Abstract
Tick salivary gland (SG) proteins possess powerful pharmacologic properties that facilitate tick feeding and pathogen transmission. For the first time, SG transcriptomes of Ixodes ricinus, an important disease vector for humans and animals, were analyzed using next-generation sequencing. SGs were collected from different tick life stages fed on various animal species, including cofeeding of nymphs and adults on the same host. Four cDNA samples were sequenced, discriminating tick SG transcriptomes of early- and late-feeding nymphs or adults. In total, 441,381,454 pyrosequencing reads and 67,703,183 Illumina reads were assembled into 272,220 contigs, of which 34,560 extensively annotated coding sequences are disclosed; 8686 coding sequences were submitted to GenBank. Overall, 13% of contigs were classified as secreted proteins that showed significant differences in the transcript representation among the 4 SG samples, including high numbers of sample-specific transcripts. Detailed phylogenetic reconstructions of two relatively abundant SG-secreted protein families demonstrated how this study improves our understanding of the molecular evolution of hematophagy in arthropods. Our data significantly increase the available genomic information for I. ricinus and form a solid basis for future tick genome/transcriptome assemblies and the functional analysis of effectors that mediate the feeding physiology and parasite-vector interaction of I. ricinus.—Schwarz, A., von Reumont, B.M., Erhart, J., Chagas, A.C., Ribeiro, J.M.C., Kotsyfakis, M. De novo Ixodes ricinus salivary gland transcriptome analysis using two next-generation sequencing methodologies.
Keywords: high-throughput annotation, molecular evolution, cDNA, public database, tick life stages, tick feeding
The most common tick species in Europe, Ixodes ricinus, serves as a vector of different human and animal pathogens, such as piroplasms (e.g., Babesia and Theileria spp.; ref. 1), viruses (e.g., louping ill virus and tick-borne encephalitis virus; refs. 2, 3) and bacteria (e.g., Anaplasma, Rickettsia and Borrelia spp.; ref. 4). Over recent decades, increases in I. ricinus abundance and prevalence of I. ricinus transmitted pathogens, as reported from Germany (5) and Sweden (6), have led to an increased risk of human exposure to tick bites and increased infection incidences of related vector-borne diseases. Changes in abiotic and biotic factors such as the microclimate, habitat, host cenosis, and human activities are assumed to have caused changes in the enzootic cycle of I. ricinus (7, 8).
Hard (Ixodidae) and soft ticks (Argasidae) are successful ectoparasites due to their morphological and physiological adaptations to survive off the host and to attach for a prolonged time to the host during feeding (9). These two major tick families have evolved different feeding strategies; namely, hard ticks feed for a prolonged time on their hosts, and soft ticks usually complete a blood meal in <1 h (10). Nevertheless, protracted attachment and blood feeding is possible in both tick families because they have evolved—like other hematophagous arthropods—a wide range of salivary proteins that are injected into their hosts while feeding to counteract vertebrate hemostasis (11), inflammation, and immunity (9, 12). These proteins are injected into the host throughout the entire feeding period and facilitate tick feeding-cavity maintenance and transmission of pathogens (13, 14).
Nowadays, next-generation sequencing technologies have replaced classical Sanger DNA sequencing techniques. They have revolutionized the field of genomics and transcriptomics, enabling high-throughput DNA sequencing that facilitates projects of entire genomes and deep coverage of transcriptomes (15). In the present study, we employed, for the first time, a combination of high-throughput 454 pyrosequencing and Illumina sequencing to study the SG transcriptome of I. ricinus and thus to greatly increase the genomic information about this important tick disease vector in Europe. The 454 pyrosequencing allowed us to obtain relatively long sequence read lengths (up to 500 bp) in combination with a high number of sequencing reads obtained by Illumina sequencing (∼68×106 assembled reads) when compared to the previous classical Sanger sequencing project of the I. ricinus transcriptome (16). Accordingly, different de novo dataset assemblies were produced in the absence of a reference genome for I. ricinus.
It has been proposed that different tick feeding conditions might affect the salivary gland (SG) proteome of hard ticks (17). Different tick feeding conditions (different vertebrate hosts, developmental stages) can lead to changes in salivary transcriptome dynamics, as already shown for the sister tick species Ixodes scapularis (18). Thus, to increase the transcript repertoire in our samples and compared to Chmelar et al. (16), SGs were dissected from adults and nymphs that were fed on various animal species under different feeding conditions, including cofeeding experiments. For our study, all ticks were collected from nature, because genetic variability (decreased polymorphism) might be lost in inbreeding species, similar to the inbreeding depression that has been reported for laboratory colonies inbreeding for several generations (19, 20). Only 3 tick sialome studies, from I. scapularis (18), Ixodes pacificus (21), and Hyalomma marginatum rufipes (22), followed the same approach in using only ticks from the field.
Our tick-feeding strategy and sample preparation combined with the technical advances of next-generation methodologies yielded large numbers of novel SG transcripts from nymphal and female I. ricinus and thus allowed us an approximation of the I. ricinus transcriptome dynamics on attachment to the host and until blood meal completion. Our large array of SG transcripts is publicly available in the U.S. National Center for Biotechnology Information (NCBI) database, and the extensive SG transcript annotations are also presented in the Supplemental Material. Moreover, we phylogenetically reconstructed the relationship of two relatively abundant I. ricinus SG-secreted protein families, the basic tail and Kunitz domain families, previously shown to modulate the vertebrate hemostatic immune response (23, 24) in order to demonstrate how our data improves our understanding of the molecular evolution of hematophagy in arthropods.
MATERIALS AND METHODS
Tick feeding experiments
Nymphal and adult (females, males) I. ricinus were collected in the Branišov forestal area of České Budějovice, Czech Republic. A total of 360 nymphs was divided into 3 groups of 120 nymphs, of which the first group was fed on 1 laboratory rabbit (Velaz, Únětice, Czech Republic), the second group on 2 guinea pigs (60 nymphs/guinea pig) obtained from a private guinea pig breeder (Ševětí, Czech Republic), and the third group on 6 mice (20 nymphs/mouse, AnLab, Prague, Czech Republic). Similarly, 90 female I. ricinus were divided into 3 groups of 30 females, of which the first and second groups were fed on a rabbit and a guinea pig, respectively, and the third group of females was allowed to feed once on 10 mice (3 females/mouse).
The remaining 420 nymphs and 96 females were used in cofeeding experiments. Therefore, one group of 120 nymphs and 30 females was fed on a laboratory rabbit. A second group of 120 nymphs and 30 females cofed on 2 guinea pigs (60 nymphs and 15 females/guinea pig), and a third group of 180 nymphs and 36 females was fed on 18 mice (10 nymphs and 2 females/mouse). In all experiments with female I. ricinus, females were always fed in the presence of equal numbers of male ticks in order to mate on the host, although the introduction of males may not reflect completely the mating behavior of I. ricinus (on—but more often off—host mating is common; ref. 25). Mating of ixodid ticks, such as Ixodes hexagonus or Dermacentor variabilis, accelerates the rate of blood feeding until repletion, among other things (26, 27), which was also observed for I. ricinus females when mating while blood feeding on the host in our experiments. Thus, we could ensure that all ticks had fed until blood meal completion. All animal experiments presented in this study were in accordance with the Animal Protection Law of the Czech Republic (§17, Act No. 246/1992 Sb) and with the approval of the Akademie Věd České Republiky (approval no. 161/2010).
