Abstract
Some of the best biomarkers of age-related cognitive decline are closely linked to synaptic function and plasticity. This review highlights several age-related synaptic alterations as they relate to Ca2+ dyshomeostasis, through elevation of intracellular Ca2+, and neuroinflammation, through production of pro-inflammatory cytokines including interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α). Though distinct in many ways, Ca2+ and neuroinflammatory signaling mechanisms exhibit extensive cross-talk and bidirectional interactions. For instance, cytokine production in glial cells is strongly dependent on the Ca2+ dependent protein phosphatase calcineurin, which shows elevated activity in animal models of aging and disease. In turn, pro-inflammatory cytokines, such as TNF, can augment the expression/activity of L-type voltage sensitive Ca2+ channels in neurons, leading to Ca2+ dysregulation, hyperactive calcineurin activity, and synaptic depression. Thus, in addition to discussing unique contributions of Ca2+ dyshomeostasis and neuroinflammation, this review emphasizes how these processes interact to hasten age-related synaptic changes.
Keywords: Aging, plasticity, synapse, Ca2+, neuroinflammation, cytokine
1. Introduction
Severe deficits in cognition (i.e. dementia) are not inevitable outcomes of brain aging, but are more symptomatic of neurodegenerative diseases such as Alzheimer’s disease (AD). Brain aging is generally associated with mild impairments in cognitive function, especially in situations or tasks that depend on the hippocampus. Declarative (semantic/episodic) memory undergoes a normal age-related decline (Fleischman et al., 2004; Margolis and Scialfa, 1984; Ronnlund et al., 2005) that is structurally linked to the hippocampus and associated regions (Leritz et al., 2006). The connections of neural circuits within the hippocampus change in strength with behavioral inputs to store and retrieve memories, but these abilities shift with age, tending to favor reductions in synaptic strength and more rapid forgetting (for review, see Burke and Barnes, 2010; Foster, 2012).
This review highlights evidence linking age-related cognitive decline to changes in hippocampal synaptic function and how these changes can arise specifically from the dysregulation of Ca2+ and neuroinflammatory signaling mechanisms. Though decades old, the Ca2+ and neuroinflammation hypotheses remain viable and highly useful frameworks for guiding experimental gerontology research and for developing nootropics and other treatments for improving quality-of-life in the elderly. Moreover, increasing evidence suggests that Ca2+ and neuroinflammatory signaling mechanisms interact extensively, with distinct outcomes in different neural cell types. These observations should stimulate new avenues of research and provide new opportunities to develop and test more integrative theories of brain aging.
2. Synaptic plasticity
2.1 Long-term potentiation and long-term depression
Most synapses in mammals rapidly adjust their signaling properties in response to changes in cellular activity, such as sensory inputs from environmental stimuli. This plasticity is absolutely necessary for establishing appropriate synaptic connections during neural development and is widely regarded as a physiological mechanism for learning and memory (for review, see Bailey et al., 2000). Activity-dependent strengthening of synaptic connections was envisioned more than 60 years ago by Donald Hebb, who proposed that the presynaptic neuron’s repeated firing could exert lasting effects in the postsynaptic neuron and the whole synapse, resulting in increased synaptic stability (Hebb, 1949).
The hippocampus is widely studied with respect to synaptic function and plasticity because of its critical involvement in learning/memory, its discrete cytoarchitecture, and its vulnerability to aging, injury, and neurodegenerative disease. Moreover, hippocampal slices from isolated tissue are relatively easy to prepare, retain much of their basic circuitry, and remain viable for hours. Two well-known and well-characterized types of synaptic plasticity contribute to activity-dependent adaptation of neuronal circuits in the hippocampus—long-term potentiation (LTP) and long-term depression (LTD). LTP is the strengthening of synapses, and typically requires high frequency activation of presynaptic afferents, while LTD is the weakening of synapses usually resulting from lower levels of presynaptic activation.
Bidirectional modulation of synaptic efficacy provides an ideal mechanism for learning and memory. LTP/LTD, like memory, are long-lasting, enduring for hours in acute slices or for days in intact animals (Barrionuevo and Brown, 1983; Bliss and Lomo, 1973; Morris, 1989; Dudek and Bear, 1992; Mulkey and Malenka, 1992; O’Dell and Kandel, 1994). Learning and LTP/LTD are also input specific, associative, and reversible (Levy and Steward, 1979; McNaughton et al., 1978; Mulkey and Malenka, 1992). Abundant experimental evidence shows that synaptic plasticity and learning/memory share the same fundamental mechanisms. For instance, blockade of hippocampal N-methyl-D-aspartic acid receptors (NMDARs) impairs learning in rodents when given prior to training (Morris, 1989; Morris et al., 1986), but enhances memory when given after training on hippocampal dependent tasks (Norris and Foster, 1999; Villarreal et al., 2002). Thus, like LTP and LTD, learning and forgetting each depend critically on NMDARs (discussed below). Learning and synaptic plasticity also occlude one another under certain conditions (Barnes et al., 1994; Moser et al., 1998; Rioult-Pedotti et al., 2000). Moreover, acquisition of spatial learning tasks and/or exposure to enriched environments is associated with increased synaptic responses in the hippocampus, similar to LTP (Foster et al., 1996; Moser et al., 1993; Sharp et al., 1985). Because of these observations and many others, changes in hippocampal synaptic plasticity and function have received much attention as a neurologic mechanism for impaired cognition in animal models of aging, injury, and disease.
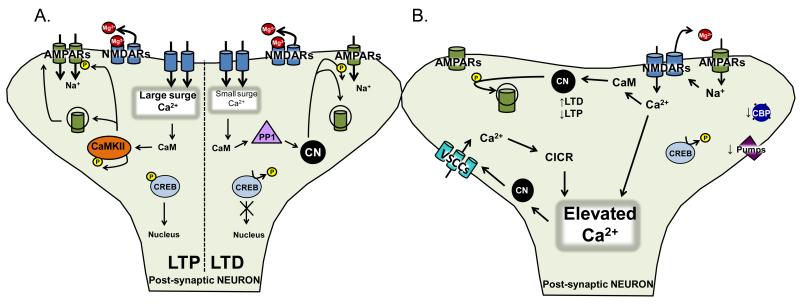
At glutamatergic synapses of the hippocampus, the best characterized forms of LTP and LTD involve two key receptor subtypes: α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs) and NMDARs. Other forms of LTP/LTD that depend on voltage-sensitive Ca2+ channels (VSCCs), metabotropic glutamate receptors, and other mechanisms have also been described. For a review of NMDAR-independent forms of LTP and LTD, see Citri and Malenka (2008) and Johnston et al. (1992). AMPARs and NMDARs are both subtypes of glutamate receptors, permeable to Na+ and K+. A subgroup of AMPARs and all NMDARs are also permeable to Ca2+. When the postsynaptic membrane is hyperpolarized to near resting values, NMDARs are unable to conduct current due to blockade of its channel pore with Mg2+. Local membrane depolarizations, triggered primarily by AMPAR currents, expel Mg2+ from the NMDAR channel, and permit Ca2+ entry into the postsynaptic cell. LTP and LTD both require Ca2+ influx through NMDARs, though to differing levels. LTP generally necessitates extensive membrane depolarization and a large surge in postsynaptic Ca2+, while LTD occurs in response to more modest depolarizations and smaller Ca2+ influxes. Differing Ca2+ signals recruit divergent biochemical cascades. Protein kinases, especially Ca2+/calmodulin-dependent protein kinase II (CaMKII), promote LTP induction and maintenance by increasing the activity and/or levels of glutamate receptors in the plasma membrane through direct actions on receptor proteins, cytoskeletal elements, or other accessory proteins, or transcriptional regulators, such as the cAMP response element binding protein (CREB) (Josselyn and Nguyen, 2005; Malinow and Malenka, 2002). For LTD, smaller elevations in Ca2+ favor the dephosphorylation of many of the same substrates through phosphatase activation, namely the Ca2+/calmodulin dependent protein phosphatase, calcineurin (CN), and protein phosphatase 1 (PP1), which is indirectly stimulated by CN activity. The relative balance in activity of protein kinases and phosphatases not only regulates the magnitude and duration of synaptic changes, but also helps maintain the functional status of the synapse under basal conditions. For comprehensive reviews on the molecular mechanisms of LTP and LTD see Citri and Malenka (2008; Malenka, 2003; Malenka and Bear, 2004). For illustration of these processes, refer to Figure 1.
Figure 1.
A. Synaptic Plasticity. Long-term potentiation (LTP) and long-term depression (LTD) involve the activation of two glutamate receptors, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs; green) and and N-methyl-D-aspartic acid receptors (NMDARs; blue). Both are permeable to Na+ and K+, while a subgroup of AMPARs and all NMDARs are permeable to Ca2+. Upon membrane depolarization by AMPARs, NMDARs expel a Mg2+-block (red), and permit Ca2+ entry into the postsynaptic cell. Both forms of plasticity require Ca2+ influx through NMDARs, though LTP generally requires a large surge in postsynaptic Ca2+, and LTD generally requires smaller Ca2+ influxes. As a result, the differing Ca2+ signals activate divergent biochemical cascades. Protein kinases, especially Ca2+/calmodulin-dependent protein kinase II (CaMKII; orange oval), have been strongly implicated in LTP, where they are involved in the phosphorylation (small yellow circle) and subsequent membrane insertion of glutamate receptors. Smaller elevations in Ca2+ are involved in LTD induction and activate protein phosphatases, namely Ca2+/calmodulin dependent protein phosphatase, calcineurin (CN; black circle), and/or protein phosphatase 1 (PP1; lavender triangle), which can be stimulated by CN activity. The balance in activity of protein kinases and phosphatases regulates the magnitude/duration of synaptic changes, and helps maintain the functional status of the synapse under basal conditions.
