) Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed., pp 305–317, Wiley-Interscience, NY [Google Scholar]
- Coultrap SJ, Bayer KU. (2011) Improving a natural CaMKII inhibitor by random and rational design. PLoS ONE 6:e25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Bayer KU. (2012a) CaMKII regulation in information processing and storage. Trends Neurosci 35:607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ and Bayer KU (2012b) Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII), in Protein Kinase Technologies (Neuromethods) (Mukai H ed) pp 49–57, Humana Press, Totowa, NJ. [Google Scholar]
- Denny JB, Polan-Curtain J, Rodriguez S, Wayner MJ, Armstrong DL. (1990) Evidence that protein kinase M does not maintain long-term potentiation. Brain Res 534:201–208 [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. (1999) Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96:3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. (1996) Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem 271:26157–26164 [DOI] [PubMed] [Google Scholar]
- Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M. (1998) Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem 71:1733–1741 [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279:870–873 [DOI] [PubMed] [Google Scholar]
- Gouet C, Aburto B, Vergara C, Sanhueza M. (2012) On the mechanism of synaptic depression induced by CaMKIIN, an endogenous inhibitor of CaMKII. PLoS ONE 7:e49293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza RF, Zhou Y, Stein IS, Qian H, Juntti S, Wojcik S, Brose N, Silva AJ, Hell JW. (2012) CaMKII binding to GluN2B is critical during memory consolidation. EMBO J 31:1203–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Quinn AM, Hunter T. (1988) The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42–52 [DOI] [PubMed] [Google Scholar]
- Hanse E, Gustafsson B. (1994) Staurosporine impairs both short-term and long-term potentiation in the dentate gyrus in vitro. Neuroscience 58:263–274 [DOI] [PubMed] [Google Scholar]
- Hanson PI, Meyer T, Stryer L, Schulman H. (1994) Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron 12:943–956 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287:2262–2267 [DOI] [PubMed] [Google Scholar]
- Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. (1984) Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036–5041 [DOI] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. (2005) A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci 25:6971–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598 [DOI] [PubMed] [Google Scholar]
- Jalan-Sakrikar N, Bartlett RK, Baucum AJ, 2nd, Colbran RJ. (2012) Substrate-selective and calcium-independent activation of CaMKII by α-actinin. J Biol Chem 287:15275–15283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. (2011) Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DSF, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. (2002) Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 5:295–296 [DOI] [PubMed] [Google Scholar]
- Lou LL, Lloyd SJ, Schulman H. (1986) Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci USA 83:9497–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Li D, Cortright JJ, Wilke G, Jeyifous O, Neve RL, Bayer KU, Vezina P. (2013) Persistent reversal of enhanced amphetamine intake by transient CaMKII inhibition. J Neurosci 33:1411–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. (1989) Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245:862–866 [DOI] [PubMed] [Google Scholar]
- Marsden KC, Shemesh A, Bayer KU, Carroll RC. (2010) Selective translocation of Ca2+/calmodulin protein kinase IIalpha (CaMKIIalpha) to inhibitory synapses. Proc Natl Acad Sci USA 107:20559–20564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H, Jr, Behnisch T, Kase H, Matthies H, Reymann KG. (1991) Differential effects of protein kinase inhibitors on pre-established long-term potentiation in rat hippocampal neurons in vitro. Neurosci Lett 121:259–262 [DOI] [PubMed] [Google Scholar]
- Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P, et al. (1995) Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem 234:317–322 [DOI] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. (2005) Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci 26:645–653 [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. (1986) Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44:861–870 [DOI] [PubMed] [Google Scholar]
- Morrison JF. (1969) Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta 185:269–286 [DOI] [PubMed] [Google Scholar]
- Nakano H, Kobayashi E, Takahashi I, Tamaoki T, Kuzuu Y, Iba H. (1987) Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 40:706–708 [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Cobb SR, Lu B, Bullmore ET, Davies CH. (2011) Studying synaptic plasticity in the human brain and opportunities for drug discovery. Curr Opin Pharmacol 11:540–548 [DOI] [PubMed] [Google Scholar]
- O’Leary H, Liu WH, Rorabaugh JM, Coultrap SJ, Bayer KU. (2011) Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the N-methyl-D-aspartate (NMDA) receptor subunit GluN2B. J Biol Chem 286:31272–31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S, Sasaki Y, Iwai Y, Takeshima H. (1995) Staurosporine, a potentially important gift from a microorganism. J Antibiot (Tokyo) 48:535–548 [DOI] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. (2010) CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67:239–252 [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Griffith LC, Lisman JE. (1997) Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J Neurosci 17:5357–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313:1141–1144 [DOI] [PubMed] [Google Scholar]
- Prade L, Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. (1997) Staurosporine-induced conformational changes of cAMP-dependent protein kinase catalytic subunit explain inhibitory potential. Structure 5:1627–1637 [DOI] [PubMed] [Google Scholar]
- Rich RC, Schulman H. (1998) Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 273:28424–28429 [DOI] [PubMed] [Google Scholar]
- Sanhueza M, Fernandez-Villalobos G, Stein IS, Kasumova G, Zhang P, Bayer KU, Otmakhov N, Hell JW, Lisman J. (2011) Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J Neurosci 31:9170–9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Lisman J. (2013) The CaMKII/NMDAR complex as a molecular memory. Mol Brain 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer CM, Colbran RJ, Keefer JR, Soderling TR. (1988) Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J Biol Chem 263:13486–13489 [PubMed] [Google Scholar]
- She K, Rose JK, Craig AM (2012) Differential stimulus-dependent synaptic recruitment of CaMKIIα by intracellular determinants of GluN2B. Mol Cell Neurosci 51:68–78. [DOI] [PubMed]
- Shen K, Meyer T. (1999) Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284:162–166 [DOI] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA. (2000) Postsynaptic protein phosphorylation and LTP. Trends Neurosci 23:75–80 [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. (1998) Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 273:20689–20692 [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 275:23798–23806 [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. (1991) The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181:968–975 [DOI] [PubMed] [Google Scholar]
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. (1986) Staurosporine, a potent inhibitor of phospholipid/Ca++-dependent protein kinase. Biochem Biophys Res Commun 135:397–402 [DOI] [PubMed] [Google Scholar]
- Thiel G, Czernik AJ, Gorelick F, Nairn AC, Greengard P. (1988) Ca2+/calmodulin-dependent protein kinase II: identification of threonine-286 as the autophosphorylation site in the alpha subunit associated with the generation of Ca2+-independent activity. Proc Natl Acad Sci USA 85:6337–6341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O’Leary H, Port JD, Bayer KU. (2007) Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell 18:5024–5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, O’Leary H, Coultrap SJ, Kindy MS, Bayer KU. (2010) Effective post-insult neuroprotection by a novel Ca(2+)/ calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem 285:20675–20682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara N, Tachikawa E, Izumi F, Yasugawa S, Yamamoto H, Miyamoto E. (1991) Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II. J Neurochem 56:294–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.