SG dissections and total RNA isolation
After attachment of ticks to the animals, groups of either 15 nymphal and/or 3 female ticks were removed from the animals after 6, 12, and 24 h, followed by additional tick collections every 24 h until nymphs (after 7 d) and females (between 3 and 4 d) dropped off the experimental animals. SGs of all collected ticks were immediately dissected in sterile DEPC/PBS buffer and transferred into lysis buffer RA1 of the NucleoSpin RNA XS kit (Macherey-Nagel, Düren, Germany). A total of 180 female and 630 nymphal SG pairs were dissected, and the glands were stored at −70°C.
Since we were especially interested in the discovery of transcripts expressed shortly after tick attachment, namely early during blood feeding, compared to all other transcripts expressed during the entire feeding period, we set up RNA samples from nymphal and adult ticks discriminating between the early and late phase of tick feeding according to the definition previously used in the Sanger-sequenced I. ricinus transcriptome study (16). Accordingly, 4 pooled total RNA samples were prepared for cDNA library constructions and sequencing following the NucleoSpin RNA XS kit manufacturer's instructions. The first RNA sample, from early-feeding nymphs (EN), contained SG RNA from nymphs (360 SG pairs) attached to animals during the early phase of feeding from 6 to 24 h as a 24-h time period was defined by Chmelar et al. (16), including unfed nymphs (Supplemental Fig. S1). Accordingly, the second sample of total RNA, from early-feeding adults (EA), was obtained from RNA of female adult ticks (90 SG pairs) that were fed between 6 and 48 h, including unfed females. The period of female feeding was prolonged up to 48 h because females searched much longer on the host for an attractive attachment site compared to nymphs during our experiments and thus initiated gene expression in the SGs later. The third and fourth RNA samples, from late-feeding nymphs (LN) and late-feeding adults (LA), contained SG RNA from nymphs collected between 2 and 4 d (including naturally dropped off ticks; 270 SG pairs) during the late period of tick feeding and from adult females that fed between 3 and 7 d (90 SG pairs) including dropped off ticks, respectively (Supplemental Fig. S1). Total RNA concentration of all 4 samples was measured using the NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and the RNA of each sample was precipitated with ethanol using a final concentration of 0.3 M Na-acetate, pH 5.2. Precipitated RNA was overlaid with 80% ethanol and stored at −70°C.
cDNA library constructions and next-generation sequencing
Precipitated total SG RNA samples were further processed for cDNA library constructions by Bio S&T Inc. (Montreal, QC, Canada). A total of 16 μg EA RNA, 10 μg of LA RNA, 4.2 μg of EN RNA, and 3 μg of LN RNA was used for cDNA synthesis, respectively. A modified SMART cDNA library construction method (Clontech Laboratories, Mountain View, CA, USA) was carried out (28), and driver and tester cDNA populations were normalized as described elsewhere (29, 30).
Next-generation sequencing of the 4 SG cDNA libraries (EN, EA, LN, LA) was carried out by the Genomic Sciences Laboratory of the North Carolina State University (Raleigh, NC, USA). In total, 1 μg of each cDNA library was used to prepare 454 cDNA sequencing libraries according to the GS FLX Titanium Roche Rapid Library Preparation kit (Roche, Florence, SC, USA). Similarly, 1 μg was used to generate cDNA libraries for Illumina sequencing following the standard TruSeq DNA v1 sample preparation guide (Illumina, San Diego, CA, USA). For 454 pyrosequencing, all 4 SG cDNA libraries were sequenced with a Roche GS FLX instrument. In total, 441,903 reads with an average read length of 362 bp were obtained by 454 pyrosequencing. All Illumina cDNA libraries were sequenced on the Illumina's Genome Analyzer IIx, and the resulting fastq libraries contained ∼1.3 Gb of sequences with a minimum quality score of Q20 across 11.9 × 106 108-bp reads. A detailed description of the cDNA library constructions and next generation sequencing is given in Supplemental Text S1.
Assembly and annotation of next-generation sequencing data
The assembly of the 454 pyrosequences was performed as described previously (31). All raw Illumina sequences were trimmed off sequences with low quality (Q<10, average <10 bases). Short-read sequences were assembled using the ABYSS assembler with the ABYSS-P option (32, 33). The resulting fasta files were trimmed off adapter sequences, combined, and processed (blastn/CAP3 cycles) as described previously for the assembly of the pyrosequencing data (31). Finally, the 454 and Illumina assemblies were again combined and processed as described by Karim et al. (31), resulting in the final contig assembly used in this work.
The contigs of the assembled expressed sequence tags (ESTs) were annotated as described previously (31). All annotated contigs are compiled in a hyperlinked Excel spreadsheet (Microsoft, Redmond, WA, USA) that can be accessed in Supplemental File S1, and all coding sequences (CDSs) of Supplemental File S1 were extracted and deposited into a new hyperlinked Excel spreadsheet (Supplemental File S2).
The individual reads from the 454 or Illumina platforms were blasted against the DNA sequences with a word size of 25 characters (−W 25 blastn switch) and a recovery of a maximum of 3 matches (−v3 switch) using the tabular blastn format (−m 8 switch) and by employing a parallel array of blasting machines. The resulting matches were mapped into the contigs if they showed a ≥97% sequence identity with one gap allowance. If more than one match was found with the same score as the best score, then this result was also mapped to the corresponding sequence, with a maximum of 3 events. Because many sequences represent splice variants and very similar gene products, some short Illumina reads could be mapped into more than one contig. A detailed description of the assembly and annotation of the sequencing data is provided in Supplemental Text S1.
Calculation and comparison of contig and transcript representation between the 4 SG libraries
χ2 tests were performed based on the number of reads of each contig from the different SG libraries (Supplemental File S3). Differences in the number of reads of each contig between the different libraries were accepted as statistically significant if P < 0.05 and if the minimum expected value for any category was ≥5. These statistical tests were implemented as macros in the Excel spreadsheets (Supplemental Files S1 and S2).
Three different comparisons were carried out between the 4 Illumina libraries: the total number of contigs per protein family of each library was compared; the total number of sequencing reads per protein family of each library was compared; and the total number of library-specific contigs was calculated and compared among the different libraries. See Supplemental Text S1 for a detailed description of the calculations for all 3 analyses.
