Abstract
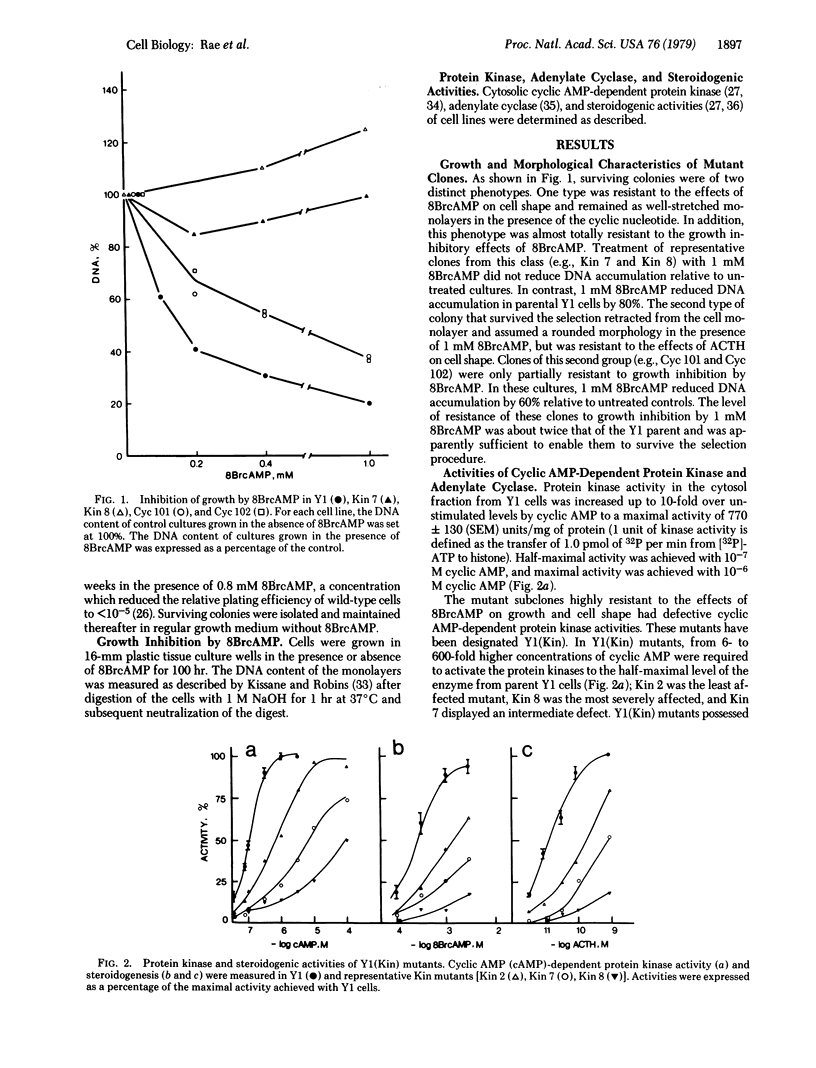
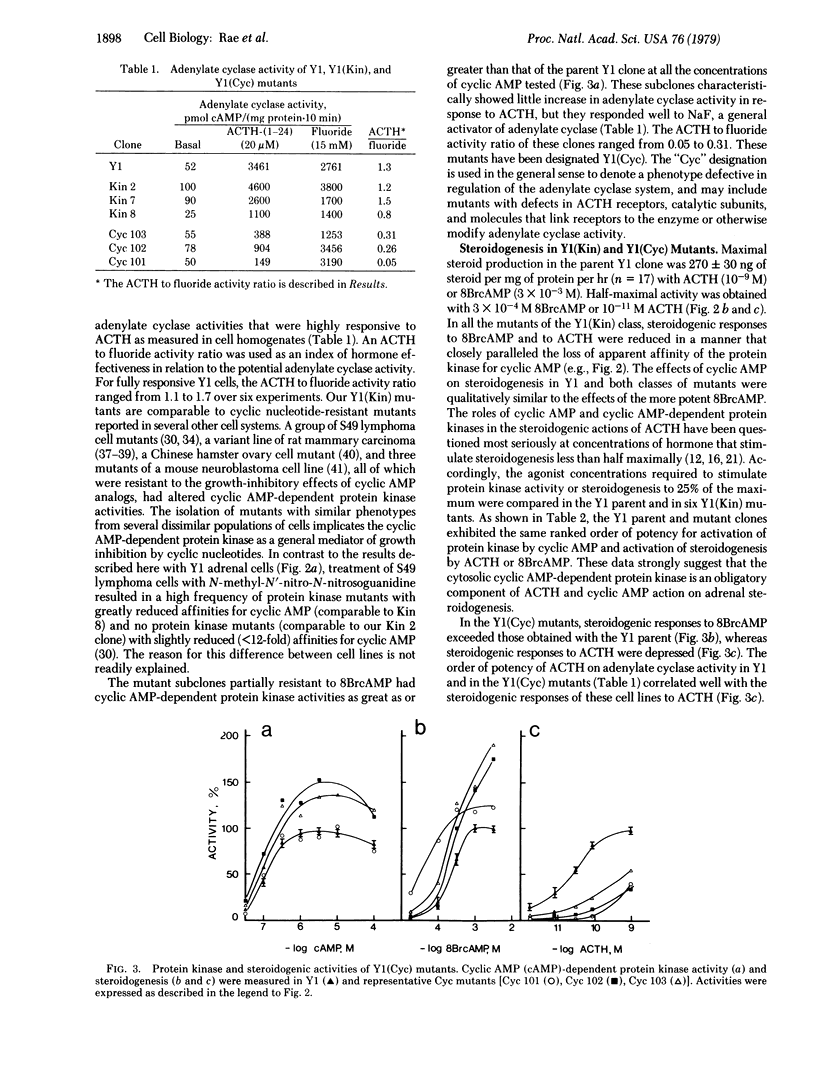
Two groups of mutant clones were isolated from YI adrenocortical tumor cells. One group, Y1(Kin), exhibited altered cytosolic cyclic AMP-dependent protein kinase activity; the second group, Y1(Cyc), exhibited diminished corticotropin-responsive adenylate cyclase activity. Steroidogenic responses to corticotropin and cyclic nucleotides closely paralleled cyclic AMP-dependent protein kinase activity in the Y1(Kin) mutants. In Y1(Cyc) mutants, corticotropin had little effect on steroidogenesis, whereas cyclic nucleotides were fully active. These data imply that adenylate cyclase and cyclic AMP-dependent protein kinase are obligatory components of the corticotropin-stimulated steroidogenic pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Beckett G. J., Boyd G. S. Purification and control of bovine adrenal cortical cholesterol ester hydrolase and evidence for the activation of the enzyme by a phosphorylation. Eur J Biochem. 1977 Jan;72(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11243.x. [DOI] [PubMed] [Google Scholar]
- Caron M. G., Goldstein S., Savard K., Marsh J. M. Protein kinase stimulation of a reconstituted cholesterol side chain cleavage enzyme system in the bovine corpus luteum. J Biol Chem. 1975 Jul 10;250(13):5137–5143. [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Huffman P. Loss of nuclear cyclic AMP binding in cyclic AMP-unresponsive Walker 256 mammary carcinoma. J Biol Chem. 1977 Sep 25;252(18):6349–6355. [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Porper R. Cyclic AMP-binding proteins and protein kinase during regression of Walker 256 mammary carcinoma. J Biol Chem. 1977 Sep 25;252(18):6342–6348. [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Yi P. N., Parkison C. Comparative studies on cyclic AMP binding and protein kinase in cyclic AMP-responsive and -unresponsive Walker 256 mammary carcinomas. J Biol Chem. 1977 Sep 25;252(18):6335–6341. [PubMed] [Google Scholar]
- Chu E. H., Malling H. V. Mammalian cell genetics. II. Chemical induction of specific locus mutations in Chinese hamster cells in vitro. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1306–1312. doi: 10.1073/pnas.61.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U., Coffino P. Mutagenesis in S49 mouse lymphoma cells: induction of resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1977 Feb;74(2):679–683. doi: 10.1073/pnas.74.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Gutmann N. S., Rae P. A., Schimmer B. P. Altered cyclic AMP-dependent protein kinase activity in a mutant adrenocortical tumor cell line. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):451–460. doi: 10.1002/jcp.1040970320. [DOI] [PubMed] [Google Scholar]
