Abstract
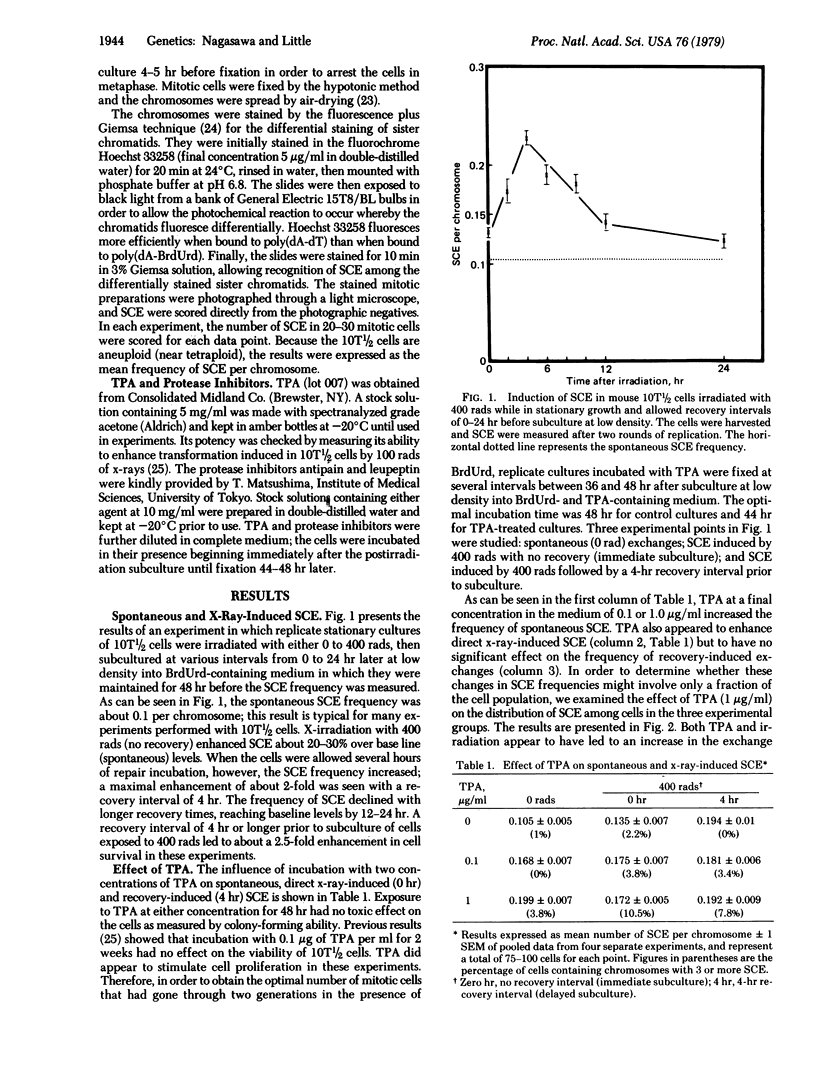
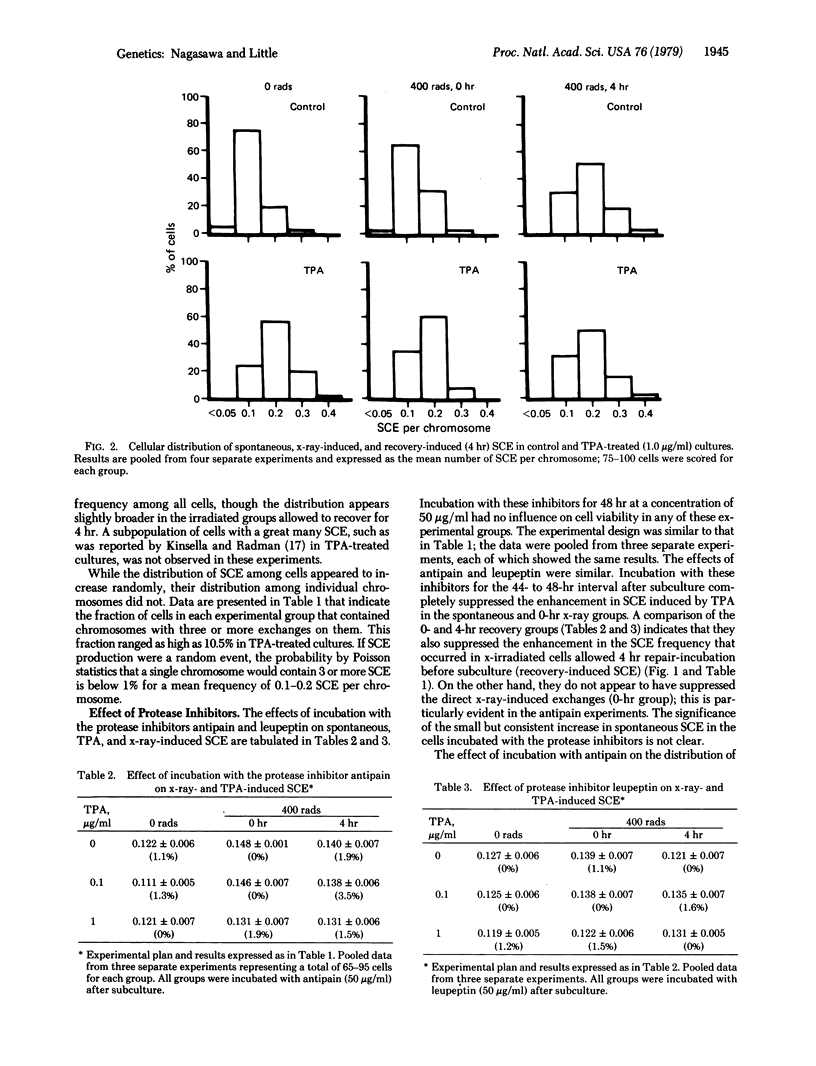
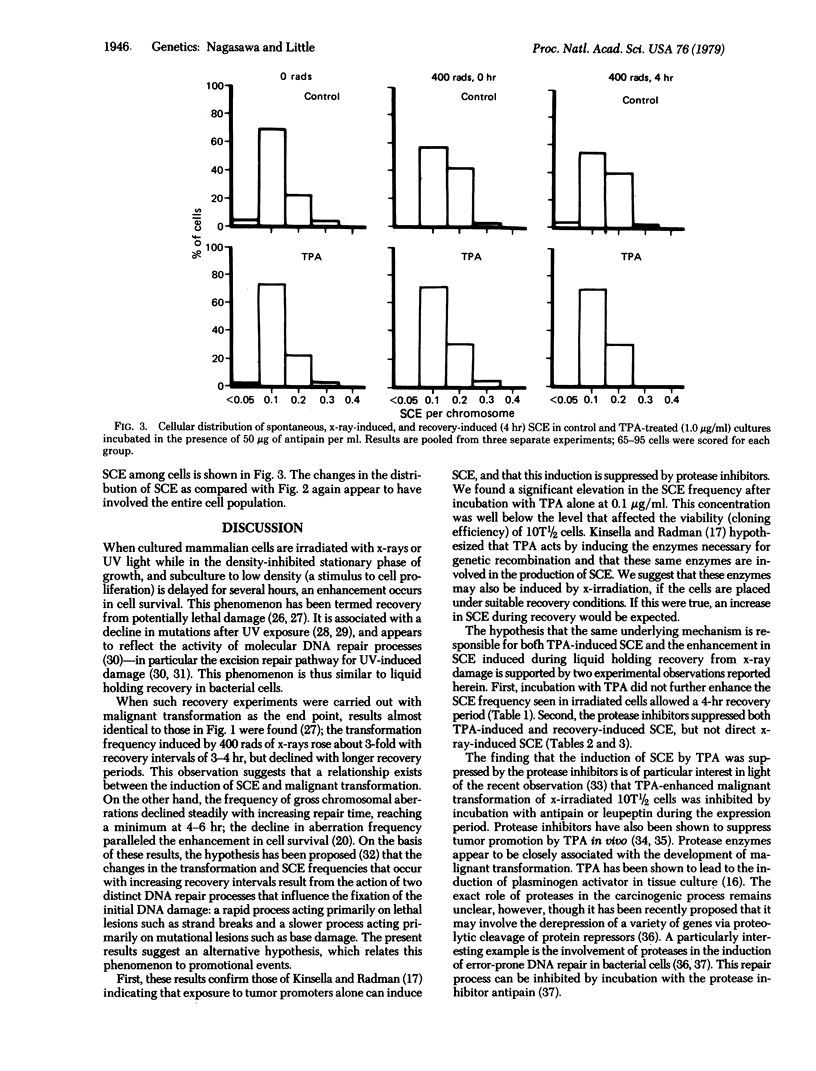
The induction of sister chromatid exchanges (SCE) in the second postirradiation mitosis was studied in mouse 10T1/2 cells irradiated with 400 rads (4 grays) and maintained in stationary growth for several hours after x-ray exposure (similar to liquid holding recovery experiments in bacterial cells). X-irradiation with no recovery period induced few SCE. With short recovery intervals, however, the SCE frequency rose in parallel with the increase in survival, reaching a maximum increase of 2-fold after 4 hr; SCE declined with longer recovery intervals. The influence of postirradiation incubation with the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) and with the protease inhibitors antipain and leupeptin was studied on spontaneous, x-ray-induced (no recovery), and recovery-induced (4 hr) SCE. TPA (0.1 microgram/ml and 1.0 microgram/ml) increased the frequency of both spontaneous and direct x-ray-induced SCE, but not of recovery-induced SCE. Incubation with the protease inhibitors suppressed both TPA- and recovery-induced SCE, but had no effect on direct x-ray-induced SCE. These results are discussed in relation to the hypothesis that promotional events in carcinogenesis may involve the expression of mutational damage in cells by mitotic segregation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird W. M., Sedgwick J. A., Boutwell R. K. Effects of phorbol and four diesters of phorbol on the incorporation of tritiated precursors into DNA, RNA, and protein in mouse epidermis. Cancer Res. 1971 Oct;31(10):1434–1439. [PubMed] [Google Scholar]
