Abstract
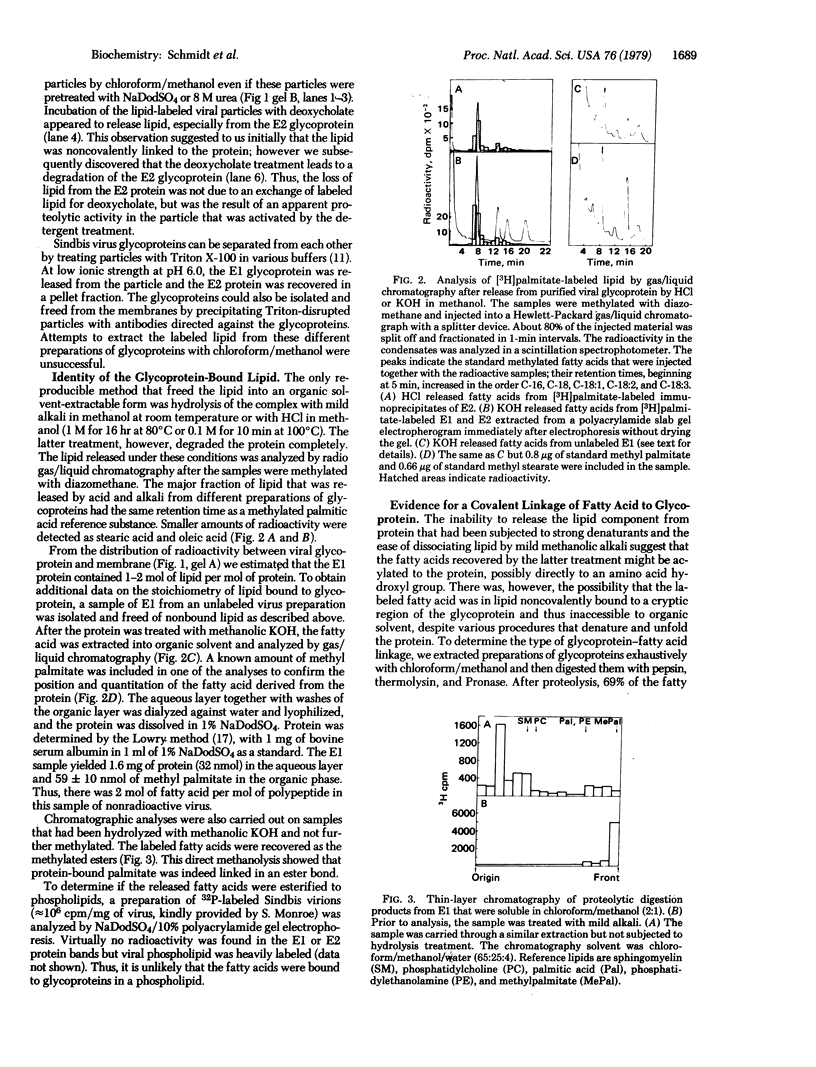
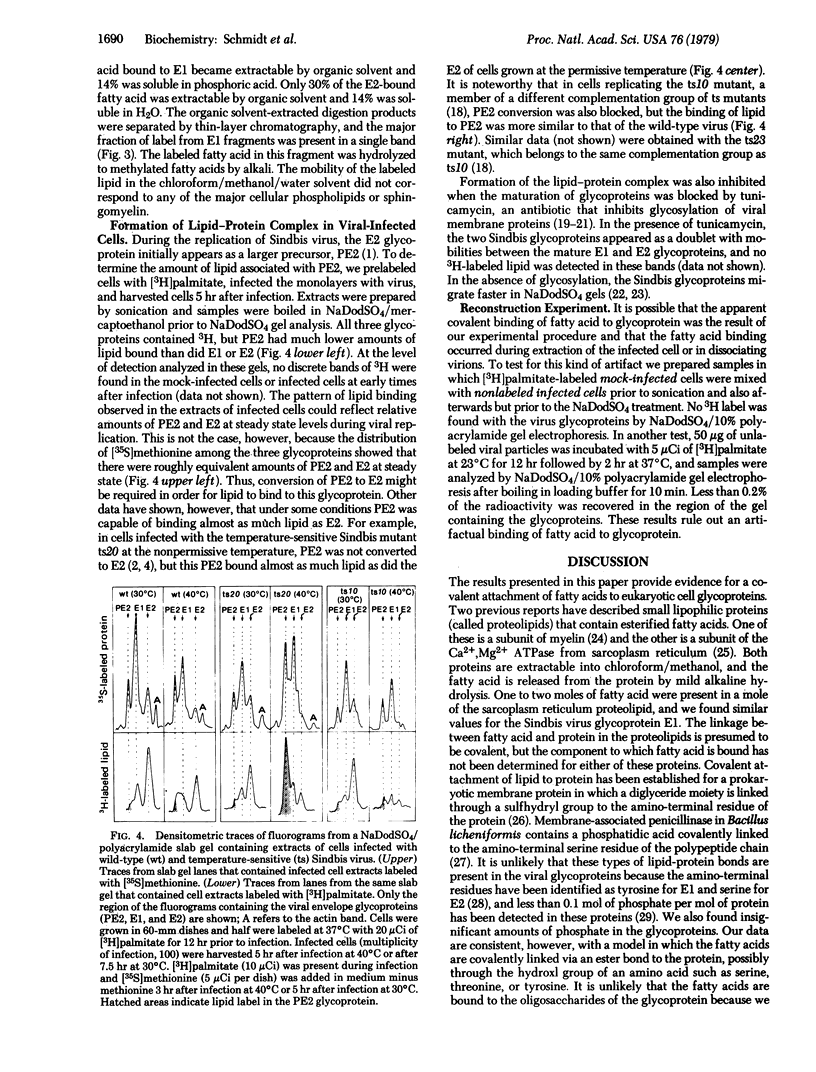
Selective binding of lipid to glycoprotein was detected when [3H]palmitate-labeled Sindbis virus particles or viral-infected cells were disrupted by heating with sodium dodecyl sulfate, and glycoproteins were isolated by electrophoresis in sodium dodecyl sulfate/10% polyacrylamide gels. The smaller glycoprotein (E2) retained 2 to 3 times more labeled lipid than did the larger EI glycoprotein, and the cell-associated glycoprotein precursor (PE2) bound even less lipid. No lipid was associated with the nonglycosylated glycoproteins that accumulated in infected cells treated with tunicamycin. The labeled lipid remained bound to the glycoproteins after exhaustive extraction with chloroform/methanol of virus particles, infected-cell extracts, or isolated glycoproteins, but it could be extracted by chloroform/methanol after treating glycoproteins with mild alkali. Analysis by gas/liquid chromatography showed that 60% of the label was in palmitate and the balance of label was distributed between oleate and stearate. There were approximately 2 mol of fatty acid bound per mol of E1 glycoprotein. Proteolysis of the fatty acid-labeled glycoprotein with pepsin, thermolysin, and Pronase degraded the polypeptide to fragments that retained the fatty acids in an alkali-labile state. These data suggest that a covalent attachment of fatty acid may occur during maturation of the viral glycoproteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. R., Hunkapiller M. W., Hood L. E., Strauss J. H. Amino-terminal sequence analysis of the structural proteins of Sindbis virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2722–2726. doi: 10.1073/pnas.75.6.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Sagher D., Schlesinger M. J. Reaction of the protease inhibitor p-nitrophenyl-p'-guanidinobenzoate with Sindbis virus. Virology. 1977 Dec;83(2):246–253. doi: 10.1016/0042-6822(77)90169-6. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Inhibition of Sindbis virus replication by zinc ions. Virology. 1976 Jul 1;72(1):272–277. doi: 10.1016/0042-6822(76)90330-5. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Complementation between temperature-sensitive mutants of Sindbis virus. Virology. 1966 Oct;30(2):214–223. doi: 10.1016/0042-6822(66)90097-3. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Keegstra K. Purification and composition of the proteins from Sindbis virus grown in chick and BHK cells. J Virol. 1976 Dec;20(3):676–686. doi: 10.1128/jvi.20.3.676-686.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman M. W., Hirschberg C. B. Biosynthesis of phytosphingosine by the rat. J Biol Chem. 1977 Aug 25;252(16):5815–5819. [PubMed] [Google Scholar]
- Duda E., Schlesinger M. J. Alterations in Sindbis viral enbelope proteins by treating BHK cells with glucosamine. J Virol. 1975 Feb;15(2):416–419. doi: 10.1128/jvi.15.2.416-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Garoff H., Söderlund H. The amphiphilic membrane glycoproteins of Semliki Forest virus are attached to the lipid bilayer by their COOH-terminal ends. J Mol Biol. 1978 Sep 25;124(3):535–549. doi: 10.1016/0022-2836(78)90186-9. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Jones K. J., Waite M. R., Bose H. R. Cleavage of a viral envelope precursor during the morphogenesis of Sindbis virus. J Virol. 1974 Apr;13(4):809–817. doi: 10.1128/jvi.13.4.809-817.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polokoff M. A., Bell R. M. Limited palmitoyl-CoA penetration into microsomal vesicles as evidenced by a highly latent ethanol acyltransferase activity. J Biol Chem. 1978 Oct 25;253(20):7173–7178. [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Schmidt M. F., Anwer U., Klenk H. D. Carbohydrates of influenza virus. I. Glycopeptides derived from viral glycoproteins after labeling with radioactive sugars. J Virol. 1977 Aug;23(2):217–226. doi: 10.1128/jvi.23.2.217-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffyn P., Folch-Pi J. On the type of linkage binding fatty acids present in brain white matter proteolipid apoprotein. Biochem Biophys Res Commun. 1971 Jul 2;44(1):157–161. doi: 10.1016/s0006-291x(71)80172-9. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Lubin M., Jones K. J., Bose H. R. Phosphorylated proteins of Sindbis virus. J Virol. 1974 Jan;13(1):244–246. doi: 10.1128/jvi.13.1.244-246.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Lampen J. O. The hydrophobic membrane penicillinase of Bacillus licheniformis 749/C. Characterization of the hydrophilic enzyme and phospholipopeptide produced by trypsin cleavage. J Biol Chem. 1976 Jul 10;251(13):4102–4110. [PubMed] [Google Scholar]