B. Ca2+ dysregulation. Synaptic plasticity requires the activation of the appropriate biochemical cascades, and exquisite Ca2+ regulation. Under normal conditions, there are numerous mechanisms regulating intracellular Ca2+ concentrations. These include: voltage sensitive Ca2+ channels (VSCCs; aqua), a variety of receptors on the cell surface (NMDARs; light blue) or receptors on intracellular organelles which store Ca2+ (IP3Rs and ryanodine receptors), pumps (SERCA Ca2+; purple), binding proteins (calreticulin, BiP/grp78, grp94, and calnexin; royal blue), and intracellular organelles which store Ca2+ (endoplasmic reticulum and mitochondria). All of these mechanisms are impaired with aging, leading to increases in Ca2+ entry/release, and decreased Ca2+ expulsion/sequestration, leading to an elevation in Ca2+ concentration.
2.2 Aging changes in synaptic plasticity
It has been more than 30 years since synaptic plasticity alterations were first reported in the aged hippocampus (Barnes, 1979; Landfield and Lynch, 1977). Early studies reported that aged animals could express hippocampal synaptic LTP at levels comparable to that seen in younger animals; however, LTP in aged animals was labile and tended to decay much more rapidly. Importantly, this decay was shown to correlate very well to forgetting on hippocampal-dependent memory tasks (Barnes, 1979; Barnes and McNaughton, 1985). While studies have shown that LTP induction and magnitude remain intact during aging, especially with intense stimulation protocols (Chapman et al., 2010; Deupree et al., 1991; Kumar et al., 2007; Landfield et al., 1978; Moore et al., 1993; Norris et al., 1996), others have reported aging-related deficits, including an increased induction threshold (Burgdorf et al., 2011; Deupree et al., 1993; Moore et al., 1993). While these differences may be attributable to methodological variation, such as different stimulation protocols, different ion concentrations in buffers, or different animal strains, the general consensus is that there is an LTP deficit with age, particularly in regard to threshold of induction and maintenance.
The first investigations of LTD during aging were conducted in the mid-1990s (Foster and Norris, 1997; Norris et al., 1996). These studies showed that aged rats are more susceptible to LTD-induction, relative to adult rats. Through similar mechanisms, the more labile LTP at hippocampal synapses of aged rats is prone to reversal during bouts of low frequency synaptic activation (Norris et al., 1996). Later work showed that the magnitude of LTD in aged rats correlates with the extent of memory dysfunction, as measured by the Morris Water Maze (Foster and Kumar, 2007). While these observations have been largely confirmed by subsequent studies from several different research groups, (Foy et al., 2008; Hsu et al., 2002; Norris et al., 1998a; Sama et al., 2012; Vouimba et al., 2000) other studies did not detect LTD in aged animals or did not find an age difference (Billard and Rouaud, 2007; Kollen et al., 2010; Lee et al., 2005). Like LTP, these discrepancies may be attributable to differences in experimental protocol, especially in regard to synaptic stimulation frequencies and extracellular Ca2+ content (see Norris et al., 1996; Foster and Norris, 1997; Kumar et al., 2007; Mathis et al., 2011). For stimulus frequencies near the induction threshold and for extracellular Ca2+/Mg2+ ratios close to 1:1, aged animals generally show increased susceptibility to LTD, without a change in maximal levels of depression (Kumar et al., 2007).
3. Ca2+ dysregulation
3.1 Mechanisms for neuronal Ca2+ dysregulation during aging
For synaptic plasticity to proceed in a discrete and efficient manner, it requires the activation of the appropriate biochemical cascades, and exquisite Ca2+ regulation— a process that is markedly compromised during aging. Compared to hippocampal neurons from young adult animals, neurons from aged animals show elevations in Ca2+ levels during repetitive activation (for review, see Thibault et al., 2007). Aging-related changes in Ca2+ dynamics have been measured in soma and dendritic compartments of hippocampal pyramidal neurons (Thibault et al., 2001; Hemond and Jaffe, 2005; Oh et al., 2013) and are at least partly attributable to increased Ca2+ influx across the plasma membrane (Oh et al., 2013). Elevated Ca2+ transients have also been observed in the dendritic spines of young adult mice with AD pathology (Goussakov et al., 2010), but it’s unclear whether similar spine Ca2+ changes occur as a result of normal aging. Interestingly, elevated Ca2+ influx during short-duration bursts of action potentials appears to be compensated by an age-related increase in Ca2+ buffering. Nonetheless, the buffering capacity of aged neurons becomes “overwhelmed” by elevated Ca2+ influx during sustained activity (Oh et al., 2013) leading to Ca2+ dysregulation. A primary source for elevated Ca2+ in aged hippocampal neurons appears to be ryanodine-receptor (RyR) gated intracellular stores (Gant et al., 2006), which are positively coupled to L-type VSCCs. It has been shown that the expression and/or function of L-VSCCs is elevated in the hippocampus during aging (Norris et al., 2010; Thibault and Landfield, 1996; Veng et al., 2003) and inversely correlated to cognitive status (Thibault and Landfield, 1996). Moreover, blockade of L-VSCCs improves neurologic function in aged subjects across multiple species including rats, rabbits, mice, humans, and non-human primates (Deyo et al., 1989; Moyer and Disterhoft, 1994; Norris et al., 1998b; Paran et al., 2010; Sandin et al., 1990; Straube et al., 1990; Thompson et al., 1990; Tsukuda et al., 2008; Veng et al., 2003; Watfa et al., 2010). In addition to L-VSCCs and ryanodine receptors, other Ca2+ signaling/regulatory mechanisms including a variety of receptors, pumps, binding proteins, and intracellular organelles undergo alterations with aging (for reviews, see Magnusson, 2012; Michaelis et al., 1984; Murchison and Griffith, 2007; Stutzmann and Mattson, 2011; Thibault et al., 2007; Toman and Fiskum, 2011; Yamaguchi, 2012). Changes in any, or all, of these mechanisms is likely to influence the amount of Ca2+ in the cell and disrupt the normal function of Ca2+-dependent synaptic plasticity mechanisms (Foster, 2007; Foster and Norris, 1997). These observations are consistent with the long-standing hypothesis (see Disterhoft et al., 1994; Gibson and Peterson, 1987; Khachaturian, 1987; Landfield, 1987) that Ca2+ dyshomeostasis during aging involves the disruption of many regulatory mechanisms, ultimately causing neurologic dysfunction and increasing vulnerability to neurodegenerative disease (also see Green and LaFerla, 2008; Stutzmann, 2007). Refer to Figure 1 for a depiction of these regulatory mechanisms.
3.2. Neuronal Ca2+ dysregulation and synaptic alterations with aging
Extensive evidence suggests that age-related alterations in synaptic plasticity are directly linked to Ca2+ dyshomeostasis. In hippocampal slice experiments, age-related changes in synaptic plasticity are highly sensitive to bath Ca2+/Mg2+ ratios. Increasing bath Mg2+ levels improves short-term frequency potentiation and blocks LTD in aged rats, while increasing bath Ca2+ in adult rat slices causes “aging-like” alterations in the synaptic measures (Landfield et al., 1986; Norris et al., 1996). Chelation of intracellular Ca2+ using BAPTA-AM in aged rat hippocampal slices also improves LTP induction (Tonkikh et al., 2006). At least some of these effects are linked to the function of L-VSCCs and resulting changes in Ca2+-induced calcium release. Indeed, blockade of L-VSCCs and RyRs normalizes membrane excitability and short-term plasticity, lowers the threshold for LTP-induction, and prevents LTD in aged rats (Moyer et al., 1992; Norris et al., 1998b; Thibault et al., 2001; Thompson et al., 1990), suggesting that age-related elevations in neuronal Ca2+ shift the plasticity thresholds in favor of LTD.
Changes in Ca2+ regulation during aging appear to affect synaptic plasticity thresholds through disruption of protein kinase/phosphatase signaling cascades. The protein phosphatase CN has more rapid activation kinetics than other Ca2+ dependent enzymes, including protein kinases involved in LTP (e.g. CaMKII) (Klee, 1991). Age-related elevations in Ca2+ levels therefore appear to favor the activation of CN-dependent signaling cascades and the dephosphorylation of critical plasticity regulators, such as CREB (Foster, 2007; Foster et al., 2001; Foster and Norris, 1997). Similar to raising the bath Ca2+ level, overexpression of active CN in young animals induces aging-like deficits in LTP (Winder et al., 1998). Conversely, blockade of CN activity and its downstream regulators in vivo increases synaptic strength in aged animals (Hsu et al., 2002; Norris et al., 1998a), facilitates LTP (Jouvenceau and Dutar, 2006), and leads to improvements in spatial memory tasks (Graff et al., 2010; Malleret et al., 2001). In addition to increases in phosphatase activity, age-related alterations in synaptic plasticity may also relate to changes in Ca2+-dependent protein kinases (Govoni et al., 2010). Impaired membrane localization and/or shifts in the dendritic-to-somal ratios of protein kinase C (PKC) have been reported in the hippocampus with aging and is associated age-related spatial cognition deficits (Battaini et al., 1995; Colombo et al., 1997; Fordyce and Wehner, 1993; Pascale et al., 1998). CaMKII mRNA and protein expression decrease in hippocampus and cortex with normal aging, though they increase in animal models of disease (Zhang et al., 2009). Moreover, the autophosphorylated, activated form of CaMKII is redistributed from synapses and dendritic arbors to the perikarya in postmortem hippocampal tissue from humans with mild cognitive impairment and AD (Reese et al., 2011). Together, these results highlight the importance of the phosphatase/kinase balance in maintaining synaptic plasticity thresholds throughout life.
The age-related shift in plasticity thresholds towards higher phosphatase activity and lower LTD induction thresholds may also have major ramifications on Ca2+ regulation. CN stimulates the upregulation of numerous proteins involved in Ca2+ homeostasis and signaling, including inositol (1, 4, 5) triphosphate receptors (IP3Rs) and several Ca2+ transporters (Carafoli et al., 1999; Graef et al., 1999). Moreover, CN activity appears to be permissive for L-VSCC activity with aging in neurons in vitro and in vivo (Norris et al., 2002; Norris et al., 2010). Elevated CN activity and expression in brain therefore may be a cause and consequence of Ca2+ dysregulation during aging.