Phylogenetic reconstruction of secreted protein families
Full-length basic tail and Kunitz domain protein sequences (including translated start and stop codons) of hematophagous arthropods, arachnids, and nematodes from GenBank (NCBI; http://www.ncbi.nlm.nih.gov/genbank/), as well as CDSs from Supplemental File S2, were used for phylogenetic reconstructions; only CDSs that were represented ≥10-fold more frequently in a certain SG library (EN, EA, LN, LA) were included in the analyses. As an exception, truncated sequences were also included if they were highly represented in any of the 4 libraries (from 1000- to 15,000-fold higher overrepresented). All basic tail and Kunitz domain protein sequences were separately aligned using a homology and structure profile alignment strategy based on published protein sequences, secondary protein structures, and 3D protein structures. The sequences were aligned using PROMALS3D (34) and/or MAFFT with the iterative refinement method (L-INS-I or E-INS-I) and the BLOSUM 62 matrix (35). The best-fitting amino acid substitution model for each alignment was inferred applying Prottest 3.0 (36). The WAG model (37) with γ-distributed heterogeneity rates was used for both protein families, and estimated empirical residue frequencies were additionally assumed for the phylogenetic analysis of the Kunitz domain family. Phylogenetic trees were reconstructed under the maximum likelihood optimality criterion using RAxML HPC-PTHREADS 7.3.0 (38). Tree searching and bootstrapping were performed simultaneously (−f a, 1000 bootstrap replicates).
Bayesian phylogenetic analyses were also performed using the parallel computing mpi version of MrBayes 3.1 (39). Amino acid substitution models were chosen as listed above, and each protein family analysis was carried out with 4 independent Markov-chain Monte Carlo (MCMC) chains and 107 generations. The starting trees were randomly generated, and the trees were sampled every 1000 generations until the average sd of split frequencies was stationary and <0.01. Distribution of log likelihood scores and MCMC summary data were examined using Tracer 1.5 (http://tree.bio.ed.ac.uk/software/tracer). Posterior probabilities were estimated on the final 9 × 106 trees (burn-in=106 trees).
RESULTS
Comparison of 454 pyrosequencing and Illumina data assemblies
In total, 441,381,454 pyrosequences (after adapter primer and vector sequence removal) with an average read size of 518 nt and 228,684,681 nt residues were produced from all 4 SG libraries (EN, LN, EA, LA; Table 1). Of these, 120,373,454 pyrosequencing reads corresponded to the EN library (average read length: 371 nt), and 67,441 reads corresponded to the EA library (average read length: 358 nt). A further 72,256 reads (average read length: 346 nt) derived from the LN library and the LA library yielded 181,833 reads (average read length: 372 nt). All 454 pyrosequencing reads were assembled into 93,331 contigs and singletons. The assembly of 67,703,183 Illumina sequences with an average read size of 90 nt (after trimming off any low-quality bases) and 6,075,545,289 total nucleotide residues resulted in 269,600 contigs (Table 1).
Table 1.
Raw sequence data and assembly characteristics
| Contigs and sequences | Dataset |
||
|---|---|---|---|
| 454 | Illumina | 454+Illumina | |
| Reads | 441,381 | 67,703,183 | |
| Total nucleotide residues | 228,684,681 | 6,075,545,289 | |
| Total contigs | 93,331 | 269,600 | 272,220 |
| Total contig residues | 60,450,377 | 54,028,787 | 84,935,511 |
| Contig sequence size | |||
| <100 nt | 965 | 40,518 | 38,463 |
| 101–200 nt | 2,062 | 166,150 | 136,409 |
| 201–300 nt | 1,818 | 25,069 | 13,358 |
| 301–400 nt | 3,549 | 12,482 | 6,920 |
| 401–500 nt | 14,426 | 7,472 | 11,591 |
| 501–600 nt | 30,088 | 4,690 | 22,745 |
| 601–700 nt | 13,456 | 2,971 | 12,155 |
| 701–800 nt | 8,814 | 2,071 | 8,171 |
| 801–900 nt | 6,326 | 1,560 | 6,162 |
| 901–1000 nt | 3,846 | 1,240 | 4,012 |
| 1001–2000 nt | 7,288 | 4,326 | 9,898 |
| 2001–3000 nt | 567 | 874 | 1,795 |
| 3001–5000 nt | 98 | 161 | 450 |
| 5001–10000 nt | 27 | 14 | 72 |
| >10001 nt | 1 | 2 | 19 |
The final assembly of 454 pyrosequencing and Illumina sequences produced 272,220 contigs (Table 1). The combined assembly resulted in an increased contig number with consensus sequences longer than 900 nt compared to the individual (454 pyrosequencing or Illumina) sequencing assemblies. However, decreased numbers of contigs in the read-size range of 400–900 nt were present in the combined assembly compared to the individual 454 pyrosequencing assembly, as a consequence of their assembly into higher order contigs (Table 1). Similarly, compared to the individual Illumina assembly, fewer contigs in the read-size range of 100–300 nt were produced in the combined 454 pyrosequencing and Illumina assembly. Thus, the final combined 454 pyrosequencing and Illumina sequence assembly resulted in an improved quantitative and qualitative dataset compared to the individual assemblies of each sequencing dataset. From all 272,220 contigs of the combined sequencing assembly, 82,907 contigs were extracted containing sequences of a minimal length of 150 nt, ≥3 reads from the 454 pyrosequencing SG libraries, or ≥5 reads from the Illumina libraries. The hyperlinked and annotated spreadsheet containing the 82,907 contigs can be browsed in Supplemental File S1. In total, 34,560 CDSs were extracted from those 82,907 contigs and annotated (Supplemental File S2). Finally, a total of 8686 CDSs were submitted to GenBank [Short Read Archives (SRA), BioProject PRJNA177622].
Functional annotation of SG transcripts: a brief overview of the publicly available sequence databases
All 82,907 contigs from the combined 454 pyrosequencing and Illumina assemblies were functionally annotated using an automated pipeline (Supplemental File S1). A detailed description of the annotated database is provided in Supplemental Text S2. All contig-encoded proteins were classified into 25 main protein families based on Basic Local Alignment Search Tool (BLAST; NCBI) searches against a set of different public databases. The contigs of Supplemental File S1 can be conceptually grouped into 3 categories (Fig. 1 and Supplemental Table S1): unknown housekeeping products and transposable elements (TEs; nonsecreted proteins); secreted proteins; and sequences of pathogens.
Figure 1.

Functional classification of contig-encoded I. ricinus proteins. Pie chart represents the functional classification of all contig-encoded I. ricinus proteins from the combined assembly of the Illumina and 454 sequencing datasets of all 4 SG libraries (Supplemental File S1). The overall contig proportion of the different protein families (%) is shown, and all secreted SG proteins were further divided into different subfamilies.
Nonsecreted proteins
Briefly, in total, 49,475 of 82,907 contigs (59.7%) and ∼30% of all Illumina reads encoded for unknown products based on the similarity of BLAST searches (Fig. 1 and Supplemental Table S1). The largest group of functionally annotated proteins belonged to housekeeping products representing 23.5% of all contigs (n=19,491) and, similar to the unknown products, 33.6% of all Illumina reads. A minor proportion of contigs (1.3%) with a total of 0.2% Illumina reads in the I. ricinus transcriptome accounted for TEs.
Secreted proteins
Thirteen percent of 82,907 contigs and 34.5% of all Illumina reads were annotated as secreted proteins (Fig. 1 and Supplemental Table S1). Protease inhibitors were the largest group of secreted proteins (2.6% of all contigs) and the most frequent transcripts encoding for secreted proteins (8.6% of all Illumina reads) in the SG transcriptome of I. ricinus.
