Abstract
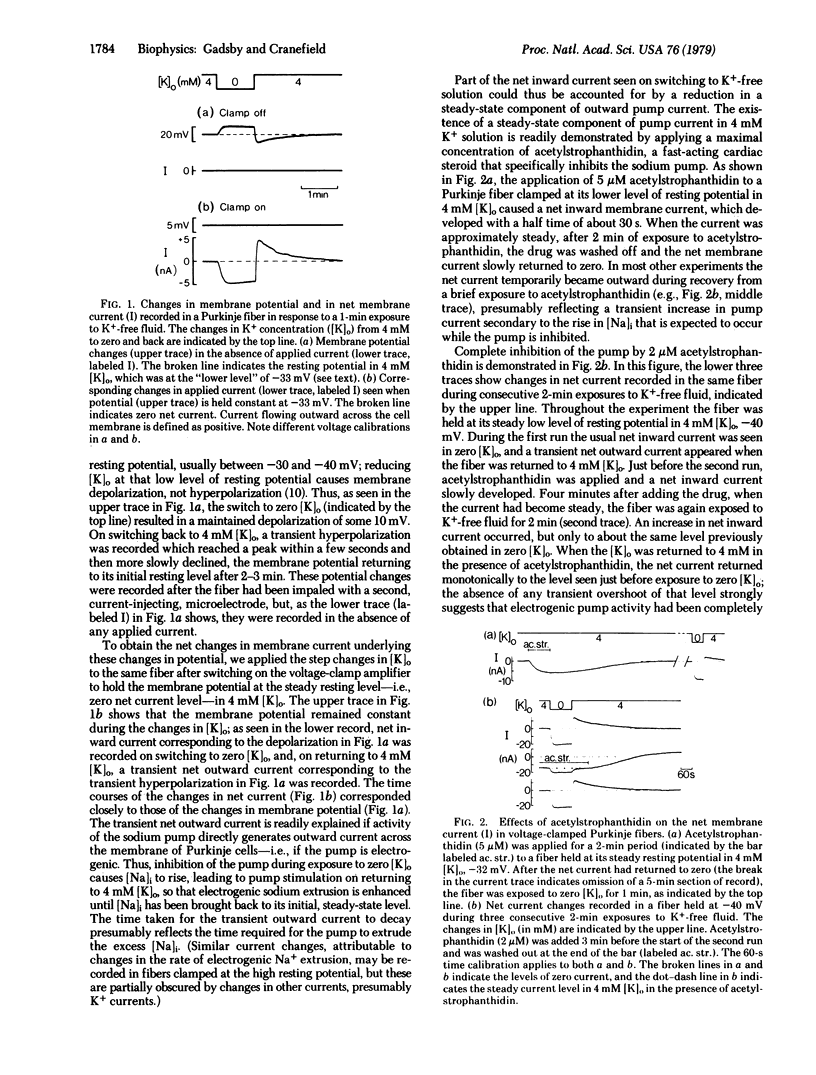
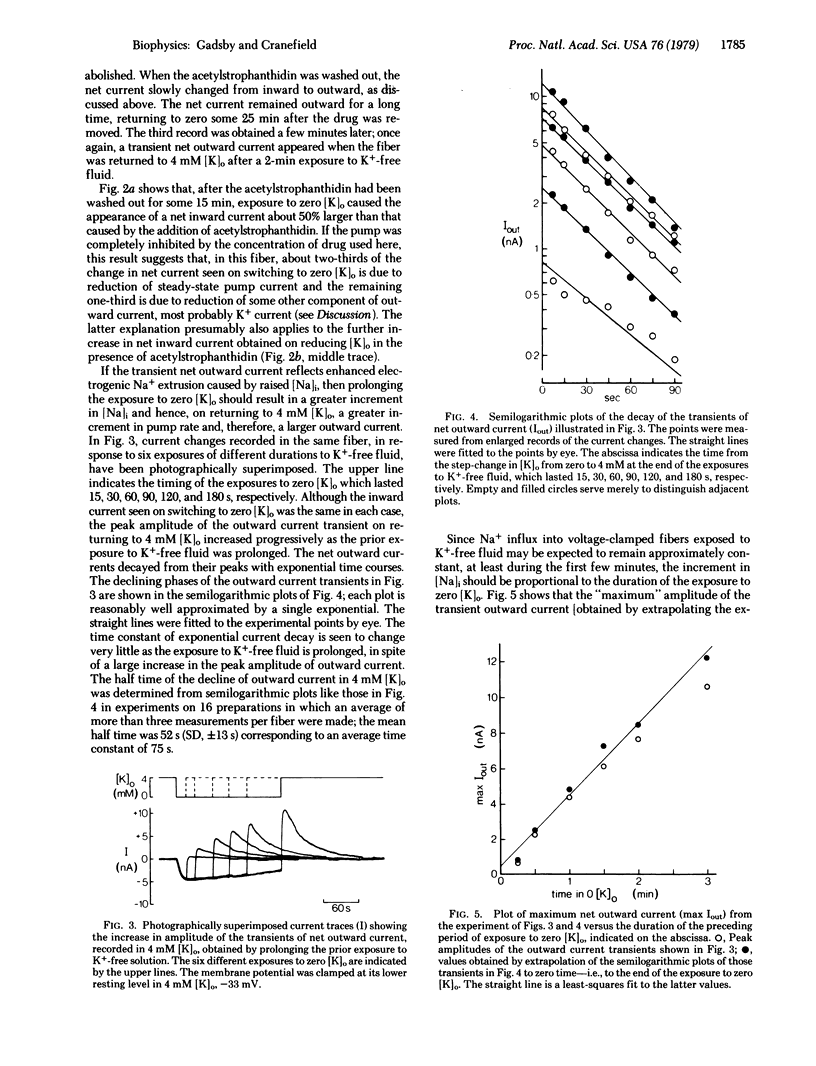
Purkinje fibers from dog hearts may have either a “high“ resting potential of about -90 mV or a “low“ resting potential of about -40 mV when immersed in low-Cl- solution containing 4 mM K+. Brief exposure of Purkinje fibers at the low level of resting potential to K+-free fluid causes further depolarization, and return to K+-containing solution elicits a transient hyperpolarization which reaches a peak within a few seconds and then declines within a few minutes. Repeating these changes in K+ concentration after clamping the membrane potential at its steady resting level in K+-containing fluid allows the changes in net membrane current presumably underlying the depolarization and transient hyperpolarization to be measured. Net inward current is recorded when the fiber is exposed to K+-free solution, and a transient net outward current arises when it is returned to K+-containing solution. The transient net outward current reflects a temporary increase in the rate of electrogenic Na+ extrusion caused by the rise in intracellular Na+ concentration that occurs while the sodium pump is slowed in K+-free fluid. Sodium extrusion remains enhanced, presumably until the internal Na+ concentration has been brought back to its resting level. The transient outward current is completely abolished by the cardiac steroid acetylstrophanthidin, and its amplitude is increased as the prior exposure to K+-free fluid is prolonged. The decay of the transient outward current and the decline in intracellular Na+ concentration both appear to follow first-order kinetics.