4. Neuroinflammation
4.1. Mechanisms and role in synaptic dysfunction
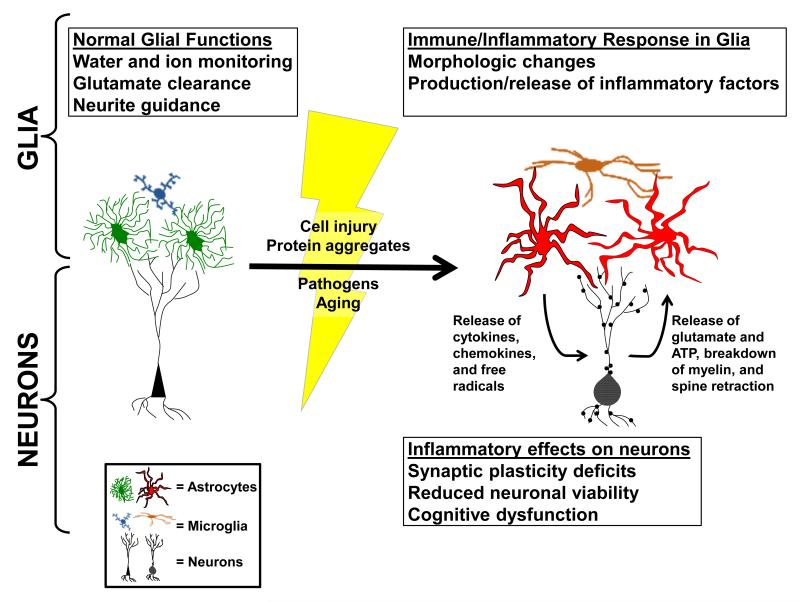
In addition to neuronal Ca2+ dysregulation, another commonly investigated mechanism for altered synaptic function in aging and age-related diseases is increased neuroinflammation (Rao et al., 2012). Glial cells, especially microglia and astrocytes, are the major effectors of immune/inflammatory signaling in the brain (Akiyama et al., 2000) (see Figure 2). Both cell types undergo characteristic morphologic changes with neuroinflammation, a process generally referred to as glial activation (or glial reactivity, or gliosis) which is found in most forms of neurologic injury and disease (Verkhratsky et al., 2012b). Activated microglia typically proliferate with injury/disease and can take on any number of morphologic phenotypes ranging from hypertrophied and ramified to amoeboid in shape (Colton and Wilcock, 2010; Olah et al., 2011; Varnum and Ikezu, 2012). As myeloid lineage cells, microglia are immune/inflammatory powerhouses that release a broad array of cytokines and free radicals. In their amoeboid form, microglia can serve as macrophages to promote the removal of dead cells, protein aggregates, and other debris from the CNS (McGeer and McGeer, 1995). Unlike microglia, astrocytes have a neural lineage, are far more abundant, and provide numerous indispensable metabolic functions that promote neuronal viability and CNS homeostasis (Verkhratsky et al., 2012b). Though astrocytes have historically received less attention than microglia in the realm of neuroinflammation, astrocytes also undergo clear morphological changes with injury and disease (e.g. hypertrophy of cell body and processes) and release a wide array of cytokines and other immune/inflammatory factors (Ridet et al., 1997; Sofroniew, 2009). As such, astrocytes are increasingly recognized for their ability to coordinate immune/inflammatory signaling in the CNS and also for their potential to drive neurologic dysfunction due to aging and/or disease (Brambilla et al., 2009; Farina et al., 2007; Furman et al., 2012; Okada et al., 2006; Sofroniew, 2009; Verkhratsky et al., 2012b).
Figure 2. Neuroinflammation.
Many astrocytes and microglia evidently morphologic changes from a “resting state” (green/blue) to an “activated state” (red/brown) under conditions of injury, disease, or aging (lightning bolt, yellow). When in a “resting state,” normal functions of glia include ion monitoring, glutamate clearance, and neurite guidance. Once astrocytes and microglia undergo a phenotype switch these functions are impaired and glia begin to produce and release immune/inflammatory mediators. The loss of glutamate transport and ion monitoring, together with the release of immune/inflammatory mediators, can lead to impaired synaptic plasticity, neuronal viability, and cognitive function.
A strong association between chronic neuroinflammation and neurologic dysfunction has been established in multiple experimental models across numerous labs. Early studies found that stimulation of glial activation and cytokine production in otherwise healthy adult animals using lipopolysaccharide endotoxins impairs performance on a variety of cognitive tasks (Hauss-Wegrzyniak et al., 2000a, 2000b) and disrupts LTP at hippocampal synapses (Hauss-Wegrzyniak et al., 2002; Kelly et al., 2003). Conversely, inhibition of neuroinflammation in animal models of injury and disease generally helps to preserve cognitive and synaptic integrity. For instance, non-steroidal anti-inflammatory drugs (NSAIDs), and other novel anti-inflammatory reagents have been shown to prevent LTP deficits and/or ameliorate hippocampal-dependent cognitive impairment in AD mouse models (Kotilinek et al., 2008; Bachstetter et al., 2012). Similar effects in AD mice have been reported with anti-inflammatory polyphenolic compounds found in a variety of fruits and vegetables, (Gong et al., 2013; Wang et al., 2012; Ho et al., 2012). Improvements in synaptic strength and LTP with polyphenols were specifically associated with elevations in phospho-CREB levels (Ho et al., 2012; Wang et al., 2012). Synaptic and cognitive enhancement in AD mice have also been demonstrated to result from the selective inhibition of astrocyte activation using an adeno-associated virus-mediated gene delivery approach (Furman et al., 2012).
Of the major cytokines linked to neuroinflammation, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFσ) are perhaps the best characterized. Both factors play a role in normal brain development (Merrill, 1992) and have numerous beneficial effects on neuronal viability and neurologic function when present at low concentrations (Goshen et al., 2007; Schmid et al., 2009; Stellwagen and Malenka, 2006). However, with heightened neuroinflammation, elevated levels of IL-1β and TNFα can trigger and maintain numerous deleterious changes in the brain, many of which extend to synapses and synaptic function (Barnum and Tansey, 2011; Lynch, 1998; Macdonald et al., 2000; Mrak and Griffin, 2000; Pickering et al., 2005). Alterations in synaptic plasticity, including reduced LTP have been observed in healthy CNS tissue following acute application of IL-1β or TNFα (Bellinger et al., 1993; Cunningham et al., 1996; Katsuki et al., 1990; Tancredi et al., 1992). Conversely, treatments that reduce IL-1β or TNFα levels, or inhibit cytokine receptor activation, generally improve indices of synaptic function and/or neuronal viability in experimental models of neuroinflammation and disease (Bachstetter et al., 2012; Kelly et al., 2001; McCoy et al., 2006; Sama et al., 2012; Sama et al., 2008). These effects are likely caused by signaling within the neurons and surrounding glial cells.
Similar to other cell types, neurons express receptors for IL-1β, TNFα, and many other cytokines (Czirr and Wyss-Coray, 2012) that, in turn, stimulate the activation of one or more members of the mitogen activated protein (MAP) kinase family (Kelly et al., 2003; Pickering et al., 2005). Several studies have described interactions between MAP kinases and glutamate receptors and other classic plasticity-related pathways shown in Figure 1 (e.g. see Pickering et al., 2005). Activation of p38 MAP kinase, in particular, seems to play important roles in the internalization of AMPA-type glutamate receptors (Boudreau et al., 2007; Zhong et al., 2008) and the interference of CREB phosphorylation (Tong et al., 2012), suggesting an important role in the weakening of synaptic efficacy, symptomatic of aging and memory dysfunction. Consistent with this possibility, an early study by Lynch and colleagues reported elevated p38 activity in the hippocampus of aged rats in conjunction with reduced LTP amplitude (O’Donnell et al., 2000). Subsequent work has shown that LTP deficits due to inflammatory mediators, including cytokines and amyloid beta peptides, are strongly attenuated by p38-inhibiting compounds (Butler et al., 2004; Li et al., 2011; Wang et al., 2004; Wang et al., 2005). Conversely, activation of p38 appears to synergize with CN activity to induce LTD and synapse loss in experimental models of AD (Hsieh et al., 2006).
4.2 Role of neuroinflammatory mechanisms in age-related synaptic dysfunction
Though neuroinflammation has been most commonly investigated in CNS injury and disease, it has also been long appreciated as a consequence of “normal” aging (Bilbo et al., 2012; Mrak and Griffin, 2005; Pizza et al., 2011). The first reports of glial activation in the aging brain were published decades ago (Geinisman et al., 1978; Landfield et al., 1977; Samorajski, 1976; Sturrock, 1977; Vaughan and Peters, 1974). Subsequently, numerous studies have described age-related changes in other glial activation markers including expression of pro-inflammatory cytokines, such as IL-1β and TNFα (Murray and Lynch, 1998; Sheng et al., 1996; Tha et al., 2000). In the early 2000s, massively parallel technologies, such as gene microarrays, demonstrated that the global transcriptional signature of aging brain is dominated by elevations in inflammatory signaling pathways (Blalock et al., 2003; Lee et al., 2000). Changes in inflammatory markers can arise as early as middle age (Blalock et al., 2003; Kadish et al., 2009) and are correlated with impaired synaptic function and/or cognitive impairment (Blalock et al., 2003; Lynch, 1999; Murray and Lynch, 1998). Intervention-type studies performed on aged animals suggest that neuroinflammation is not only a correlate, but a likely cause of impaired neurologic function. Non-steroidal anti-inflammatory drugs, like celecoxib, have been shown to improve hippocampal-dependent learning in middle aged rats concurrent with a reduction in hippocampal cytokine levels (Casolini et al., 2002). Improvements in cognitive function, synaptic strength, and/or LTP have also been reported for aged rodents exposed to a variety of other anti-inflammatory treatments including minocycline (Griffin et al., 2006), resveratrol (Joseph et al., 2008; Ranney and Petro, 2009) and flavonoid-rich diets (Coultrap et al., 2008; Goyarzu et al., 2004; Malin et al., 2011).