- HAYNES R. C., Jr, KORITZ S. B., PERON F. G. Influence of adenosine 3',5'-monophosphate on corticoid production by rat adrenal glands. J Biol Chem. 1959 Jun;234(6):1421–1423. [PubMed] [Google Scholar]
- HAYNES R. C., Jr The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J Biol Chem. 1958 Nov;233(5):1220–1222. [PubMed] [Google Scholar]
- Haksar A., Maudsley D. V., Péron F. G. Stimulation of cyclic adenosine 3':5'-monophosphate and corticosterone formation in isolated rat adrenal cells by cholera enterotoxin. Comparison with the effects of ACTH. Biochim Biophys Acta. 1975 Feb 13;381(2):308–323. doi: 10.1016/0304-4165(75)90237-8. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Bourne H. R., Coffino P., Tomkins G. M. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science. 1975 Nov 28;190(4217):896–898. doi: 10.1126/science.171770. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kowal J., Fiedler R. P. Adrenal cells in tissue culture. II. Steroidogenic responses to nucleosides and nucleotides. Endocrinology. 1969 May;84(5):1113–1117. doi: 10.1210/endo-84-5-1113. [DOI] [PubMed] [Google Scholar]
- Kowal J., Fiedler R. Arenal cells in tissue culture. I. Assay of steroid products; steroidogenic responses to peptide hormones. Arch Biochem Biophys. 1968 Nov;128(2):406–421. doi: 10.1016/0003-9861(68)90047-7. [DOI] [PubMed] [Google Scholar]
- Kowal J., Horst I., Pensky J., Alfonzo M. A comparison of the effects of ACTH, vasoactive intestinal peptide, and cholera toxin on adrenal cAMP and steroid synthesis. Ann N Y Acad Sci. 1977 Oct 28;297:314–328. doi: 10.1111/j.1749-6632.1977.tb41863.x. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., De Renzo E. C. A comparison of the effects of lipolytic and antilipolytic agents on adenosine 3',5'-monophosphate levels in adipose cells as determined by prior labeling with adenine-8-14C. J Biol Chem. 1969 May 10;244(9):2252–2260. [PubMed] [Google Scholar]
- Macho L., Saffran M. Metabolism of fatty acids in the rat adrenal gland. Endocrinology. 1967 Aug;81(2):179–185. doi: 10.1210/endo-81-2-179. [DOI] [PubMed] [Google Scholar]