Sequences of pathogens
Several transcripts possibly originating from pathogens or symbionts were found in the SG of I. ricinus, because all ticks used for this transcriptome study were field-collected and thus could be infected with different disease agents. This is the first tick SG transcriptome that discloses pathogenic sequences in detail (see Supplemental Text S2). Of all transcriptome contigs, 2.5% contigs originated from pathogens (Fig. 1) and mostly encoding for piroplasm proteins (∼80% of all pathogen contigs; Supplemental Table S1) with high similarity to Babesia bovis and Theileria spp.
Supplemental File S2 of the functionally annotated and extracted CDSs provides, in addition to the information given in Supplemental File S1, biochemical and biophysical data about the transcriptome-encoding proteins.
Comparison of transcript representation in the 4 I. ricinus SG Illumina libraries
Our SG transcript data set obtained by Illumina sequencing provides a rough estimate of the I. ricinus SG transcriptome dynamics and by definition (given the already known effects of tick saliva) uncovers transcripts that might mediate hematophagy and/or pathogen transmission in different developmental stages of I. ricinus (Supplemental File S2). Notice that despite library normalization, large differences were frequently found in Illumina read abundance of particular contigs, in particular pairwise library comparisons. The read differences thus should be understood as an underestimation of the real read coverage, and they can serve only as the basis for future studies; no attempts were made to validate the differences of the herein reported abundances by other means, such as qPCR. Our comparisons of the transcript representation between the 4 Illumina libraries are based on statistical tests performed with the Illumina datasets (Supplemental File S3). From these statistical analyses, only contig transcripts that were significantly at least 10-fold more frequently sequenced (10xcontigs) in a certain SG library, e.g., EN library, compared to the pairmate library, e.g., LN library, (10-fold higher read numbers of a contig in one library compared to a second library) were chosen (Supplemental File S2) and compared. After the initial pairwise library comparison, the comparisons were reversed, e.g., LN library vs. EN library. A detailed description of the library comparisons is provided in Supplemental Text S3. To summarize our findings, great statistically significant differences in numbers of contigs and numbers of reads per protein family between nymphal and adult Illumina libraries could be detected (Supplemental File S2, Supplemental Fig. S2, and Supplemental Table S2). Several protein families were exclusively represented in one of the two compared libraries. For example, in the EN vs. LN library comparison of Supplemental Table S3, ∼17% of all 10xcontigs (contigs that were significantly at least 10-fold more frequently sequenced in a certain SG library compared to its mate pair library) of all protein families were EN specific, and thus for these contigs no Illumina reads were obtained by sequencing from the LN library (Supplemental Text S3: see pairwise library comparisons EN vs. LN, LN vs. EN).
Molecular evolution of basic tail salivary effectors
The transcriptome sequences can serve as a basis to study the molecular evolution of tick salivary effectors, and here, we phylogenetically analyzed members of the basic tail and Kunitz domain families. These two secreted protein families belong to the class of protease inhibitors that are the most abundant secreted group of proteins represented in the I. ricinus SG transcriptome. Due to the large amount of transcripts sequenced for these encoding proteins we could perform a thoroughly phylogenetic analysis, especially compared to the number of sequences retrieved by classical Sanger sequencing and then used for phylogenetic reconstructions. Since basic tail and Kunitz domain proteins play an important role in the blood meal acquisition of ticks, their phylogenetic reconstruction within hematophagous arthropods improves our knowledge about the evolution of hematophagy in ticks. The resulting phylogenetic trees also displayed differences in the transcript frequency in the four I. ricinus SG libraries.
The phylogenetic reconstruction of basic tail proteins revealed several clades that were well supported using maximum likelihood (ML) and bayesian methods, but the overall relationship among the different basic tail proteins was not resolved because the nodes of deeper, internal clades on the tree basis were poorly supported (Fig. 2; see Supplemental Fig. S3 for the original bayesian tree). Dipteran proteins were clearly separated from soft and hard tick proteins (Diptera I group) with the exception of a protein from Simulium vittatum and Glossina morsitans morsitans (Diptera II, III) in the ML tree (Fig. 2).
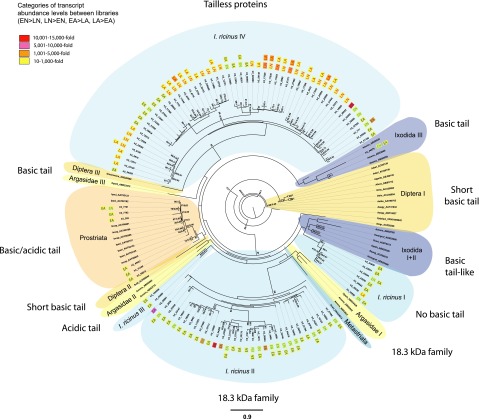
Figure 2.
Phylogram of the basic tail family. Basic tail-encoding protein sequences of different hematophagous arthropods (defined by GenBank accession numbers) and of all 4 SG Illumina libraries that were significantly ≥10-fold more frequent in a certain SG library compared to a second SG library (EN vs. LN, LN vs. EN, EA vs. LA, LA vs. EA) were used for phylogenetic reconstruction by ML and bayesian methods. Abundance levels of each protein in a certain SG library are also shown. The presented ML tree was rooted with Aedes aegypti (GenBank: AAY41832), and only strongly supported nodes with bootstrap values ≥ 50% are given. Bayesian posterior probabilities ≥ 0.5 are also displayed for clade nodes that showed the same topology in the ML and bayesian trees. Scale bar (mean amino acid substitution/site) in the ML tree is at the bottom center of the figure.
Classical basic tail proteins
Only a few proteins possessing the classical basic tail of lysines (K) with 6 cysteine residue proteins appeared in the phylogram (Supplemental Fig. S4). Of these, the largest resolved group was the Prostriata clade, with I. ricinus transcripts encoding for proteins that frequently expressed in the SGs of early-feeding ticks (Fig. 2).
Basic tail-like proteins
Heterogeneous taxa clades of metastriate and soft ticks (Ixodida I and II) consisted of basic tail-like proteins with 6 cysteines, but a less compact basic tail compared to classical basic tail proteins (Fig. 2 and Supplemental Fig. S4). Well supported in the tree was the I. ricinus I clade, with protein encoding transcripts mainly expressed in the EN and EA libraries.
Acidic tail proteins
Acidic tail proteins of the basic tail family with glutamate (E)- and/or aspartate (D)-rich C termini were also present in the I. ricinus sialome and appeared as a sister group in the Prostriata clade (Fig. 2 and Supplemental Fig. S4). In addition, two further clades of I. ricinus (I. ricinus III) and Argas monolakensis (Argasidae II) also included acidic tail proteins; their position in the phylogenetic tree is unclear.
Tailless proteins
The largest and well-resolved group of exclusively I. ricinus proteins (I. ricinus IV) shared with basic tail proteins the conserved cysteine residues, but they were tailless and of shorter amino acid sequence. I. ricinus IV contained mainly transcripts that were highly frequent in the LN and LA libraries (Fig. 2).