Pro-inflammatory cytokines affect baseline synaptic activity in a variety of conditions, and they appear likely to mediate at least some of the detrimental effects of neuroinflammation on aging synapses (Lynch, 1998; Pickering et al., 2005). Antagonizing IL-1β, either with the IL-1 receptor antagonist, or with the anti-inflammatory cytokine, IL-4, reverses LTP deficits (Nolan et al., 2005) and improves memory performance in an aging-dependent manner (Frank et al., 2010). Similar benefits have also been reported for aged animals treated with TNF inhibiting drugs (Paredes et al., 2010; Sama et al., 2012).
Several recent studies suggest that different receptor mechanisms for IL-1β and TNFα could play unique roles in the modulation of synaptic function and neuronal viability during aging. Signaling through the IL-1 receptor relies heavily on other partnering proteins, such as the IL-1 receptor accessory protein (IL-1RAcP) (Cullinan et al., 1998). Its newly characterized “b” isoform, termed IL-1RAcPb, is present only in neurons, preferentially responds to low concentrations of IL-1β, and preferentially activates Src kinase pathways associated with enhanced NMDAR activity (Huang et al., 2011). In contrast, the more conventional IL-1RAcP, which is ubiquitously expressed, appears to preferentially activate p38 cascades when IL-1 levels are elevated, leading to impaired synaptic function and viability (Huang et al., 2011). For the TNF system, different receptors (TNFRs) similarly regulate distinct intracellular signaling cascades. TNFR1, which shows greater affinity for soluble TNF (Grell et al., 1998), contains a cell death domain in its cytosolic tail, and is coupled to cytotoxic caspase-dependent signaling cascades. Conversely, TNFR2, which binds more tightly to transmembrane TNF (Grell et al., 1995), is preferentially coupled to pro-survival pathways. For a review of TNFR signaling mechanisms and functions see McCoy and Tansey (2008). Consistent with these divergent signaling mechanisms, primary neurons that express TNFR1 in the absence of TNFR2, show enhanced susceptibility to excitotoxicity in the presence of TNF, whereas TNFR2-expressing neurons show increased resistance to excitotoxicity when TNF is present (Marchetti et al., 2004). Interestingly, recent work revealed an aging-related reduction in hippocampal protein levels for TNFR2 but not TNFR1 (Sama et al., 2012), suggesting that TNFR1 is the dominant pathway for TNF signaling during aging. Furthermore, chronic intracranial infusions of the novel TNF/TNFR1 inhibitor XPro1595 improved learning acquisition rates, blocked the induction of LTD, and prevented the loss of GluR1 type glutamate receptors in the hippocampus of aged rats (Sama et al., 2012). The results highlight the complexity of neuroinflammatory signaling in brain and suggest that the development of highly specific, and perhaps more efficacious anti-inflammatory treatments, could result from an increased understanding of individual cytokine messengers and their pathway specific interactions.
5. Interactions between Ca2+ dysregulation and neuroinflammation
5.1 Neuroinflammation and the link to Ca2+ dysregulation in neurons
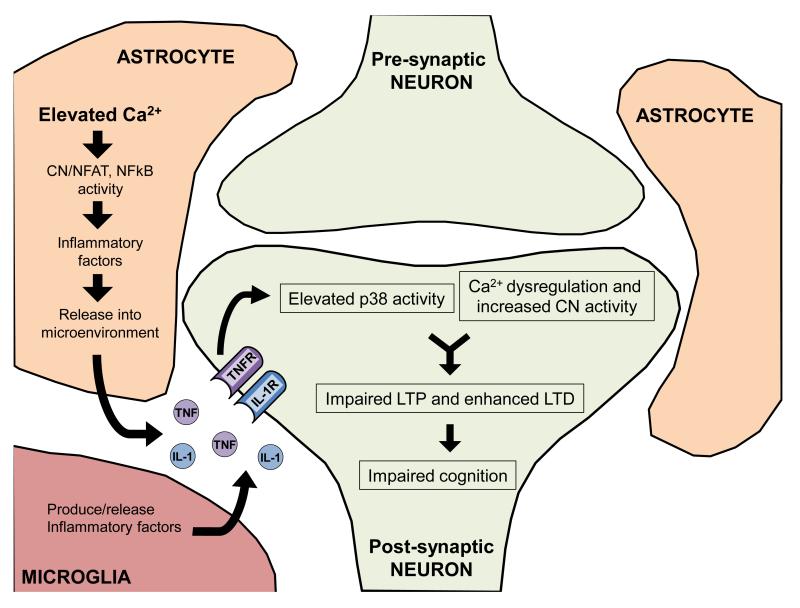
As described above, neuroinflammation can adversely affect synaptic function and plasticity through the activation of neuronal MAP kinases. However, it deserves noting that neuroinflammatory mediators can also strongly modulate neuronal Ca2+ signaling and homeostasis, which can, in turn, impact synapses. Numerous Ca2+ signaling mechanisms in neurons including IP3Rs, RyRs, NMDARs, and VSCCs appear to be sensitive to local cytokine levels and/or cytokine receptor activation (see Table 1). Sensitivity of Ca2+ signaling mechanisms to cytokines may partly account for the beneficial effects of polyphenol rich diets on Ca2+ clearance in aged rat synaptosomal preparations (Joseph et al., 1999). For some major cytokine species, the impact on neuronal Ca2+ signaling is highly similar to the effects of brain aging. For instance, elevated TNF levels trigger enhanced Ca2+ release from intracellular stores (Park et al., 2008; Pollock et al., 2002) and increase L-type VSCC expression/function in primary neuronal cultures (Furukawa and Mattson, 1998). Moreover, chronic blockade of TNF/TNFR1 signaling in the hippocampus of intact aging rats reduces L-VSCC activity in CA1 neurons and suppresses the induction of hippocampal LTD (Sama et al., 2012), shown previously to have a key L-VSCC-sensitive component (Norris et al., 1998b). That neuronal TNF receptors appear necessary for LTD induction in aged animals (also see Albensi and Mattson, 2000) suggests a close relationship to neuronal CN signaling pathways. In non-neuronal cell types, like astrocytes, CN activation is strongly induced by multiple cytokine factors, including TNF (Fernandez et al., 2007; Furman et al., 2012; Sama et al., 2008). TNF also appears to regulate CN-dependent apoptotic cascades in neuroblastoma cells (Alvarez et al., 2011). TNF, CN, and L-VSCC interactions in neurons could provide yet another critical junction for the Ca2+ and neuroinflammation hypotheses of aging (for an overview, see Figure 3). However, while existing data implicates TNF in L-VSCC regulation, no studies to our knowledge have investigated the specific linkage between neuronal TNF receptors and elevated CN activity with brain aging.
Table 1. Effects of inflammatory modulators on mechanisms of Ca2+ regulation in neurons.
This table highlights references that have investigated the impact of pro- or anti-inflammatory modulators on neuronal Ca2+ signaling. While the interaction of these signaling pathways has support in the literature, it is noteworthy that most mechanisms have only been investigated in one or a few studies.
Figure 3. The intersection of Ca2+ dysregulation and neuroinflammation.
Ca2+ dysregulation in glia, especially astrocytes, can increase calcineurin (CN) activity and lead to the production and release of inflammatory factors. These factors can then initiate activation of the MAP kinase signaling pathways (p38) and Ca2+ dysregulation in neurons, leading to increased CN activity. Changes in neuronal Ca2+ signaling can further perpetuate Ca2+ dysregulation, synaptic plasticity deficits, and cognitive impairments.
5.2. Ca2+ dysregulation and the link to neuroinflammatory signaling in glial cells
In an interesting parallel to neurons, changes in neuroinflammation with aging and disease may have strong ties to altered Ca2+ signaling mechanisms in glia. Glia are generally considered electrically non-excitable cells. Nevertheless, both astrocytes and microglia express diverse and highly regulated Ca2+ signaling machinery that allows them to respond to their local environment and to communicate with neighboring cells via gap junction networks and releasable factors (Farber and Kettenmann, 2006; Verkhratsky et al., 2012a). Elevated levels of glutamate, ATP, cytokines, and numerous other inflammatory mediators in the extracellular milieu all can induce elevated Ca2+ levels in glial cells (e.g. see Beskina et al., 2007; Cornell-Bell et al., 1990; Glaum et al., 1990; Holliday and Gruol, 1993; James et al., 2011; Koller et al., 1996). Astrocytes, which are in close contact to nearly every excitatory synapse in the brain, seem to be especially reliant on Ca2+ signaling and show marked Ca2+ dysregulation as a function of aging and/or disease.
Relative to astrocytes in healthy nervous tissue, activated astrocytes in aged animals, or in experimental models of amyloid pathology exhibit higher resting Ca2+ levels, bigger Ca2+ transients, and/or more frequent Ca2+ fluctuation (Kuchibhotla et al., 2009; Lin et al., 2007; Vincent et al., 2010). For aged animals, elevated Ca2+ responses in astrocytes have been attributed to reduced uptake into mitochondrial stores (Lin et al., 2007). However, it remains possible that other Ca2+ regulatory mechanisms, such as IP3Rs, RyRs, Ca2+-ATPases, and VSCCs are also disrupted in astrocytes during aging, injury, and disease. For instance, L-VSCCs, which exhibit low expression levels in “resting” astrocytes, are prominently expressed in activated astrocytes following acute CNS injury (Westenbroek et al., 1998). This observation is intriguing because, as discussed earlier, L-VSCCs appear to play a predominant role in neuronal Ca2+ dysregulation during aging. However, it remains unclear whether astrocytic L-VSCC expression is increased as a function of aging.