Keywords: electrogenic sodium extrusion, voltage clamp, acetylstrophanthidin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke I. M., Leblanc G., Tauc L. Sodium pump stoichiometry in Aplysia neurones from simultaneous current and tracer measurements. Nature. 1974 Sep 20;251(5472):254–256. doi: 10.1038/251254a0. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Two levels of resting potential in cardiac Purkinje fibers. J Gen Physiol. 1977 Dec;70(6):725–746. doi: 10.1085/jgp.70.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol. 1956 Mar 28;131(3):592–616. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. Temperature sensitivity of outward current in cardiac Purkinje fibers. Evidence of electrogenicity of active transport. Pflugers Arch. 1975 Jul 28;358(3):225–234. doi: 10.1007/BF00587219. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. Contribution of an electrogenic sodium pump to the membrane potential in rabbit sinoatrial node cells. Pflugers Arch. 1975 Aug 12;358(4):289–301. doi: 10.1007/BF00580527. [DOI] [PubMed] [Google Scholar]
- Noma A., Irisawa H. Electrogenic sodium pump in rabbit sinoatrial node cell. Pflugers Arch. 1974;351(2):177–182. doi: 10.1007/BF00587436. [DOI] [PubMed] [Google Scholar]
- PAGE E., GOERKE R. J., STORM S. R. CAT HEART MUSCLE IN VITRO. IV. INHIBITION OF TRANSPORT IN QUIESCENT MUSCLES. J Gen Physiol. 1964 Jan;47:531–543. doi: 10.1085/jgp.47.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]