18.3-kDa family
The second-largest group of exclusively I. ricinus proteins (I. ricinus II) in the phylogram belonged to the 18.3-kDa family, a subfamily of the basic tail class (Fig. 2). Most proteins did not possess a basic C terminus, and their amino acid sequence was longer compared with classical basic tail proteins (Supplemental Fig. S4). Transcripts (18.3 kDa) appeared only frequently in early-feeding ticks.
Molecular evolution of Kunitz domain salivary effectors
Similar to the phylogenetic analysis of the basic tail family, several monophyletic groups of Kunitz domain proteins were well distinguishable in ML and bayesian analyses, but the relationship on the base between the main clades is not resolved (Fig. 3; see Supplemental Fig. S5 for the original bayesian tree).
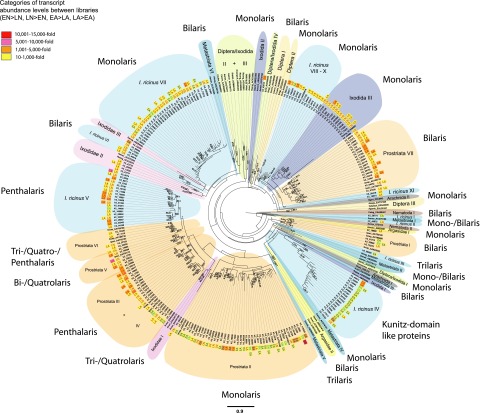
Figure 3.
Phylogram of the Kunitz domain family. Kunitz domain-encoding proteins of hematophagous arthropods, of nematodes (defined by GenBank accession numbers), and of all 4 SG Illumina libraries that were significantly ≥10-fold more frequent in a certain SG library compared to a second SG library (EN vs. LN, LN vs. EN, EA vs. LA, LA vs. EA) were used for phylogenetic reconstruction by ML and bayesian analyses. Abundance levels of each protein from a certain SG library are displayed. The presented ML tree was rooted with Caenorhabditis elegans (GenBank: CAA98467), and only strongly supported nodes with bootstrap values ≥ 50% are shown. Bayesian posterior probabilities ≥ 0.5 are also given for clade nodes that had the same topology in the ML and bayesian phylograms. Scale bar (mean amino acid substitution/site) of the ML tree is at the bottom center of the figure.
Monolaris
Most clades in the phylogram contained proteins with only a single Kunitz domain (Monolaris) of typically 6 cysteine residues that form 3 disulfide bridges (Supplemental Fig. S6). These Monolaris proteins were widespread throughout the different tick species, and they were also found in Diptera (Diptera II, III clades), in Hadrurus gertschi (Arachnida II clade), and in Anisakis simplex (Nematoda II clade) (Fig. 3). Of these, Prostriata II was the largest clade of exclusively tick Monolaris proteins, and most transcripts encoding for these proteins were frequently expressed in the SGs of early-feeding ticks. A few Prostriata II transcripts were frequently detected in the early and late SG libraries, and Ir2_27722 was additionally expressed in different developmental stages (LN, EA libraries). The second-largest tick Monolaris clade was made up of exclusively I. ricinus (I. ricinus VII) proteins with 5 to 6 conserved glycines between the C1 and C2 residues (Supplemental Fig. S6). Transcripts encoding for these Monolaris proteins were mostly expressed late in the tick SGs during blood feeding (Fig. 3).
Bilaris
Bilaris proteins with the classical 2 Kunitz domains (12-cysteine framework) were mainly found in ticks, but also in Diptera (Diptera I clade), in Haplopelma hainanum (Arachnida I clade) and in Caenorhabditis elegans (Nematoda I clade). The largest, well-supported tick Bilaris group in the phylogram represented the monophyletic clade Prostriata VII (Fig. 3). Only Kunitz transcripts expressed late in the SGs during adult feeding were assigned to Prostriata VII.
Trilaris, Quatrolaris, and Penthalaris
Only the Trilaris proteins Ir2_58580 (I. ricinus III) and Dathoxin-3 of Dermacentor andersoni (Metastriata V) featuring 3 Kunitz domains appeared in the Kunitz phylogram, and Quatrolaris Kunitz only clustered together with Bilaris, Trilaris, and Penthalaris (5 Kunitz domain proteins; Prostriata V, VI) proteins in the ML tree (Fig. 3). Both heterogeneous Quatrolaris clades reassembled well supported in the phylogram, and all I. ricinus Kunitz transcripts were lately expressed in the SGs during tick feeding. Penthalaris proteins clustered in 3 clades (Prostriata III, IV; I. ricinus V) and were highly supported in the phylogram (Fig. 3). Most proteins were encoded by transcripts frequently sequenced from the LN and LA libraries.
Kunitz domain-like proteins
A third major I. ricinus clade (I. ricinus IV), which was basically not well supported in the tree, could not be classified as typical Kunitz domain proteins, but their amino acid sequence was similar to Kunitz domain proteins. Interestingly, this I. ricinus IV clade is the second of two tick clades that contained Kunitz transcripts frequently expressed in the SGs of early feeding ticks.
DISCUSSION
Advantages of next-generation sequencing methodologies employed in tick sialome studies
Several salivary transcriptomes (sialomes) from hard and soft ticks (e.g., refs 16, 18, 21, 40–43) were already published. All these transcriptome studies yielded a few thousand ESTs by classical Sanger sequencing, with the exception of the A. maculatum sialotranscriptome study employing 454 pyrosequencing methods (31). Although a total of 72,441 contigs containing 1,498,171 reads were assembled by Karim et al. (31), the SG transcriptome coverage is limited due to the experimental design of the sialome study. The experimental design considered only female adult ticks from a tick-rearing facility, and neither were cofeeding experiments performed nor different host species used in all experiments. Moreover, only one cDNA library was sequenced, and thus the transcriptome dynamics were not analyzed.
A comparison between the transcriptomes of I. ricinus using classical (16) and next-generation sequencing methodologies demonstrates clearly that the higher transcriptome coverage of our sequencing approach changes the current knowledge about the SG transcript dynamics of I. ricinus, this important disease vector. Although ∼30% of all Illumina reads in our study and ∼30% of all ESTs in the previous I. ricinus transcriptome (16) were assigned into the three main classes encoding for secreted, housekeeping, and unknown proteins, both studies varied greatly in the distribution of sequencing reads within these main three classes and in the revealed patterns of gene expression during tick feeding. For example, according to Chmelar et al. (16), the most abundant ESTs, encoding for salivary-secreted proteins, were transcripts encoding for collagen-like proteins (209 ESTs) that were up-regulated during tick feeding. In our transcriptome study, the most frequently sequenced transcripts encoded for lipocalins (5.76% total read proportion of the entire transcriptome), and the second-largest transcript group encoded for metalloproteases (5.04% total read proportion), a group that was negligible by Chmelar et al. (16) (only 18 ESTs). Furthermore, feeding-associated regulation of metalloproteases was not detected in the previous I. ricinus transcriptome, although a strong difference in the frequency of metalloprotease transcripts between early- and late-feeding ticks appeared in our study. The observed differences are clearly explainable due to the different sequencing methodologies, the differences in experimental tick feedings, and the differences in the cDNA library construction methodologies used in the two I. ricinus transcriptome studies.