In addition to regulating the release of neurotransmitters and neuropeptides from astrocytes (i.e. gliotransmission), elevated glial Ca2+ transients also modulate inflammatory responses, including cytokine production and release (Canellada et al., 2008; Choi et al., 2001; Fernandez et al., 2007; Norris et al., 1994; Sama et al., 2008). Some inflammatory-related mediators, such as the S100 family members, are themselves Ca2+-binding proteins that exhibit increased activity in the presence of Ca2+ (Donato, 2001). Most other cytokines and inflammatory mediators are indirectly induced by Ca2+ through the activation of a variety of signaling cascades involving CN, CaM kinases, and PKC, among others. CN is an interesting case because it is highly expressed in activated astrocytes during aging, injury, and disease (Abdul et al., 2010; Abdul et al., 2009; Celsi et al., 2007; Hashimoto et al., 1998; Norris et al., 2005), but is only weakly expressed in astrocytes of young healthy animals (Goto et al., 1986; Norris et al., 2005). Thus, astrocytic CN appears to be a biomarker of sorts for astrocyte activation. CN induces cytokine expression in astrocytes and microglia through the activation of the nuclear factor of activated T-cells (NFAT) (Canellada et al., 2008; Fernandez et al., 2007; Nagamoto-Combs and Combs, 2010; Sama et al., 2008): a transcription factor well-characterized for its pivotal roles in lymphocyte activation, immune responses, and phenotype switching (Crabtree and Olson, 2002; Horsley and Pavlath, 2002; Macian, 2005). Hyperactivation of CN/NFAT activity in normal astrocytes recapitulates many of the loss- and gain-of function phenotypes associated with astrocyte activation including cellular hypertrophy, increased expression of intermediate filament proteins, loss of high affinity glutamate transport, and induction of numerous inflammatory mediators and cytokines (Abdul et al., 2009; Canellada et al., 2006; Fernandez et al., 2007; Norris et al., 2005; Perez-Ortiz et al., 2008; Sama et al., 2008). Since many of the cytokines induced by CN also stimulate Ca2+ elevations in glial cells, the CN/NFAT pathway can maintain its own activation and propagate to other nearby glial populations through positive feedback signaling (Sama et al., 2008). Thus, similar to its positive-feedback role in neuronal Ca2+ dysregulation, CN appears to be both a cause and a consequence of glial activation, and as such, may be a central mechanism for chronic neuroinflammation found in aging and age-related neurodegenerative disease.
6. Summary and Conclusions
The Ca2+ and neuroinflammation hypotheses provide alternate explanations for increased susceptibility to synaptic dysfunction, cognitive decline and neurodegenerative disease with aging. Both hypotheses have been supported by abundant data collected from diverse experimental models, and both undergo continual refinement. Though discrete in nature, Ca2+ and neuroinflammatory signaling mechanisms also interact at multiple levels in different neural cell types. One of the many questions that remain unanswered is: Which comes first: Ca2+ dysregulation or increased neuroinflammation? Gene microarray studies on transcriptional changes across the lifespan show that elevations in immune/inflammatory regulators (including levels for TNF family members) begin to emerge in rat hippocampus as early as six-months-of-age (Kadish et al., 2009). In contrast, common indices of CA1 neuronal Ca2+ dysregulation (i.e. increased CICR and augmented Ca2+-dependent afterhyperpolarizations) do not appear until approximately 12-months-of-age in the same animal model. These observations would seem to suggest that low levels of neuroinflammation are present before Ca2+ dysregulation arises, which, in turn, is consistent with the hypothesis that neuroinflammation precipitates Ca2+ dysregulation. However, caution is needed with this interpretation as Ca2+ measurement technology, as applied to in situ brain slices, may lack the sensitivity to detect very subtle changes in Ca2+ dynamics. Of course, it’s also possible that Ca2+ dysregulation in neuronal microdomains (e.g. dendritic spines) or in non-neuronal cell types (e.g. astrocytes and microglia) occur as early, or even earlier, than inflammatory transcriptional changes. Further investigation of Ca2+/neuroinflammatory interactions, the temporal progression of these interactions, and the specific molecular pathways/cell types involved will surely lead to more comprehensive theories of brain aging and perhaps stimulate novel strategies for treating age-related memory decline and/or reducing the risk of developing neurodegenerative diseases.
Highlights.
Age related cognitive deficits are linked to alterations in synaptic function and plasticity
The Ca2+ and neuroinflammation hypotheses have provided frameworks for synaptic dysfunction
Though discrete in nature, Ca2+ signaling and inflammatory mechanisms are also intertwined
Understanding Ca2+/inflammatory interactions may lead to the development of novel nootropics
Acknowledgments
Work supported by NIH grants AG02729 (CMN), AG000242 (DMS), and an award from the Kentucky Spinal Cord and Head Injury Research Trust (CMN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul HM, Furman JL, Sama MA, Mathis DM, Norris CM. NFATs and Alzheimer’s Disease. Mol Cell Pharmacol. 2010;2:7–14. [PMC free article] [PubMed] [Google Scholar]
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SH, Kim HJ, Jeong I, Hong YJ, Kim MJ, Rhie DJ, Jo YH, Hahn SJ, Yoon SH. Grape seed proanthocyanidin extract inhibits glutamate-induced cell death through inhibition of calcium signals and nitric oxide formation in cultured rat hippocampal neurons. BMC neuroscience. 2011;12:78. doi: 10.1186/1471-2202-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Blanco A, Fresno M, Munoz-Fernandez MA. TNF-alpha contributes to caspase-3 independent apoptosis in neuroblastoma cells: role of NFAT. PLoS One. 2011;6:e16100. doi: 10.1371/journal.pone.0016100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Norris CM, Sompol P, Wilcock DM, Goulding D, Neltner JH, Clair D, Watterson DM, Van Eldik LJ. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer’s disease-related pathology. J Neurosci. 2012;32:10201–10210. doi: 10.1523/JNEUROSCI.1496-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JH, Mun KC, Park WK, Lee SR, Suh SI, Baek WK, Yim MB, Kwon TK, Song DK. EGCG attenuates AMPA-induced intracellular calcium increase in hippocampal neurons. Biochem Biophys Res Commun. 2002;290:1506–1512. doi: 10.1006/bbrc.2002.6372. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci. 2000;1:11–20. doi: 10.1038/35036191. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Tansey MG. The duality of TNF signaling outcomes in the brain: potential mechanisms? Exp Neurol. 2011;229:198–200. doi: 10.1016/j.expneurol.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Brown TH. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983;80:7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaini F, Elkabes S, Bergamaschi S, Ladisa V, Lucchi L, De Graan PN, Schuurman T, Wetsel WC, Trabucchi M, Govoni S. Protein kinase C activity, translocation, and conventional isoforms in aging rat brain. Neurobiol Aging. 1995;16:137–148. doi: 10.1016/0197-4580(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. American journal of physiology. Cell physiology. 2007;293:C1103–1111. doi: 10.1152/ajpcell.00249.2007. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J Neuroimmune Pharmacol. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. The European journal of neuroscience. 2007;25:2260–2268. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, Moskal JR. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011;32:698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Canellada A, Cano E, Sanchez-Ruiloba L, Zafra F, Redondo JM. Calcium-dependent expression of TNF-alpha in neural cells is mediated by the calcineurin/NFAT pathway. Mol Cell Neurosci. 2006;31:692–701. doi: 10.1016/j.mcn.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Canellada A, Ramirez BG, Minami T, Redondo JM, Cano E. Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1-4 and cyclooxygenase-2 as NFAT target genes. Glia. 2008;56:709–722. doi: 10.1002/glia.20647. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Genazzani A, Guerini D. Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Commun. 1999;266:624–632. doi: 10.1006/bbrc.1999.1879. [DOI] [PubMed] [Google Scholar]
- Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–343. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- Celsi F, Svedberg M, Unger C, Cotman CW, Carri MT, Ottersen OP, Nordberg A, Torp R. Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol Dis. 2007;26:342–352. doi: 10.1016/j.nbd.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Krushel LA, Crossin KL. NF-kappaB activation by N-CAM and cytokines in astrocytes is regulated by multiple protein kinases and redox modulation. Glia. 2001;33:45–56. doi: 10.1002/1098-1136(20010101)33:1<45::aid-glia1005>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. Involvement of microglial P2×7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain, behavior, and immunity. 2010;24:1176–1189. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci U S A. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Cullinan EB, Kwee L, Nunes P, Shuster DJ, Ju G, McIntyre KW, Chizzonite RA, Labow MA. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol. 1998;161:5614–5620. [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- De A, Churchill L, Obal F, Jr., Simasko SM, Krueger JM. GHRH and IL1beta increase cytoplasmic Ca(2+) levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Turner DA, Watters CL. Spatial performance correlates with in vitro potentiation in young and aged Fischer 344 rats. Brain Res. 1991;554:1–9. doi: 10.1016/0006-8993(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Moyer JR, Jr, Thompson LT. The calcium rationale in aging and Alzheimer’s disease. Evidence from an animal model of normal aging. Ann NY Acad Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Zhang B, Shu HF, Zhang XY, Wang X, Kuang F, Liu L, Peng ZW, Wu R, Zhou Z, Wang BR. Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J Neurosci Res. 2009;87:2757–2762. doi: 10.1002/jnr.22107. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–665. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Feng W, Cherednichenko G, Ward CW, Padilla IT, Cabrales E, Lopez JR, Eltit JM, Allen PD, Pessah IN. Green tea catechins are potent sensitizers of ryanodine receptor type 1 (RyR1) Biochemical pharmacology. 2010;80:512–521. doi: 10.1016/j.bcp.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Fernandez S, Carrero P, Garcia-Garcia M, Torres-Aleman I. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci. 2007;27:8745–8756. doi: 10.1523/JNEUROSCI.1002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JD, Bienias JL, Bennett DA. A longitudinal study of implicit and explicit memory in old persons. Psychol Aging. 2004;19:617–625. doi: 10.1037/0882-7974.19.4.617. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res. 1996;736:243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87:522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–73. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17beta-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci. 2008;122:301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344×BN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, Van Eldik LJ, Norris CM. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70:1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- Gambino F, Pavlowsky A, Begle A, Dupont JL, Bahi N, Courjaret R, Gardette R, Hadjkacem H, Skala H, Poulain B, Chelly J, Vitale N, Humeau Y. IL1-receptor accessory protein-like 1 (IL1RAPL1), a protein involved in cognitive functions, regulates N-type Ca2+-channel and neurite elongation. Proc Natl Acad Sci U S A. 2007;104:9063–9068. doi: 10.1073/pnas.0701133104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- Gardam KE, Geiger JE, Hickey CM, Hung AY, Magoski NS. Flufenamic acid affects multiple currents and causes intracellular Ca2+ release in Aplysia bag cell neurons. Journal of neurophysiology. 2008;100:38–49. doi: 10.1152/jn.90265.2008. [DOI] [PubMed] [Google Scholar]
