Abstract
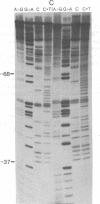
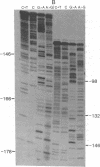
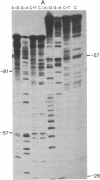
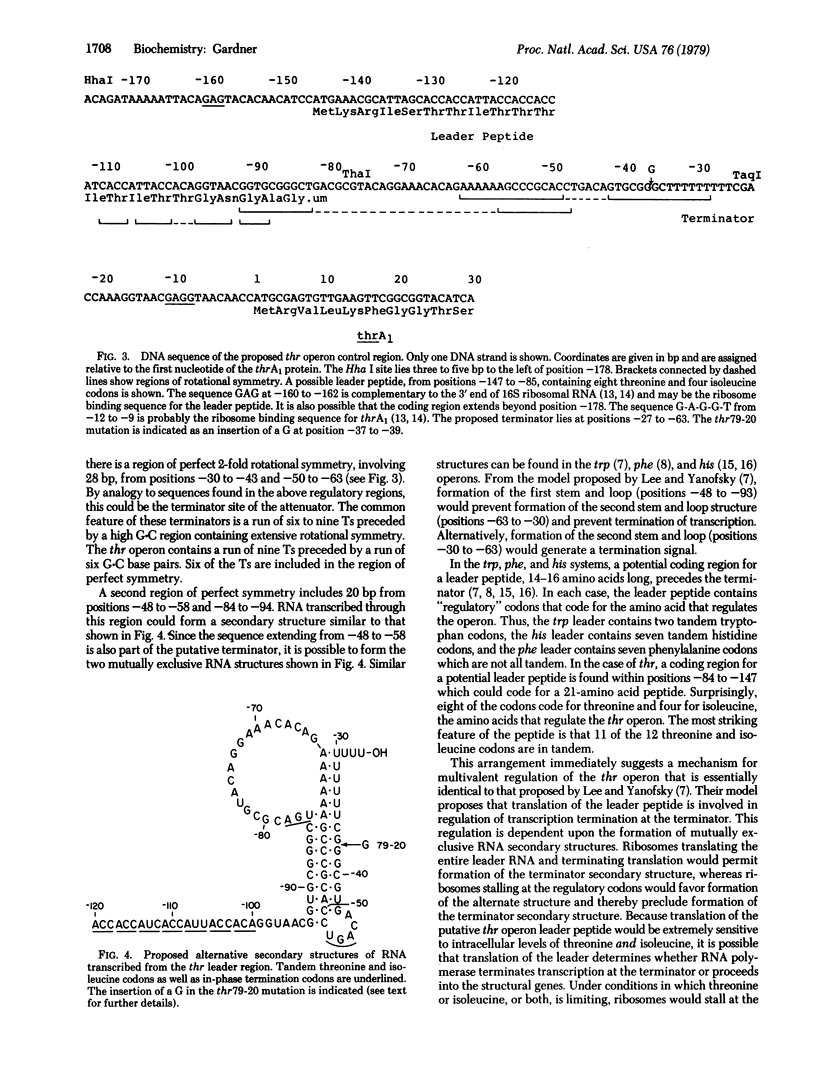
The DNA sequence of 178 base pairs preceding the first structural gene of the threonine operon of Escherichia coli has been determined. A region of perfect 2-fold rotational symmetry, involving 28 base pairs, precedes the first structural gene. The structural similarity of this sequence to known RNA polymerase termination sites suggests that this region is the termination site of the threonine operon leader RNA. Moreover a mutation (thr 79-20), which confers a depressed, constitutive phenotype, was sequenced and found to be a G.C insertion in the putative terminator. A potential coding region for a 21-amino acid leader peptide ends approximately 18 base pairs before the terminator. This peptide contains eight threonine and four isoleucine codons. Eleven of these codons are in tandem. A model for threonine operon regulation, involving alternative secondary RNA structures and translation of leader RNA, is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Blasi F., Di Lauro R., Frunzio R., Bruni C. B. Nucleotide sequence of the attenuator region of the histidine operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4276–4280. doi: 10.1073/pnas.75.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Gardner J. F., Reznikoff W. S. Identification and restriction endonuclease mapping of the threonine operon regulatory region. J Mol Biol. 1978 Dec 5;126(2):241–258. doi: 10.1016/0022-2836(78)90361-3. [DOI] [PubMed] [Google Scholar]
- Gardner J. F., Smith O. H., Fredricks W. W., McKinney M. A. Secondary-site attachment of coliphage lambda near the thr operon. J Mol Biol. 1974 Dec 25;90(4):613–631. doi: 10.1016/0022-2836(74)90528-2. [DOI] [PubMed] [Google Scholar]
- Gardner J. F., Smith O. H. Operator-promoter functions in the threonine operon of Escherichia coli. J Bacteriol. 1975 Oct;124(1):161–166. doi: 10.1128/jb.124.1.161-166.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Chow L., Helinski D. R. Characterization of a mini-ColC1 plasmid. J Bacteriol. 1976 Apr;126(1):447–453. doi: 10.1128/jb.126.1.447-453.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., Poralla K., Zachau H. G., Zähner H. Stoffwechselprodukte von Mikroorganismen. 51. Uber die Wirkungsweise von Borrelidin-Hemmung des Threonineinbaus in sRNA. Biochem Z. 1966 Mar 28;344(2):190–196. [PubMed] [Google Scholar]
- Johnson E. J., Cohen G. N., Saint-Girons I. Threonyl-transfer ribonucleic acid synthetase and the regulation of the threonine operon in Escherichia coli. J Bacteriol. 1977 Jan;129(1):66–70. doi: 10.1128/jb.129.1.66-70.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G., Poralla K., Zähner H. Effect of the antibiotic Borrelidin on the regulation of threonine biosynthetic enzymes in E. coli. Biochem Biophys Res Commun. 1969 Jan 6;34(1):84–91. doi: 10.1016/0006-291x(69)90532-4. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Margarita D. Operator-constitutive mutants in the threonine operon of Escherichia coli K-12. J Bacteriol. 1975 Dec;124(3):1137–1141. doi: 10.1128/jb.124.3.1137-1141.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I. New regulatory mutations affecting the expression of the threonine operon in Escherichia coli K-12. Mol Gen Genet. 1978 Jun 1;162(1):95–100. doi: 10.1007/BF00333855. [DOI] [PubMed] [Google Scholar]
- Sato S., Hutchinson C. A., 3rd, Harris J. I. A thermostable sequence-specific endonuclease from Thermus aquaticus. Proc Natl Acad Sci U S A. 1977 Feb;74(2):542–546. doi: 10.1073/pnas.74.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thèze J., Saint-Girons I. Threonine locus of Escherichia coli K-12: genetic structure and evidence for an operon. J Bacteriol. 1974 Jun;118(3):990–998. doi: 10.1128/jb.118.3.990-998.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]