We considered, for our sequencing approach, different life stages of I. ricinus collected from nature, in contrast to previous studies that mostly used inbred ticks from laboratory colonies. Although inbred species from a colony should be rather ideal for de novo transcriptome assemblies, computer simulations confirmed that even the usage of wild species produce reliable transcriptomes with a realistic polymorphism level (44). To our knowledge, this is the first study of a tick sialome employing 454 pyrosequencing in combination with an Illumina methodology.
A sufficient number of sequence reads employing next-generation sequencing techniques has been proven to produce a robust and reliable transcriptome assembly even in the absence of a reference genome (44); our transcriptome study is another example that supports this observation. As a consequence, our de novo transcriptome assembly can serve as a valuable reference for the assembly and annotation of future tick genome projects or even for improving the annotation of the already publicly disclosed I. scapularis genome, as well as a reference protein database of I. ricinus for future microarray and proteomics studies. Attempts to use transcriptome data to support genome studies are currently carried out for the sequencing of the Rhipicephalus microplus genome. Sequencing of the whole R. microplus genome has turned out difficult due to its large size (estimated 7.1 Gbp) and complexity (more than 70% repetitive DNA) (45). Thus, a consortium of researchers attempts to produce different datasets, e.g., transcriptomes, gene expression data, to assist the assembly the R. microplus genome.
Phylotranscriptomics of important gene families that mediate hematophagy
Next-generation sequencing data provide us with valuable raw material for detailed phylogenetic reconstructions of large gene families. Our datasets contribute to the better understanding of the evolution of gene families that might mediate hematophagy in different blood-feeding arthropods.
Our phylogenetic analysis showed that classical basic tail proteins, which possess the 6-cysteine framework and a basic tail of lysines, are exclusively found in ticks; their C termini appear to be very variable. NP7, a salivary nitrophorin of Rhodnius prolixus that contained a positively charged membrane interaction surface (due to its basic lysine residues), could target the negatively charged surfaces of activated platelets and other cells and thus act as an antihemostatic protein (46). Until now, limited functional data are available about basic tail proteins, and only the protease inhibitors salp9Pac and salp14 of I. scapularis were analyzed to function as anticoagulants (24, 47).
On the contrary, Kunitz domain proteins, such as Monolaris proteins that are widespread throughout hematophagous arthropods, are more intensively studied. To date, about a dozen Monolaris proteins of hard and soft ticks have been functionally characterized that mainly function as anticlotting agents or as antiplatelet inhibitors or ion channel modulators (e.g., refs. 48–51). Our phylogenetic analysis of the Kunitz domain family revealed that multiple Kunitz domain proteins with ≥3 Kunitz domains appeared widely distributed in different tick species. The absence of these multiple Kunitz domain proteins in Diptera or other taxa suggests that these proteins have evolved only in ticks, most likely from Monolaris and Bilaris proteins. Monolaris proteins of I. scapularis clustered extensively with multiple Kunitz domain peptides in a previous analysis (52). These multiple Kunitz domain proteins were split into single-domain peptides, realigned with Monolaris proteins, and phylogenetically reconstructed. Three main Monolaris types (I–III) were identified in I. scapularis (52), and their putative homologs are also present in our I. ricinus SG transcriptome. In the study by Dai et al. (52), Monolaris group I proteins of I. scapularis [-C-X(8)-C-X(15)-C-X(7)-C-X(12)-C-X(3)-C-] clustered with I. scapularis multiple Kunitz domain peptides. In agreement, I. ricinus Monolaris type I homologs coclustered with I. ricinus multiple Kunitz domain proteins, but we also identified various I. ricinus Monolaris that were rather unrelated to the I. ricinus multidomain Kunitz proteins. In our phylogram, several clades of I. ricinus Monolaris type I homologs (I. ricinus VIII-X, Ixodida III, and Prostriata VII) cogrouped with dipteran type I Kunitz homologs (Diptera/Ixodida II-IV and Diptera II). Homologs of these Monolaris type I proteins were also present in an arachnid species and appeared as slightly modified form in a nematode. Similar proteins were also detected in sea anemones (53). Thus, we assume that these Monolaris type I proteins represent the ancestral group of all Kunitz in I. ricinus, as was also suggested for I. scapularis (52). In addition, a new and specific group of I. ricinus Monolaris proteins (I. ricinus VII clade) was discovered in our transcriptome that it was not previously described in I. scapularis (52).
Next-generation sequencing techniques combined with our comprehensive experimental design allowed us the discovery of numerous, novel SG transcripts and thus the expansion of our knowledge about I. ricinus genomics. Our large dataset of annotated transcripts provides a solid platform for future tick genome and transcriptome assemblies and annotations, as well as for the employment of high-throughput methodologies (e.g., proteomics, microarrays) to discover and functionally analyze the effectors that mediate biology, feeding physiology, and parasite-vector interaction of I. ricinus.
Our methodology allowed us to perform detailed comparisons of the SG transcript representation between the nymphal and adult SG libraries. Any user of our publicly disclosed data can have an idea of the transcripts encoding for developmental specific proteins that are relevant to tick physiology because they might facilitate tick feeding, as well as transcripts originating from pathogen species transmitted on tick bites.
Last but not least, the transcriptome data proved to be valuable raw material for the phylogenetic reconstruction of large protein families that are secreted by I. ricinus SGs. Phylogenetic reconstructions using large datasets can improve our understanding of the evolution of these protein families that might mediate hematophagy in different blood-feeding arthropods. In our study, newly discovered proteins of the 18.3-kDa basic tail subfamily or Monolaris proteins of the Prostriata II clade that are overrepresented in the tick SGs during the early stage of blood feeding and are tick-specific (absent in other hematophagous arthropods) can be important antigens toward the development of antitick vaccines and tick exposure markers. They may be additionally of further interest for the development of pharmacologic applications due to their biochemical properties as potential protease inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the Grant Agency of the Czech Republic (GACR; grant P502/12/2409), the Academy of Sciences of the Czech Republic (grant Z60220518), and the 7th Framework Program of the European Union (Marie Curie Reintegration grant PIRG07-GA-2010-268177). A.S. was funded by the Alexander von Humboldt Foundation (Feodor Lynen Research Fellowship), and B.M.V.R. received financial support from the German Research Foundation [DFG; grants WA-530/34 at the Zoologisches Forschungsmuseum Alexander Koenig (ZFMK; Bonn, Germany) and RE-3454/1-1 at the Natural History Museum (London, UK)]. J.M.C.R. was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; USA). M.K. received support from the Academy of Sciences of the Czech Republic (Jan Evangelista Purkyne Fellowship) and the European Molecular Biology Organization [EMBO; Application Short Term Fellowship (ASTF) 482-2012].