- Bogart B., Prutkin L., Ocken P. R. The localization of phorbol ester 14C acetate in papillomas that were initiated with 7,12 DMBA and promoted with phorbol ester. An electron microscopic autoradiography study. J Invest Dermatol. 1971 Feb;56(2):140–146. doi: 10.1111/1523-1747.ep12260717. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The biochemistry of preneoplasia in mouse skin. Cancer Res. 1976 Jul;36(7 Pt 2):2631–2635. [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- HSU T. C., KLATT O. Mammalian chromosomes in vitro. IX. On genetic polymorphism in cell populations. J Natl Cancer Inst. 1958 Sep;21(3):437–473. [PubMed] [Google Scholar]
- Hozumi M., Ogawa M., Sugimura T., Takeuchi T., Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972 Aug;32(8):1725–1728. [PubMed] [Google Scholar]
- Kato H. Spontaneous and induced sister chromatid exchanges as revealed by the BUdR-labeling method. Int Rev Cytol. 1977;49:55–97. doi: 10.1016/s0074-7696(08)61947-6. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Little J. B. Protease inhibitors suppress radiation-induced malignant transformation in vitro. Nature. 1978 Dec 21;276(5690):825–826. doi: 10.1038/276825a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Mondal S., Heidelberger C., Little J. B. Enhancement of X-ray transformation by 12-O-tetradecanoyl-phorbol-13-acetate in a cloned line of C3H mouse embryo cells. Cancer Res. 1978 Feb;38(2):439–443. [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. B. Repair of sub-lethal and potentially lethal radiation damage in plateau phase cultures of human cells. Nature. 