- Ge QF, Hu X, Ma ZQ, Liu JR, Zhang WP, Chen Z, Wei EQ. Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons. Pharmacological research: the official journal of the Italian Pharmacological Society. 2007;55:148–157. doi: 10.1016/j.phrs.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Bondareff W, Dodge JT. Hypertrophy of astroglial processes in the dentate gyrus of the senescent rat. Am J Anat. 1978;153:537–543. doi: 10.1002/aja.1001530405. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Peterson C. Calcium and the aging nervous system. Neurobiol Aging. 1987;8:329–343. doi: 10.1016/0197-4580(87)90072-8. [DOI] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Shi JS, Huang XN, Liu Q, Ma H. Inhibition of caspases and intracellular free Ca2+ concentrations are involved in resveratrol protection against apoptosis in rat primary neuron cultures. Acta pharmacologica Sinica. 2007;28:1724–1730. doi: 10.1111/j.1745-7254.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res. 1986;397:161–172. doi: 10.1016/0006-8993(86)91381-8. [DOI] [PubMed] [Google Scholar]
- Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci. 2010;30:12128–12137. doi: 10.1523/JNEUROSCI.2474-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni S, Amadio M, Battaini F, Pascale A. Senescence of the brain: focus on cognitive kinases. Curr Pharm Des. 2010;16:660–671. doi: 10.2174/138161210790883732. [DOI] [PubMed] [Google Scholar]
- Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- Graff J, Koshibu K, Jouvenceau A, Dutar P, Mansuy IM. Protein phosphatase 1-dependent transcriptional programs for long-term memory and plasticity. Learn Mem. 2010;17:355–363. doi: 10.1101/lm.1766510. [DOI] [PubMed] [Google Scholar]
- Green KN, LaFerla FM. Linking calcium to Abeta and Alzheimer’s disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE. Purkinje neuron physiology is altered by the inflammatory factor interleukin-6. Cerebellum. 2005;4:198–205. doi: 10.1080/14734220500199987. [DOI] [PubMed] [Google Scholar]
- Hagenacker T, Czeschik JC, Schafers M, Busselberg D. Sensitization of voltage activated calcium channel currents for capsaicin in nociceptive neurons by tumor-necrosis-factor-alpha. Brain research bulletin. 2010;81:157–163. doi: 10.1016/j.brainresbull.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Han JH, Kim KJ, Jang HJ, Jang JH, Kim MJ, Sung KW, Rhie DJ, Jo YH, Hahn SJ, Lee MY, Yoon SH. Effects of Apigenin on Glutamate-induced [Ca](i) Increases in Cultured Rat Hippocampal Neurons. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2008;12:43–49. doi: 10.4196/kjpp.2008.12.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Kawamata T, Saito N, Sasaki M, Nakai M, Niu S, Taniguchi T, Terashima A, Yasuda M, Maeda K, Tanaka C. Isoform-specific redistribution of calcineurin A alpha and A beta in the hippocampal CA1 region of gerbils after transient ischemia. J Neurochem. 1998;70:1289–1298. doi: 10.1046/j.1471-4159.1998.70031289.x. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vannucchi MG, Wenk GL. Behavioral and ultrastructural changes induced by chronic neuroinflammation in young rats. Brain Res. 2000a;859:157–166. doi: 10.1016/s0006-8993(00)01999-5. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak PD, Wenk GL. LPS-induced neuroinflammatory effects do not recover with time. Neuroreport. 2000b;11:1759–1763. doi: 10.1097/00001756-200006050-00032. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;176:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. Wiley; New York, NY: 1949. [DOI] [PubMed] [Google Scholar]
- Hemond P, Jaffe DB. Caloric restriction prevents aging-associated changes in spike-mediated Ca2+ accumulation and the slow afterhyperpolarization in hippocampal CA1 pyramidal neurons. Neuroscience. 2005;135:413–20. doi: 10.1016/j.neuroscience.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday J, Gruol DL. Cytokine stimulation increases intracellular calcium and alters the response to quisqualate in cultured cortical astrocytes. Brain Res. 1993;621:233–241. doi: 10.1016/0006-8993(93)90111-y. [DOI] [PubMed] [Google Scholar]
- Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Liang YC, Wu HM, Chen YL, Lo SW, Ho WC. Alterations in the balance of protein kinase and phosphatase activities and age-related impairments of synaptic transmission and long-term potentiation. Hippocampus. 2002;12:787–802. doi: 10.1002/hipo.10032. [DOI] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe G, Sanchez-Gomez MV, Campos-Esparza MR, Alberdi E, Matute C. Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia. 2006;53:201–211. doi: 10.1002/glia.20267. [DOI] [PubMed] [Google Scholar]
- Johnston D, Williams S, Jaffe D, Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–21. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. Creb, synapses, and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J Physiol Paris. 2006;99:154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O’Neill LA, Lynch MA. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. J Biol Chem. 2001;276:45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Hypothesis on the Regulation of Cytosol Calcium Concentration and the Aging Brain. Neurobiol Aging. 1987;8:345–346. doi: 10.1016/0197-4580(87)90073-x. [DOI] [PubMed] [Google Scholar]
- Klee CB. Concerted regulation of protein phosphorylation and dephosphorylation by calmodulin. Neurochem Res. 1991;16:1059–1065. doi: 10.1007/BF00965851. [DOI] [PubMed] [Google Scholar]
- Kohno T. Zaltoprofen inhibits bradykinin-mediated enhancement of glutamate receptor activity in substantia gelatinosa neurons. Anesthesia and analgesia. 2011;113:412–416. doi: 10.1213/ANE.0b013e31821c693c. [DOI] [PubMed] [Google Scholar]
- Kollen M, Stephan A, Faivre-Bauman A, Loudes C, Sinet PM, Alliot J, Billard JM, Epelbaum J, Dutar P, Jouvenceau A. Preserved memory capacities in aged Lou/C/Jall rats. Neurobiol Aging. 2010;31:129–142. doi: 10.1016/j.neurobiolaging.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–64. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging Effects on the Limits and Stability of Long-Term Synaptic Potentiation and Depression in Rat Hippocampal Area CA1. Journal of neurophysiology. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Lai AY, Swayze RD, El-Husseini A, Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. Journal of neuroimmunology. 2006;175:97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging. 1987;8:46–47. doi: 10.1016/0197-4580(87)90074-1. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Lynch G. Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol. 1977;32:523–533. doi: 10.1093/geronj/32.5.523. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. Journal of neurophysiology. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Rose G, Sandles L, Wohlstadter TC, Lynch G. Patterns of astroglial hypertrophy and neuronal degeneration in the hippocampus of ages, memory-deficient rats. J Gerontol. 1977;32:3–12. doi: 10.1093/geronj/32.1.3. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lee HH, Yang LL, Wang CC, Hu SY, Chang SF, Lee YH. Differential effects of natural polyphenols on neuronal survival in primary cultured central neurons against glutamate- and glucose deprivation-induced neuronal death. Brain Res. 2003;986:103–113. doi: 10.1016/s0006-8993(03)03197-4. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Zhao P, Beattie EC. Rapid tumor necrosis factor alpha-induced exocytosis of glutamate receptor 2-lacking AMPA receptors to extrasynaptic plasma membrane potentiates excitotoxicity. J Neurosci. 2008;28:2119–2130. doi: 10.1523/JNEUROSCI.5159-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leritz EC, Grande LJ, Bauer RM. Temporal lobe epilepsy as a model to understand human memory: the distinction between explicit and implicit memory. Epilepsy Behav. 2006;9:1–13. doi: 10.1016/j.yebeh.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979;175:233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Li L, Tsai HJ, Li L, Wang XM. Icariin inhibits the increased inward calcium currents induced by amyloid-beta(25-35) peptide in CA1 pyramidal neurons of neonatal rat hippocampal slice. The American journal of Chinese medicine. 2010;38:113–125. doi: 10.1142/S0192415X10007701. [DOI] [PubMed] [Google Scholar]
- Li M, Wang QS, Chen Y, Wang ZM, Liu Z, Guo SM. Resveratrol inhibits neuronal discharges in rat hippocampal CA1 area. Sheng li xue bao: [Acta physiologica Sinica] 2005;57:355–360. [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Wu J, Holstein D, Upadhyay G, Rourk W, Muller E, Lechleiter JD. Ca2+ signaling, mitochondria and sensitivity to oxidative stress in aging astrocytes. Neurobiol Aging. 2007a;28:99–111. doi: 10.1016/j.neurobiolaging.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lin F, Xin Y, Wang J, Ma L, Liu J, Liu C, Long L, Wang F, Jin Y, Zhou J, Chen J. Puerarin facilitates Ca(2+)-induced Ca(2+) release triggered by KCl-depolarization in primary cultured rat hippocampal neurons. European journal of pharmacology. 2007b;570:43–49. doi: 10.1016/j.ejphar.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Liu T, Jiang CY, Fujita T, Luo SW, Kumamoto E. Enhancement by interleukin-1beta of AMPA and NMDA receptor-mediated currents in adult rat spinal superficial dorsal horn neurons. Molecular pain. 2013;9:16. doi: 10.1186/1744-8069-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. What is the biological significance of an age-related increase in IL-1beta in hippocampus? Mol Psychiatry. 1999;4:15–18. doi: 10.1038/sj.mp.4000438. [DOI] [PubMed] [Google Scholar]
