Abstract
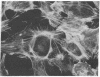
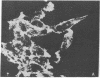
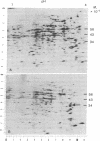
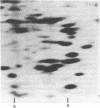
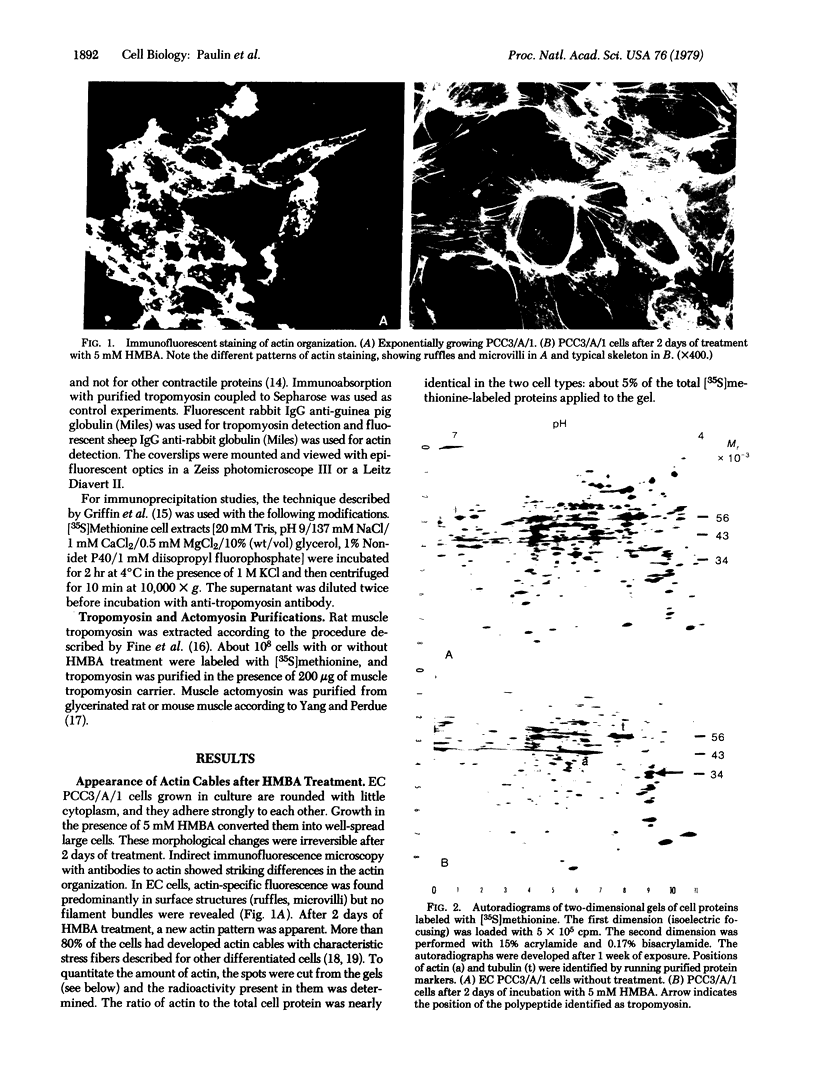
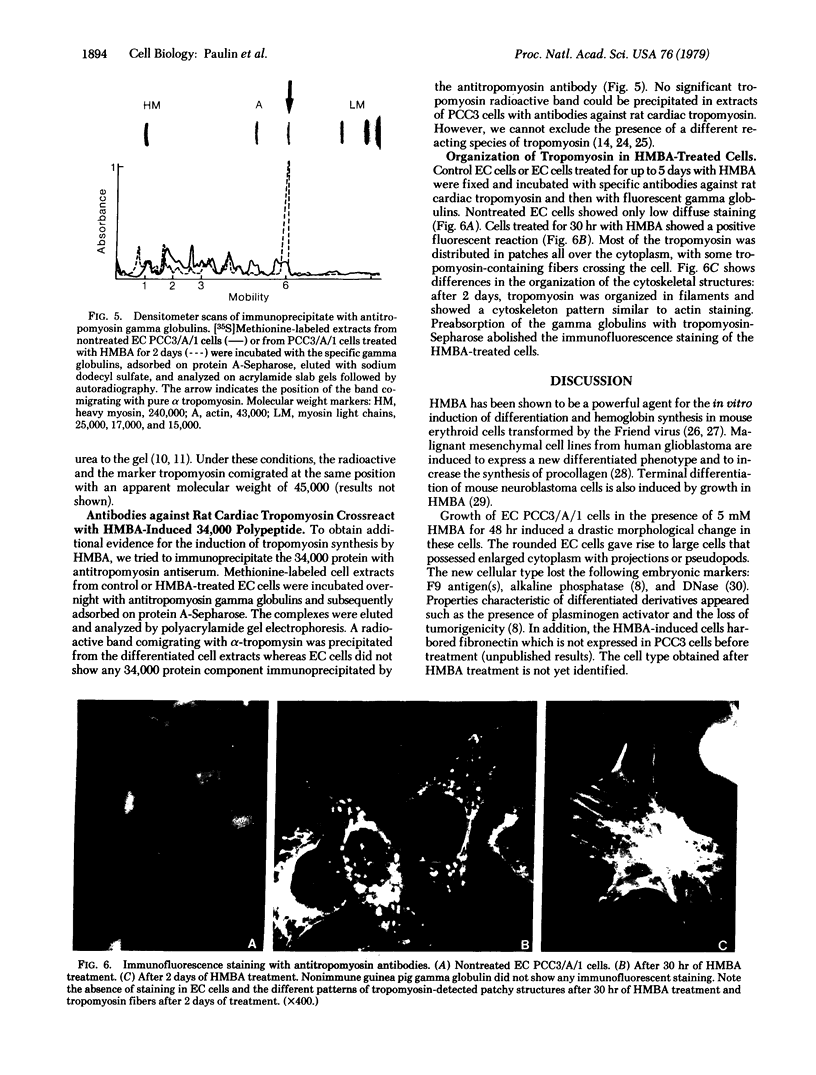
Hexamethylene bisacetamide (HMBA) induces in vitro the cytodifferentiation of PCC3/A/1 mouse embryonal carcinoma (EC) cells. In EC cells, actin is associated with surface structures but microfilament bundles are not seen. After 2 days of HMBA treatment, rounded EC cells are converted to flat adhesive ones with a developed cytoskeleton containing actin and tropomyosin. The ratio of actin to total proteins is constant in EC cells and their HMBA derivatives; but a striking difference is observed for one of the newly synthesized proteins (Mr 34,000) identified as tropomyosin. Synthesis of tropomyosin is followed by its association with actin microfilament bundles, as revealed by indirect immunofluorescence microscopy with specific antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Stromer M. H., Goll D. E., Robson R. M. Sythesis of tropomyosin in cultures of differentiating muscle cells. J Cell Biol. 1978 Jan;76(1):98–104. doi: 10.1083/jcb.76.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Tropomyosin from bovine brain contains two polypeptide chains of slightly different molecular weights. FEBS Lett. 1978 Jan 1;85(1):145–148. doi: 10.1016/0014-5793(78)81267-8. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Carmon Y., Neuman S., Yaffe D. Synthesis of tropomyosin in myogenic cultures and in RNA-directed cell-free systems: qualitative changes in the polypeptides. Cell. 1978 Jun;14(2):393–401. doi: 10.1016/0092-8674(78)90124-1. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Gröschel-Stewart U., Burnstock G. FITC-labelled antibody staining of tropomyosin-containing fibrils in smooth, cardiac and skeletal muscle cells, prefusion myoblasts, fibroblasts, endothelial cells and 3T3 cells in culture. Cell Tissue Res. 1977 Sep 26;183(2):153–166. doi: 10.1007/BF00226616. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Reactions at the 3' terminus of transfer ribonucleic acid. II. Purification and physical and chemical properties of rabbit liver transfer ribonucleic acid nucleotidyltransferase. J Biol Chem. 1972 Jan 25;247(2):450–458. [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L., Hitchcock S. E., Kaminer B. Tropomyosin in brain and growing neurones. Nat New Biol. 1973 Oct 10;245(145):182–186. doi: 10.1038/newbio245182a0. [DOI] [PubMed] [Google Scholar]