The authors thank Petr Kopáček and his research group for the supply of dissection equipment, James J. Valdés and Brenda Rae Marshall (Division of Intramural Research Program Support Staff, NIAID) for English language editing of this manuscript, and the reviewers of this article for their constructive comments. The authors further thank Tomáš Skalický for his bioinformatics support using the MetaCentrum Virtual Organization (Czech Republic). The access to computing and storage facilities owned by parties and projects contributing to the National Grid Infrastructure MetaCentrum, provided under the program Projects of Large Infrastructure for Research, Development, and Innovations (LM2010005) is highly appreciated. The authors are also grateful to Andrea Gocke (National Center for Biotechnology Information, USA) for helping with sequence submission. Because A.C.C. and J.M.C.R. are government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. Rights can be established outside of the United States subject to a government use license.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BLAST
- Basic Local Alignment Search Tool
- CDS
- coding sequence
- EA
- early-feeding adult
- EN
- early-feeding nymph
- EST
- expressed sequence tag
- LA
- late-feeding adult
- LN
- late-feeding nymph
- ML
- maximum likelihood
- SG
- salivary gland
- TE
- transposable element
REFERENCES
- 1. Florin-Christensen M., Schnittger L. (2009) Piroplasmids and ticks: a long-lasting intimate relationship. Front. Biosci. 14, 3064–3073 [DOI] [PubMed] [Google Scholar]
- 2. Mansfield K. L., Johnson N., Phipps L. P., Stephenson J. R., Fooks A. R., Solomon T. (2009) Tick-borne encephalitis virus—a review of an emerging zoonosis. J. Gen. Virol. 90, 1781–1794 [DOI] [PubMed] [Google Scholar]
- 3. Porter R., Norman R. A., Gilbert L. (2012) A model to test how ticks and louping ill virus can be controlled by treating red grouse with acaricide. [E-pub ahead of print] Med. Vet. Entomol. 10.1111/j.1365-2915.2012.01047.x [DOI] [PubMed] [Google Scholar]
- 4. Stanek G. (2009) Pandora's Box: pathogens in Ixodes ricinus ticks in Central Europe. Wien. Klin. Wochenschr. 121, 673–683 [DOI] [PubMed] [Google Scholar]
- 5. Schwarz A., Hönig V., Vavrušková Z., Grubhoffer L., Balczun C., Albring A., Schaub G. A. (2012) Abundance of Ixodes ricinus and prevalence of Borrelia burgdorferi s.l. in the nature reserve Siebengebirge, Germany, in comparison to three former studies from 1978 onwards. Parasit. Vectors 5, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaenson T. G., Jaenson D. G., Eisen L., Petersson E., Lindgren E. (2012) Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors. 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Randolph S. E. (2010) Human activities predominate in determining changing incidence of tick-borne encephalitis in Europe. Euro. Surveill. 15, 24–31 [DOI] [PubMed] [Google Scholar]
- 8. Medlock J. M., Hansford K. M., Bormane A., Derdakova M., Estrada-Pena A., George J. C., Golovljova I., Jaenson T. G., Jensen J. K., Jensen P. M., Kazimirova M., Oteo J. A., Papa A., Pfister K., Plantard O., Randolph S. E., Rizzoli A., Santos-Silva M. M., Sprong H., Vial L., Hendrickx G., Zeller H., Van Bortel W. (2013) Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit. Vectors 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Francischetti I. M., Sa-Nunes A., Mans B. J., Santos I. M., Ribeiro J. M. (2009) The role of saliva in tick feeding. Front. Biosci. 14, 2051–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mans B. J., Neitz A. W. (2004) Adaptation of ticks to a blood-feeding environment: evolution from a functional perspective. Insect Biochem. Mol. Biol. 34, 1–17 [DOI] [PubMed] [Google Scholar]
- 11. Chmelar J., Calvo E., Pedra J. H., Francischetti I. M., Kotsyfakis M. (2012) Tick salivary secretion as a source of antihemostatics. J. Proteomics 75, 3842–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chmelar J., Oliveira C. J., Rezacova P., Francischetti I. M., Kovarova Z., Pejler G., Kopacek P., Ribeiro J. M., Mares M., Kopecky J., Kotsyfakis M. (2011) A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117, 736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotsyfakis M., Horka H., Salat J., Andersen J. F. (2010) The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol. Microbiol. 77, 456–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuijt T. J., Coumou J., Narasimhan S., Dai J., Deponte K., Wouters D., Brouwer M., Oei A., Roelofs J. J., van Dam A. P., van der Poll T., Van't Veer C., Hovius J. W., Fikrig E. (2011) A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell. Host Microbe 10, 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pareek C. S., Smoczynski R., Tretyn A. (2011) Sequencing technologies and genome sequencing. J. Appl. Genet. 52, 413–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chmelar J., Anderson J. M., Mu J., Jochim R. C., Valenzuela J. G., Kopecky J. (2008) Insight into the sialome of the castor bean tick, Ixodes ricinus. BMC Genomics 9, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H., Kaufman W. R., Cui W. W., Nuttall P. A. (2001) Molecular individuality and adaptation of the tick Rhipicephalus appendiculatus in changed feeding environments. Med. Vet. Entomol. 15, 403–412 [DOI] [PubMed] [Google Scholar]
- 18. Ribeiro J. M., Alarcon-Chaidez F., Francischetti I. M., Mans B. J., Mather T. N., Valenzuela J. G., Wikel S. K. (2006) An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 36, 111–129 [DOI] [PubMed] [Google Scholar]
- 19. Reed D. H., Frankham R. (2003) Correlation between fitness and genetic diversity. Conserv. Biol. 17, 230–237 [Google Scholar]
- 20. Garcia C., Avila V., Quesada H., Caballero A. (2012) Gene-expression changes caused by inbreeding protect against inbreeding depression in Drosophila. Genetics 192, 161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Francischetti I. M., My Pham V., Mans B. J., Andersen J. F., Mather T. N., Lane R. S., Ribeiro J. M. (2005) The transcriptome of the salivary glands of the female western black-legged tick Ixodes pacificus (Acari: Ixodidae). Insect Biochem. Mol. Biol. 35, 1142–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Francischetti I. M., Anderson J. M., Manoukis N., Pham V. M., Ribeiro J. M. (2011) An insight into the sialotranscriptome and proteome of the coarse bontlegged tick, Hyalomma marginatum rufipes. J. Proteomics 74, 2892–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Islam M. K., Tsuji N., Miyoshi T., Alim M. A., Huang X., Hatta T., Fujisaki K. (2009) The Kunitz-like modulatory protein haemangin is vital for hard tick blood-feeding success. PLoS Pathog. 5, e1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narasimhan S., Koski R. A., Beaulieu B., Anderson J. F., Ramamoorthi N., Kantor F., Cappello M., Fikrig E. (2002) A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol. Biol. 11, 641–650 [DOI] [PubMed] [Google Scholar]