- Ma SH, Li B, Huang HW, Peng YP, Qiu YH. Interleukin-6 inhibits L-type calcium channel activity of cultured cerebellar granule neurons. The journal of physiological sciences: JPS. 2012;62:385–392. doi: 10.1007/s12576-012-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lee DR, Goyarzu P, Chang YH, Ennis LJ, Beckett E, Shukitt-Hale B, Joseph JA. Short-term blueberry-enriched diet prevents and reverses object recognition memory loss in aging rats. Nutrition. 2011;27:338–342. doi: 10.1016/j.nut.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Margolis RB, Scialfa CT. Age differences in Wechsler Memory Scale performance. J Clin Psychol. 1984;40:1442–1449. doi: 10.1002/1097-4679(198411)40:6<1442::aid-jclp2270400630>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Mathis DM, Furman JL, Norris CM. Preparation of acute hippocampal slices from rats and transgenic mice for the study of synaptic alterations during aging and amyloid pathology. J Vis Exp. 2011 doi: 10.3791/2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Miyazaki K, Sakai S, Yawo H, Nakata N, Moriguchi S, Fukunaga K, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus. European journal of pharmacology. 2008;578:194–200. doi: 10.1016/j.ejphar.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Matteucci A, Cammarota R, Paradisi S, Varano M, Balduzzi M, Leo L, Bellenchi GC, De Nuccio C, Carnovale-Scalzo G, Scorcia G, Frank C, Mallozzi C, Di Stasi AM, Visentin S, Malchiodi-Albedi F. Curcumin protects against NMDA-induced toxicity: a possible role for NR2A subunit. Invest Ophthalmol Vis Sci. 2011;52:1070–1077. doi: 10.1167/iovs.10-5966. [DOI] [PubMed] [Google Scholar]
- Matteucci A, Frank C, Domenici MR, Balduzzi M, Paradisi S, Carnovale-Scalzo G, Scorcia G, Malchiodi-Albedi F. Curcumin treatment protects rat retinal neurons against excitotoxicity: effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;167:641–648. doi: 10.1007/s00221-005-0068-0. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Johe K, Kitos TE. Age-dependent alterations in synaptic membrane systems for Ca2+ regulation. Mech Ageing Dev. 1984;25:215–225. doi: 10.1016/0047-6374(84)90142-8. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3:57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Synaptic potentiation in the rat dentate gyrus during exploratory learning. Neuroreport. 1993;5:317–320. doi: 10.1097/00001756-199312000-00035. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Motagally MA, Lukewich MK, Chisholm SP, Neshat S, Lomax AE. Tumour necrosis factor alpha activates nuclear factor kappaB signalling to reduce N-type voltage-gated Ca2+ current in postganglionic sympathetic neurons. The Journal of physiology. 2009;587:2623–2634. doi: 10.1113/jphysiol.2009.172312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr., Disterhoft JF. Nimodipine decreases calcium action potentials in rabbit hippocampal CA1 neurons in an age-dependent and concentration-dependent manner. Hippocampus. 1994;4:11–17. doi: 10.1002/hipo.450040104. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Jr., Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. Journal of neurophysiology. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Murakami M, Ohta T, Ito S. Lipopolysaccharides enhance the action of bradykinin in enteric neurons via secretion of interleukin-1beta from enteric glial cells. J Neurosci Res. 2009;87:2095–2104. doi: 10.1002/jnr.22036. [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell. 2007;6:297–305. doi: 10.1111/j.1474-9726.2007.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamoto-Combs K, Combs CK. Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells) J Neurosci. 2010;30:9641–9646. doi: 10.1523/JNEUROSCI.0828-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Gruol DL. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. Journal of neuroimmunology. 2004;156:74–87. doi: 10.1016/j.jneuroim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Netzeband JG, Gruol DL. Chronic interleukin-6 exposure alters metabotropic glutamate receptor-activated calcium signalling in cerebellar Purkinje neurons. The European journal of neuroscience. 2004;20:2387–2400. doi: 10.1111/j.1460-9568.2004.03706.x. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic interleukin-6 exposure alters electrophysiological properties and calcium signaling in developing cerebellar purkinje neurons in culture. Journal of neurophysiology. 2002;88:475–486. doi: 10.1152/jn.2002.88.1.475. [DOI] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Norris CM, Blalock EM, Chen KC, Porter NM, Landfield PW. Calcineurin enhances L-type Ca(2+) channel activity in hippocampal neurons: increased effect with age in culture. Neuroscience. 2002;110:213–225. doi: 10.1016/s0306-4522(01)00574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Blalock EM, Chen KC, Porter NM, Thibault O, Kraner SD, Landfield PW. Hippocampal ‘zipper’ slice studies reveal a necessary role for calcineurin in the increased activity of L-type Ca(2+) channels with aging. Neurobiol Aging. 2010;31:328–338. doi: 10.1016/j.neurobiolaging.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194–206. doi: 10.1006/nlme.1998.3864. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. Journal of neurophysiology. 1998a;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998b;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris JG, Tang LP, Sparacio SM, Benveniste EN. Signal transduction pathways mediating astrocyte IL-6 induction by IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1994;152:841–850. [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Waters J, Disterhoft JF. Altered Calcium Metabolism in Aging CA1 Hippocampal Pyramidal Neurons. J Neurosci. 2013;33:7905–7911. doi: 10.1523/JNEUROSCI.5457-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS Neurol Disord Drug Targets. 2011;10:108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB. Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotoxicity research. 2005;8:295–304. doi: 10.1007/BF03033983. [DOI] [PubMed] [Google Scholar]
- Ott D, Murgott J, Rafalzik S, Wuchert F, Schmalenbeck B, Roth J, Gerstberger R. Neurons and glial cells of the rat organum vasculosum laminae terminalis directly respond to lipopolysaccharide and pyrogenic cytokines. Brain Res. 2010;1363:93–106. doi: 10.1016/j.brainres.2010.09.083. [DOI] [PubMed] [Google Scholar]
- Paran E, Anson O, Lowenthal DT. Cognitive function and antihypertensive treatment in the elderly: a 6-year follow-up study. Am J Ther. 2010;17:358–364. doi: 10.1097/MJT.0b013e3181bf325c. [DOI] [PubMed] [Google Scholar]
- Paredes D, Acosta S, Gemma C, Bickford PC. Role of TNFalpha Induced Inflammation in Delay Eyeblink Conditioning in Young and Aged Rats. Aging Dis. 2010;1:191–198. [PMC free article] [PubMed] [Google Scholar]
- Park KM, Yule DI, Bowers WJ. Tumor necrosis factor-alpha potentiates intraneuronal Ca2+ signaling via regulation of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2008;283:33069–33079. doi: 10.1074/jbc.M802209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Govoni S, Battaini F. Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602. [DOI] [PubMed] [Google Scholar]
- Peng BG, Chen S, Lin X. Aspirin selectively augmented N-methyl-D-aspartate types of glutamate responses in cultured spiral ganglion neurons of mice. Neurosci Lett. 2003;343:21–24. doi: 10.1016/s0304-3940(03)00296-9. [DOI] [PubMed] [Google Scholar]
- Perez-Ortiz JM, Serrano-Perez MC, Pastor MD, Martin ED, Calvo S, Rincon M, Tranque P. Mechanical lesion activates newly identified NFATc1 in primary astrocytes: implication of ATP and purinergic receptors. The European journal of neuroscience. 2008;27:2453–2465. doi: 10.1111/j.1460-9568.2008.06197.x. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O’Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Pizza V, Agresta A, D’Acunto CW, Festa M, Capasso A. Neuroinflammation and ageing: current theories and an overview of the data. Rev Recent Clin Trials. 2011;6:189–203. doi: 10.2174/157488711796575577. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Ffrench-Mullen JM. Interleukin-1 beta depresses calcium currents in CA1 hippocampal neurons at pathophysiological concentrations. Brain research bulletin. 1992;29:221–223. doi: 10.1016/0361-9230(92)90029-w. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, ffrench-Mullen JM. Interleukin-2 modulates calcium currents in dissociated hippocampal CA1 neurons. Neuroreport. 1993;4:579–581. doi: 10.1097/00001756-199305000-00030. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, ffrench-Mullen JM. Interleukin-1 beta inhibits Ca2+ channel currents in hippocampal neurons through protein kinase C. European journal of pharmacology. 1994;266:1–10. doi: 10.1016/0922-4106(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42:93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Parsons KL, Gruol DL. Interleukin-6 selectively enhances the intracellular calcium response to NMDA in developing CNS neurons. J Neurosci. 1995;15:6688–6699. doi: 10.1523/JNEUROSCI.15-10-06688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Sweeney DD, Netzeband JG, Gruol DL. Chronic interleukin-6 alters NMDA receptor-mediated membrane responses and enhances neurotoxicity in developing CNS neurons. J Neurosci. 1998;18:10445–10456. doi: 10.1523/JNEUROSCI.18-24-10445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranney A, Petro MS. Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav Pharmacol. 2009;20:330–336. doi: 10.1097/FBP.0b013e32832f0193. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Kim HW, Rapoport SI, Reese EA. Neuroinflammation and synaptic loss. Neurochem Res. 2012;37:903–910. doi: 10.1007/s11064-012-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese LC, Laezza F, Woltjer R, Taglialatela G. Dysregulated phosphorylation of Ca(2+)/calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease. J Neurochem. 2011;119:791–804. doi: 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci. 2006;26:12642–12646. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnlund M, Nyberg L, Backman L, Nilsson LG. Stability, growth, and decline in adult life span development of declarative memory: cross-sectional and longitudinal data from a population-based study. Psychol Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Sama DM, Mohmmad Abdul H, Furman JL, Artiushin IA, Szymkowski DE, Scheff SW, Norris CM. Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS One. 2012;7:e38170. doi: 10.1371/journal.pone.0038170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama MA, Mathis DM, Furman JL, Abdul HM, Artiushin IA, Kraner SD, Norris CM. Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem. 2008;283:21953–21964. doi: 10.1074/jbc.M800148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samorajski T. How the human brain responds to aging. J Am Geriatr Soc. 1976;24:4–11. doi: 10.1111/j.1532-5415.1976.tb03246.x. [DOI] [PubMed] [Google Scholar]
