Abstract
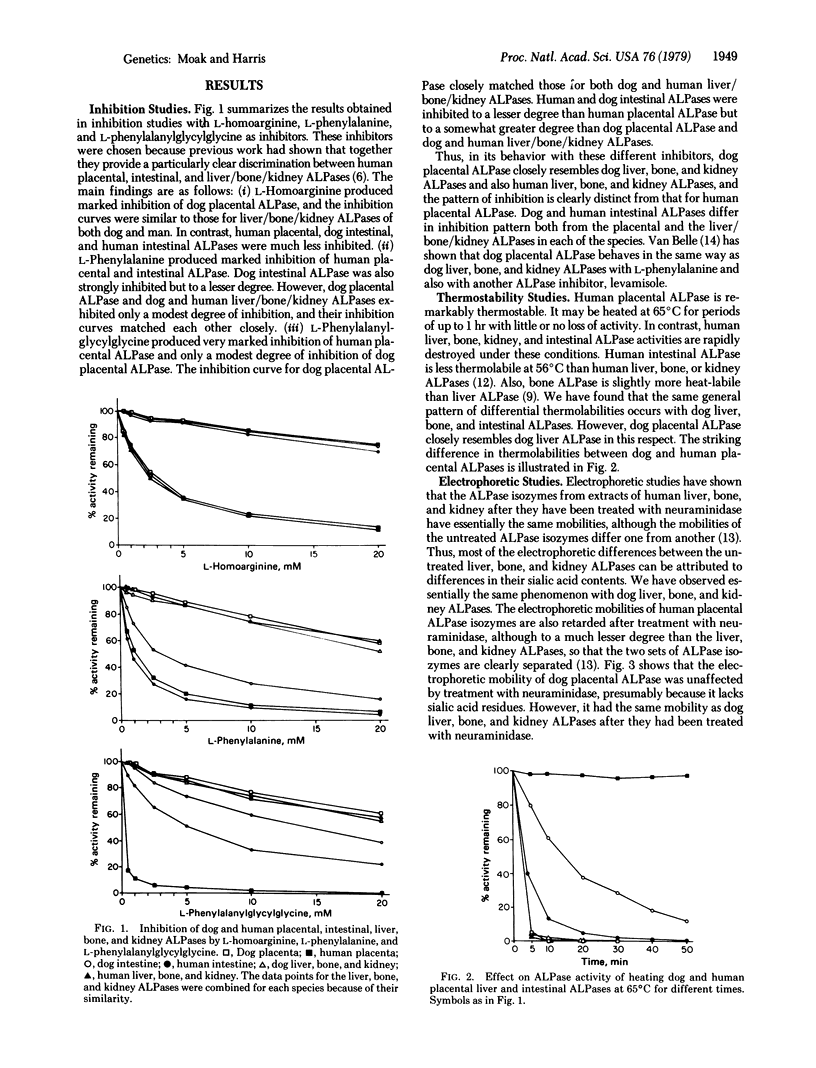
Alkaline phosphatases [ALPases; orthophosphoric-monoester phosphohydrolase (alkaline optimum), EC 3.1.3.1] from dog and human placenta, liver, bone, kidney, and intestine were investigated by inhibition studies with L-homoarginine, L-phenylalanine, and L-phenylalanylglycyl-glycine; by thermostability studies; and by electrophoresis, both before and after treatment with neuraminidase. The inhibitions obtained for each inhibitor with dog placental ALPase closely match those obtained with dog and human liver, bone, and kidney ALPases, but are quite different from those obtained with human placental ALPase. Dog placental ALPase is thermolabile, as are dog and human liver, bone, and kidney ALPases, in marked contrast to human placental ALPase, which is very thermostable. Dog placental ALPase has the same electrophoretic mobility as dog liver, bone, and kidney ALPases after removal of sialic acid residues with neuraminidase. Desialated human placental ALPase differs electrophoretically from desialated human liver, bone, and kidney ALPases, which show the same mobilities. Dog and human intestinal ALPases are distinguished by these various criteria from the liver, bone, kidney, and placental ALPases of both species, but are similar to each other. These results suggest that the ALPase gene locus expressed in dog placenta is not homologous to that expressed in human placenta. Rather, it appears to be homologous to the ALPase locus expressed in dog and human liver and possibly also bone and kidney. Other incomplete data suggest that this may also be true for placental ALPase in other mammalian species. One possible explanation is that human placental ALPase, a relatively recent newcomer on the evolutionary scene, arose from a gene duplication that occurred subsequent to the evolutionary divergence of many other mammalian species.