- Gachelin G. The cell surface antigens of mouse embryonal carcinoma cells. Biochim Biophys Acta. 1978 Sep 18;516(1):27–60. doi: 10.1016/0304-419x(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Griffin J. D., Light S., Livingston D. M. Measurements of the molecular size of the simian virus 40 large T antigen. J Virol. 1978 Jul;27(1):218–226. doi: 10.1128/jvi.27.1.218-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F. The Leeuwenhoek Lecture, 1977. Mouse teratocarcinoma and mouse embryo. Proc R Soc Lond B Biol Sci. 1978 May 16;201(1144):249–270. doi: 10.1098/rspb.1978.0044. [DOI] [PubMed] [Google Scholar]
- Jakob H., Dubois P., Eisen H., Jacob F. Effets de l'hexaméthylènebisacétamide sur la différenciation de cellules de carcinome embryonnaire. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jan;286(1):109–111. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger J., Bouveret P., Lompre A. M., Schwartz K. Species-dependent immunological differences between various mammalian cardiac tropomyosins. Biochim Biophys Acta. 1979 Feb 26;576(2):314–321. doi: 10.1016/0005-2795(79)90406-9. [DOI] [PubMed] [Google Scholar]
- Léger J. J., Berson G., Delcaryre C., Klotz C., Schwartz K., Léger J., Stephens M., Swynghedauw B. Heart contractile proteins. Biochimie. 1975 Nov-Dec;57(11-12):1249–1273. doi: 10.1016/s0300-9084(76)80538-x. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- Nicolas J. F., Dubois P., Jakob H., Gaillard J., Jacob F. Tératocarcinome de la souris: différenciation en culture d'une lignée de cellules primitives a potentialités multiples. Ann Microbiol (Paris) 1975 Jan;126(1):3–22. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Tanaka M., Terada M., Miller O. J., Bank A., Marks P., Rifkind R. A. Erythroid cell differentiation: murine erythroleukemia cell variant with unique pattern of induction by polar compounds. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1232–1236. doi: 10.1073/pnas.73.4.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Born T., Koitsch H. J., Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978 Jul;14(3):477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Palfrey C., Kimhi Y., Littauer U. Z. Induction of differentiation in mouse neuroblastoma cells by hexamethylene bisacetamide. Biochem Biophys Res Commun. 1977 Jun 6;76(3):937–942. doi: 10.1016/0006-291x(77)91592-3. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., Stern R., Tralka T. S., Costa J., Wilczek J. Hexamethylene bisacetamide induces morphologic changes and increased synthesis of procollagen in cell line from glioblastoma multiforme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5060–5064. doi: 10.1073/pnas.74.11.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano L., Paulin D. Alkaline deoxyribonuclease activity in mouse teratocarcinoma cells: variation of enzyme levels during differentiation. Biochem Biophys Res Commun. 1978 Jul 28;83(2):406–413. doi: 10.1016/0006-291x(78)91005-7. [DOI] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]