- Masui H., Garren L. D. Inhibition of replication in functional mouse adrenal tumor cells by adrenocorticotropic hormone mediated by adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3206–3210. doi: 10.1073/pnas.68.12.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlevede W., Riley G. A. The activation and inactivation of phosphorylase phosphatase from bovine adrenal cortex. J Biol Chem. 1966 Aug 10;241(15):3517–3524. [PubMed] [Google Scholar]
- Moyle W. R., Kong Y. C., Ramachandran J. Steroidogenesis and cyclic adenosine 3',5'-monophosphate accumulation in rat adrenal cells. Divergent effects of adrenocorticotropin and its o-nitrophenyl sulfenyl derivative. J Biol Chem. 1973 Apr 10;248(7):2409–2417. [PubMed] [Google Scholar]
- Moyle W. R., MacDonald G. J., Garfink J. E. Role of histone kinases as mediators of corticotropin-induced steroidogenesis. Biochem J. 1976 Oct 15;160(1):1–9. doi: 10.1042/bj1600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. Morphological responses to corticotrophin and cyclic AMP by adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Mar;56(3):529–536. doi: 10.1677/joe.0.0560529. [DOI] [PubMed] [Google Scholar]
- Palfreyman J. W., Schulster D. On the mechanism of action of cholera toxin on isolated rat adrenocortical cells. Comparison with the effects of adrenocorticotropin on steroidogenesis and cyclic AMP output. Biochim Biophys Acta. 1975 Oct 9;404(2):221–230. doi: 10.1016/0304-4165(75)90328-1. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Penman B. W., Thilly W. G. Concentration-dependent mutation of diploid human lymphoblasts by methylnitronitrosoguanidine: the importance of phenotypic lag. Somatic Cell Genet. 1976 Jul;2(4):325–330. doi: 10.1007/BF01538837. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. C., Schulster D. The role of protein kinase activation in the control of steroidogenesis by adrenocorticotrophic hormone in the adrenal cortex. Biochem J. 1973 Dec;136(4):993–998. doi: 10.1042/bj1360993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer B. P. Adenylate cyclase activity in adrenocorticotropic hormone-sensitive and mutant adrenocortical tumor cell lines. J Biol Chem. 1972 May 25;247(10):3134–3138. [PubMed] [Google Scholar]
- Schimmer B. P. Adrenocortical Y1 cells. Methods Enzymol. 1979;58:570–574. doi: 10.1016/s0076-6879(79)58173-7. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P. Phenotypically variant adrenal tumor cell cultures with biochemical lesions in the ACTH-stimulated steroidogenic pathway. J Cell Physiol. 1969 Oct;74(2):115–122. doi: 10.1002/jcp.1040740203. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P., Tsao J., Knapp M. Isolation of mutant adrenocortical tumor cells resistant to cyclic nucleotides. Mol Cell Endocrinol. 1977 Aug;8(2):135–145. doi: 10.1016/0303-7207(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Schimmer B. P., Zimmerman A. E. Steroidogenesis and extracellular cAMP accumulation in adrenal tumor cell cultures. Mol Cell Endocrinol. 1976 Mar;4(4):263–270. doi: 10.1016/0303-7207(76)90060-5. [DOI] [PubMed] [Google Scholar]
- Seelig S., Kumar S., Sayers G. Isolated adrenal cells: the partial agonists (Trp(Nps) 9 )ACTH 1-39 and (Trp(Nps) 9 )ACTH 1-24 (nitrophenyl sulfenyl derivatives of ACTH). Proc Soc Exp Biol Med. 1972 Apr;139(4):1217–1219. doi: 10.3181/00379727-139-36332. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Ahmed N. K., Shanker G. Metabolic regulation of steroidogenesis in isolated adrenal cells of rat. Relationship of adrenocorticotropin-, adenosine 3':5'-monophosphate-and guanosine 3':5'-monophosphate-stimulated steroidogenesis with the activation of protein kinase. Eur J Biochem. 1976 Nov 15;70(2):427–433. doi: 10.1111/j.1432-1033.1976.tb11033.x. [DOI] [PubMed] [Google Scholar]
- Shima S., Mitsunaga M., Nakao T. Effect of ACTH on cholesterol dynamics in rat adrenal tissue. Endocrinology. 1972 Mar;90(3):808–814. doi: 10.1210/endo-90-3-808. [DOI] [PubMed] [Google Scholar]
- Simantov R., Sachs L. Temperature sensitivity of cyclic adenosine 3':5'-monophosphate-binding proteins and the regulation of growth and differentiation in neuroblastoma cells. J Biol Chem. 1975 May 10;250(9):3236–3242. [PubMed] [Google Scholar]
- Walton G. M., Gill G. N., Abrass I. B., Garren L. D. Phosphorylation of ribosome-associated protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase: location of the microsomal receptor and protein kinase. Proc Natl Acad Sci U S A. 1971 May;68(5):880–884. doi: 10.1073/pnas.68.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Adenosine 3',5'-monophosphate and protein kinase dependent phosphorylation of ribosomal protein. Biochemistry. 1973 Jul 3;12(14):2604–2611. doi: 10.1021/bi00738a009. [DOI] [PubMed] [Google Scholar]
- Weidman R. E., Gill G. N. Differential effects of ACTH or 8-Dr-cAMP on growth and replicationin a functional adrenal tumor cell line. J Cell Physiol. 1977 Jan;90(1):91–103. doi: 10.1002/jcp.1040900112. [DOI] [PubMed] [Google Scholar]
- Yasumura Y., Buonassisi V., Sato G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966 Mar;26(3):529–535. [PubMed] [Google Scholar]