Keywords: inhibition, thermostability, electrophoresis, evolution, duplication
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fishman W. H. Perspectives on alkaline phosphatase isoenzymes. Am J Med. 1974 May;56(5):617–650. doi: 10.1016/0002-9343(74)90631-7. [DOI] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Ghosh N. K., Goldman S. S., Fishman W. H. Human placental alkaline phosphatase; a sialoprotein. Enzymologia. 1967 Aug 31;33(2):113–124. [PubMed] [Google Scholar]
- Harris H., Hopkinson D. A., Robson E. B. The incidence of rare alleles determining electrophoretic variants: data on 43 enzyme loci in man. Ann Hum Genet. 1974 Jan;37(3):237–253. doi: 10.1111/j.1469-1809.1974.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Komoda T., Sakagishi Y. Partial purification and some properties of human liver alkaline phosphatase. Biochim Biophys Acta. 1976 Jun 7;438(1):138–152. doi: 10.1016/0005-2744(76)90230-8. [DOI] [PubMed] [Google Scholar]
- Komoda T., Sakagishi Y. Partial purification of human intestinal alkaline phosphatase with affinity chromotography. Some properties and interaction of concanavalin A with alkaline phosphatase. Biochim Biophys Acta. 1976 Oct 11;445(3):645–660. doi: 10.1016/0005-2744(76)90117-0. [DOI] [PubMed] [Google Scholar]
- Komoda T., Sakagishi Y. The function of carbohydrate moiety and alteration of carbohydrate composition in human alkaline phosphatase isoenzymes. Biochim Biophys Acta. 1978 Apr 12;523(2):395–406. doi: 10.1016/0005-2744(78)90042-6. [DOI] [PubMed] [Google Scholar]
- Leroux M. L., Perry W. F. Some characteristics of placental alkaline phosphatase of a variety of mammals. Enzymologia. 1971 Oct;41(4):241–248. [PubMed] [Google Scholar]
- Lin C. W., Fishman W. H. L-Homoarginine. An organ-specific, uncompetitive inhibitor of human liver and bone alkaline phosphohydrolases. J Biol Chem. 1972 May 25;247(10):3082–3087. [PubMed] [Google Scholar]
- Manning J. P., Inglis N. R., Green S., Fishman W. H. Characterization of placental alkaline phosphatase from the rabbit, guinea pig, mouse and hamster. Enzymologia. 1970;39(5):307–318. [PubMed] [Google Scholar]
- Manning J. P., Inglis N. R., Green S., Fishman W. H. Characterization of placental alkaline phosphatase from three primates: African green and rhesus monkey and baboon. Enzymologia. 1969 Oct 31;37(4):251–261. [PubMed] [Google Scholar]
- Moss D. W. Alkaline phosphatase isoenzymes. Technical and clinical aspects. Enzyme. 1975;20(1):20–34. doi: 10.1159/000458916. [DOI] [PubMed] [Google Scholar]
- Mulivor R. A., Hannig V. L., Harris H. Developmental change in human intestinal alkaline phosphatase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3909–3912. doi: 10.1073/pnas.75.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulivor R. A., Plotkin L. I., Harris H. Differential inhibition of the products of the human alkaline phosphatase loci. Ann Hum Genet. 1978 Jul;42(1):1–13. doi: 10.1111/j.1469-1809.1978.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Singh I., Tsang K. Y. An in vitro production of bone specific alkaline phosphatase. Exp Cell Res. 1975 Oct 15;95(2):347–358. doi: 10.1016/0014-4827(75)90560-1. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- Van Belle H. Kinetics and inhibition of alkaline phosphatases from canine tissues. Biochim Biophys Acta. 1972 Nov 10;289(1):158–168. doi: 10.1016/0005-2744(72)90118-0. [DOI] [PubMed] [Google Scholar]
- Whitby L. G., Moss D. W. Analysis of heat inactivation curves of alkaline phosphatase isoenzymes in serum. Clin Chim Acta. 1975 Mar 24;59(3):361–367. doi: 10.1016/0009-8981(75)90012-1. [DOI] [PubMed] [Google Scholar]
- van Belle H. Kinetics and inhibition of rat and avian alkaline phosphatases. Gen Pharmacol. 1976;7(1):53–58. doi: 10.1016/0306-3623(76)90033-1. [DOI] [PubMed] [Google Scholar]