- 25. Kiszewski A. E., Matuschka F. R., Spielman A. (2001) Mating strategies and spermiogenesis in ixodid ticks. Annu. Rev. Entomol. 46, 167–182 [DOI] [PubMed] [Google Scholar]
- 26. Toutoungi L. N., Gern L., Aeschlimann A. (1995) Biology of Ixodes (Pholeoixodes) hexagonus under laboratory conditions. Part II. Effect of mating on feeding and fecundity of females. Exp. Appl. Acarol. 19, 233–245 [DOI] [PubMed] [Google Scholar]
- 27. Donohue K. V., Khalil S. M., Ross E., Mitchell R. D., Roe R. M., Sonenshine D. E. (2009) Male engorgement factor: Role in stimulating engorgement to repletion in the ixodid tick, Dermacentor variabilis. J. Insect. Physiol. 55, 909–918 [DOI] [PubMed] [Google Scholar]
- 28. Reddy M. K., Nair S., Sopory S. K. (2002) Global amplification of cDNA from limiting amounts of tissue. An improved method for gene cloning and analysis. Mol. Biotechnol. 22, 223–230 [DOI] [PubMed] [Google Scholar]
- 29. Soares M. B., Bonaldo M. F., Jelene P., Su L., Lawton L., Efstratiadis A. (1994) Construction and characterization of a normalized cDNA library. Proc. Natl. Acad. Sci. U. S. A. 91, 9228–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patanjali S. R., Parimoo S., Weissman S. M. (1991) Construction of a uniform-abundance (normalized) cDNA library. Proc. Natl. Acad. Sci. U. S. A. 88, 1943–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karim S., Singh P., Ribeiro J. M. (2011) A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One 6, e28525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Birol I., Jackman S. D., Nielsen C. B., Qian J. Q., Varhol R., Stazyk G., Morin R. D., Zhao Y., Hirst M., Schein J. E., Horsman D. E., Connors J. M., Gascoyne R. D., Marra M. A., Jones S. J. (2009) De novo transcriptome assembly with ABySS. Bioinformatics 25, 2872–2877 [DOI] [PubMed] [Google Scholar]
- 33. Simpson J. T., Wong K., Jackman S. D., Schein J. E., Jones S. J., Birol I. (2009) ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pei J., Kim B. H., Grishin N. V. (2008) PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katoh K., Toh H. (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 9, 286–298 [DOI] [PubMed] [Google Scholar]
- 36. Abascal F., Zardoya R., Posada D. (2005) ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104–2105 [DOI] [PubMed] [Google Scholar]
- 37. Whelan S., Goldman N. (2001) A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699 [DOI] [PubMed] [Google Scholar]
- 38. Stamatakis A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 [DOI] [PubMed] [Google Scholar]
- 39. Ronquist F., Huelsenbeck J. P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 [DOI] [PubMed] [Google Scholar]
- 40. Anatriello E., Ribeiro J. M., de Miranda-Santos I. K., Brandao L. G., Anderson J. M., Valenzuela J. G., Maruyama S. R., Silva J. S., Ferreira B. R. (2010) An insight into the sialotranscriptome of the brown dog tick, Rhipicephalus sanguineus. BMC Genomics 11, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Batista I. F., Chudzinski-Tavassi A. M., Faria F., Simons S. M., Barros-Batestti D. M., Labruna M. B., Leao L. I., Ho P. L., Junqueira-de-Azevedo I. L. (2008) Expressed sequence tags (ESTs) from the salivary glands of the tick Amblyomma cajennense (Acari: Ixodidae). Toxicon 51, 823–834 [DOI] [PubMed] [Google Scholar]
- 42. Mans B. J., Andersen J. F., Francischetti I. M., Valenzuela J. G., Schwan T. G., Pham V. M., Garfield M. K., Hammer C. H., Ribeiro J. M. (2008) Comparative sialomics between hard and soft ticks: implications for the evolution of blood-feeding behavior. Insect Biochem. Mol. Biol. 38, 42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ribeiro J. M., Labruna M. B., Mans B. J., Maruyama S. R., Francischetti I. M., Barizon G. C., de Miranda Santos I. K. (2012) The sialotranscriptome of Antricola delacruzi female ticks is compatible with non-hematophagous behavior and an alternative source of food. Insect Biochem. Mol. Biol. 42, 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vijay N., Poelstra J. W., Kunstner A., Wolf J. B. (2013) Challenges and strategies in transcriptome assembly and differential gene expression quantification. A comprehensive in silico assessment of RNA-seq experiments. Mol. Ecol. 22, 620–634 [DOI] [PubMed] [Google Scholar]
- 45. Bellgard M. I., Moolhuijzen P. M., Guerrero F. D., Schibeci D., Rodriguez-Valle M., Peterson D. G., Dowd S. E., Barrero R., Hunter A., Miller R. J., Lew-Tabor A. E. (2012) CattleTickBase: an integrated Internet-based bioinformatics resource for Rhipicephalus (Boophilus) microplus. Int. J. Parasitol. 42, 161–169 [DOI] [PubMed] [Google Scholar]
- 46. Andersen J. F., Gudderra N. P., Francischetti I. M., Valenzuela J. G., Ribeiro J. M. (2004) Recognition of anionic phospholipid membranes by an antihemostatic protein from a blood-feeding insect. Biochemistry 43, 6987–6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Das S., Banerjee G., DePonte K., Marcantonio N., Kantor F. S., Fikrig E. (2001) Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 184, 1056–1064 [DOI] [PubMed] [Google Scholar]
- 48. Paesen G. C., Siebold C., Dallas M. L., Peers C., Harlos K., Nuttall P. A., Nunn M. A., Stuart D. I., Esnouf R. M. (2009) An ion-channel modulator from the saliva of the brown ear tick has a highly modified Kunitz/BPTI structure. J. Mol. Biol. 389, 734–747 [DOI] [PubMed] [Google Scholar]
- 49. Batista I. F., Ramos O. H., Ventura J. S., Junqueira-de-Azevedo I. L., Ho P. L., Chudzinski-Tavassi A. M. (2010) A new Factor Xa inhibitor from Amblyomma cajennense with a unique domain composition. Arch. Biochem. Biophys. 493, 151–156 [DOI] [PubMed] [Google Scholar]
- 50. Alim M. A., Islam M. K., Anisuzzaman Miyoshi T., Hatta T., Yamaji K., Matsubayashi M., Fujisaki K., Tsuji N. (2012) A hemocyte-derived Kunitz-BPTI-type chymotrypsin inhibitor, HlChI, from the ixodid tick Haemaphysalis longicornis, plays regulatory functions in tick blood-feeding processes. Insect Biochem. Mol. Biol. 42, 925–934 [DOI] [PubMed] [Google Scholar]
- 51. Mans B. J., Andersen J. F., Schwan T. G., Ribeiro J. M. (2008) Characterization of anti-hemostatic factors in the argasid, Argas monolakensis: implications for the evolution of blood-feeding in the soft tick family. Insect Biochem. Mol. Biol. 38, 22–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dai S. X., Zhang A. D., Huang J. F. (2012) Evolution, expansion and expression of the Kunitz/BPTI gene family associated with long-term blood feeding in Ixodes scapularis. BMC Evol. Biol. 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schweitz H., Bruhn T., Guillemare E., Moinier D., Lancelin J. M., Beress L., Lazdunski M. (1995) Kalicludines and kaliseptine. Two different classes of sea anemone toxins for voltage sensitive K+ channels. J. Biol. Chem. 270, 25121–25126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.