1969 Nov 22;224(5221):804–806. doi: 10.1038/224804a0. [DOI] [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S., Heidelberger C. Transformation of C3H/10T1/2CL8 mouse embryo fibroblasts by ultraviolet irradiation and a phorbol ester. Nature. 1976 Apr 22;260(5553):710–711. doi: 10.1038/260710a0. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Rohrschneider L. R., Boutwell R. K. The early stimulation of phospholipid metabolism by 12-0-tetradecanoyl-phorbol-13-acetate and its specificity for tumor promotion. Cancer Res. 1973 Aug;33(8):1945–1952. [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. Repair of potentially lethal radiation damage in mammalian cells is associated with enhancement of malignant transformation. Nature. 1975 Feb 13;253(5492):548–549. doi: 10.1038/253548a0. [DOI] [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C., Yotti L. P., Chu E. H. Effect of phorbol myristate acetate on the recovery of spontaneous and ultraviolet light-induced 6-thioguanine and ouabain-resistant Chinese hamster cells. Cancer Res. 1977 Jan;37(1):188–193. [PubMed] [Google Scholar]
- Trosko J. E., Chang C. C., Yotti L. P., Chu E. H. Effect of phorbol myristate acetate on the recovery of spontaneous and ultraviolet light-induced 6-thioguanine and ouabain-resistant Chinese hamster cells. Cancer Res. 1977 Jan;37(1):188–193. [PubMed] [Google Scholar]
- Weichselbaum R. R., Nove J., Little J. B. Deficient recovery from potentially lethal radiation damage in ataxia telengiectasia and xeroderma pigmentosum. Nature. 1978 Jan 19;271(5642):261–262. doi: 10.1038/271261a0. [DOI] [PubMed] [Google Scholar]
- Wenner C. E., Hackney J., Kimelberg H. K., Mayhew E. Membrane effects of phorbol esters. Cancer Res. 1974 Jul;34(7):1731–1737. [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]