- Sandin M, Jasmin S, Levere TE. Aging and cognition: facilitation of recent memory in aged nonhuman primates by nimodipine. Neurobiol Aging. 1990;11:573–575. doi: 10.1016/0197-4580(90)90120-o. [DOI] [PubMed] [Google Scholar]
- Savina TA, Shchipakina TG, Levin SG, Godukhin OV. Interleukin-10 prevents the hypoxia-induced decreases in expressions of AMPA receptor subunit GluA1 and alpha subunit of Ca(2+)/calmodulin-dependent protein kinase II in hippocampal neurons. Neurosci Lett. 2013;534:279–284. doi: 10.1016/j.neulet.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Schmid AW, Lynch MA, Herron CE. The effects of IL-1 receptor antagonist on beta amyloid mediated depression of LTP in the rat CA1 in vivo. Hippocampus. 2009;19:670–676. doi: 10.1002/hipo.20542. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Bahia P, Spencer JP, Sheppard O, Rattray M, Cadenas E, Rice-Evans C, Williams RJ. (−)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. J Neurochem. 2007;101:1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- Sharp PE, McNaughton BL, Barnes CA. Enhancement of hippocampal field potentials in rats exposed to a novel, complex environment. Brain Res. 1985;339:361–365. doi: 10.1016/0006-8993(85)90105-2. [DOI] [PubMed] [Google Scholar]
- Shen Y, Zhu LJ, Liu SS, Zhou SY, Luo JH. Interleukin-2 inhibits NMDA receptor-mediated currents directly and may differentially affect subtypes. Biochem Biophys Res Commun. 2006;351:449–454. doi: 10.1016/j.bbrc.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Rovnaghi CR, Kozlowska E, Van Eldik LJ, Griffin WS. Human brain S100 beta and S100 beta mRNA expression increases with age: pathogenic implications for Alzheimer’s disease. Neurobiol Aging. 1996;17:359–363. doi: 10.1016/0197-4580(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Shu HF, Wang BR, Wang SR, Yao W, Huang HP, Zhou Z, Wang X, Fan J, Wang T, Ju G. IL-1beta inhibits IK and increases [Ca2+]i in the carotid body glomus cells and increases carotid sinus nerve firings in the rat. The European journal of neuroscience. 2007;25:3638–3647. doi: 10.1111/j.1460-9568.2007.05586.x. [DOI] [PubMed] [Google Scholar]
- Soliven B, Albert J. Tumor necrosis factor modulates Ca2+ currents in cultured sympathetic neurons. J Neurosci. 1992;12:2665–2671. doi: 10.1523/JNEUROSCI.12-07-02665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Lie-Cheng W, Wang GD, Zhou Z, Zhao ZQ. Interleukin-2 regulates membrane potentials and calcium channels via mu opioid receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43:1324–1329. doi: 10.1016/s0028-3908(02)00298-8. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Straube KT, Deyo RA, Moyer JR, Jr., Disterhoft JF. Dietary nimodipine improves associative learning in aging rabbits. Neurobiol Aging. 1990;11:659–661. doi: 10.1016/0197-4580(90)90033-v. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE. The pathogenesis of Alzheimers disease is it a lifelong “calciumopathy”? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tha KK, Okuma Y, Miyazaki H, Murayama T, Uehara T, Hatakeyama R, Hayashi Y, Nomura Y. Changes in expressions of proinflammatory cytokines IL-1beta, TNF-alpha and IL-6 in the brain of senescence accelerated mouse (SAM) P8. Brain Res. 2000;885:25–31. doi: 10.1016/s0006-8993(00)02883-3. [DOI] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Deyo RA, Disterhoft JF. Nimodipine enhances spontaneous activity of hippocampal pyramidal neurons in aging rabbits at a dose that facilitates associative learning. Brain Res. 1990;535:119–130. doi: 10.1016/0006-8993(90)91830-a. [DOI] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkikh A, Janus C, El-Beheiry H, Pennefather PS, Samoilova M, McDonald P, Ouanounou A, Carlen PL. Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol. 2006;197:291–300. doi: 10.1016/j.expneurol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension. 2008;51:528–533. doi: 10.1161/HYPERTENSIONAHA.107.101634. [DOI] [PubMed] [Google Scholar]
- Turovskaya MV, Turovsky EA, Zinchenko VP, Levin SG, Godukhin OV. Interleukin-10 modulates [Ca2+]i response induced by repeated NMDA receptor activation with brief hypoxia through inhibition of InsP(3)-sensitive internal stores in hippocampal neurons. Neurosci Lett. 2012;516:151–155. doi: 10.1016/j.neulet.2012.03.084. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. The European journal of neuroscience. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3:405–429. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110:193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- Vereyken EJ, Bajova H, Chow S, de Graan PN, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. The European journal of neuroscience. 2007;25:3605–3616. doi: 10.1111/j.1460-9568.2007.05615.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353:45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Villarreal DM, Do V, Haddad E, Derrick BE. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci. 2002;5:48–52. doi: 10.1038/nn776. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Gasperini R, Foa L, Small DH. Astrocytes in Alzheimer’s disease: emerging roles in calcium dysregulation and synaptic plasticity. J Alzheimers Dis. 2010;22:699–714. doi: 10.3233/JAD-2010-101089. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, Thompson RF. 17beta-estradiol suppresses expression of long-term depression in aged rats. Brain research bulletin. 2000;53:783–787. doi: 10.1016/s0361-9230(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Ferruzzi MG, Ho L, Blount J, Janle EM, Gong B, Pan Y, Gowda GA, Raftery D, Arrieta-Cruz I, Sharma V, Cooper B, Lobo J, Simon JE, Zhang C, Cheng A, Qian X, Ono K, Teplow DB, Pavlides C, Dixon RA, Pasinetti GM. Brain-targeted proanthocyanidin metabolites for Alzheimer’s disease treatment. J Neurosci. 2012;32:5144–5150. doi: 10.1523/JNEUROSCI.6437-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wu J, Rowan MJ, Anwyl R. Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci. 2005;22:2827–2832. doi: 10.1111/j.1460-9568.2005.04457.x. [DOI] [PubMed] [Google Scholar]
- Watfa G, Rossignol P, Kearney-Schwartz A, Fay R, Bracard S, Felblinger J, Boivin JM, Lacolley P, Zannad F, Benetos A. Use of calcium channel blockers is associated with better cognitive performance in older hypertensive patients with subjective memory complaints. J Hypertens. 2010;28:2485–2493. doi: 10.1097/HJH.0b013e32833e4108. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Bausch SB, Lin RC, Franck JE, Noebels JL, Catterall WA. Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. J Neurosci. 1998;18:2321–2334. doi: 10.1523/JNEUROSCI.18-07-02321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Wuchert F, Ott D, Rafalzik S, Roth J, Gerstberger R. Tumor necrosis factor-alpha, interleukin-1beta and nitric oxide induce calcium transients in distinct populations of cells cultured from the rat area postrema. Journal of neuroimmunology. 2009;206:44–51. doi: 10.1016/j.jneuroim.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lin D, Li S, Li G, Shyamala SG, Barish PA, Vernon MM, Pan J, Ogle WO. Curcumin reverses impaired cognition and neuronal plasticity induced by chronic stress. Neuropharmacology. 2009;57:463–471. doi: 10.1016/j.neuropharm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M. Role of regucalcin in brain calcium signaling: involvement in aging. Integr Biol (Camb) 2012;4:825–837. doi: 10.1039/c2ib20042b. [DOI] [PubMed] [Google Scholar]
- Yanez M, Galan L, Matias-Guiu J, Vela A, Guerrero A, Garcia AG. CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 2011;1423:77–86. doi: 10.1016/j.brainres.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX. Interleukin-1beta enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Res. 2005;1034:172–179. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yin HZ, Hsu CI, Yu S, Rao SD, Sorkin LS, Weiss JH. TNF-alpha triggers rapid membrane insertion of Ca(2+) permeable AMPA receptors into adult motor neurons and enhances their susceptibility to slow excitotoxic injury. Exp Neurol. 2012;238:93–102. doi: 10.1016/j.expneurol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin ST, Tang ML, Deng HM, Xing TR, Chen JT, Wang HL, Ruan DY. Epigallocatechin-3-gallate induced primary cultures of rat hippocampal neurons death linked to calcium overload and oxidative stress. Naunyn-Schmiedeberg’s archives of pharmacology. 2009;379:551–564. doi: 10.1007/s00210-009-0401-4. [DOI] [PubMed] [Google Scholar]
- Zhang GR, Cheng XR, Zhou WX, Zhang YX. Age-related expression of calcium/calmodulin-dependent protein kinase II A in the hippocampus and cerebral cortex of senescence accelerated mouse prone/8 mice is modulated by anti-Alzheimer’s disease drugs. Neuroscience. 2009;159:308–315. doi: 10.1016/j.neuroscience.2008.06.068. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Exp Neurol. 2008a;212:44–52. doi: 10.1016/j.expneurol.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008b;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008c;154:1533–1538. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Ma ZG, Rowlands DK, Gou YL, Fok KL, Wong HY, Yu MK, Tsang LL, Mu L, Chen L, Yung WH, Chung YW, Zhang BL, Zhao H, Chan HC. Flavonoid Myricetin Modulates GABA(A) Receptor Activity through Activation of Ca(2+) Channels and CaMK-II Pathway. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:758097. doi: 10.1155/2012/758097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Liu W, Gu Z, Yan Z. Serotonin facilitates long-term depression induction in prefrontal cortex via p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization. J Physiol. 2008;586:4465–4479. doi: 10.1113/jphysiol.2008.155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tai C, Ye HH, Ren X, Chen JG, Wang SQ, Chai Z. Interleukin-1beta downregulates the L-type Ca2+ channel activity by depressing the expression of channel protein in cortical neurons. Journal of cellular physiology. 2006a;206:799–806. doi: 10.1002/jcp.20518. [DOI] [PubMed] [Google Scholar]
- Zhou C, Ye HH, Wang SQ, Chai Z. Interleukin-1beta regulation of N-type Ca2+ channels in cortical neurons. Neurosci Lett. 2006b;403:181–185. doi: 10.1016/j.neulet.2006.04.043. [DOI] [PubMed] [Google Scholar]