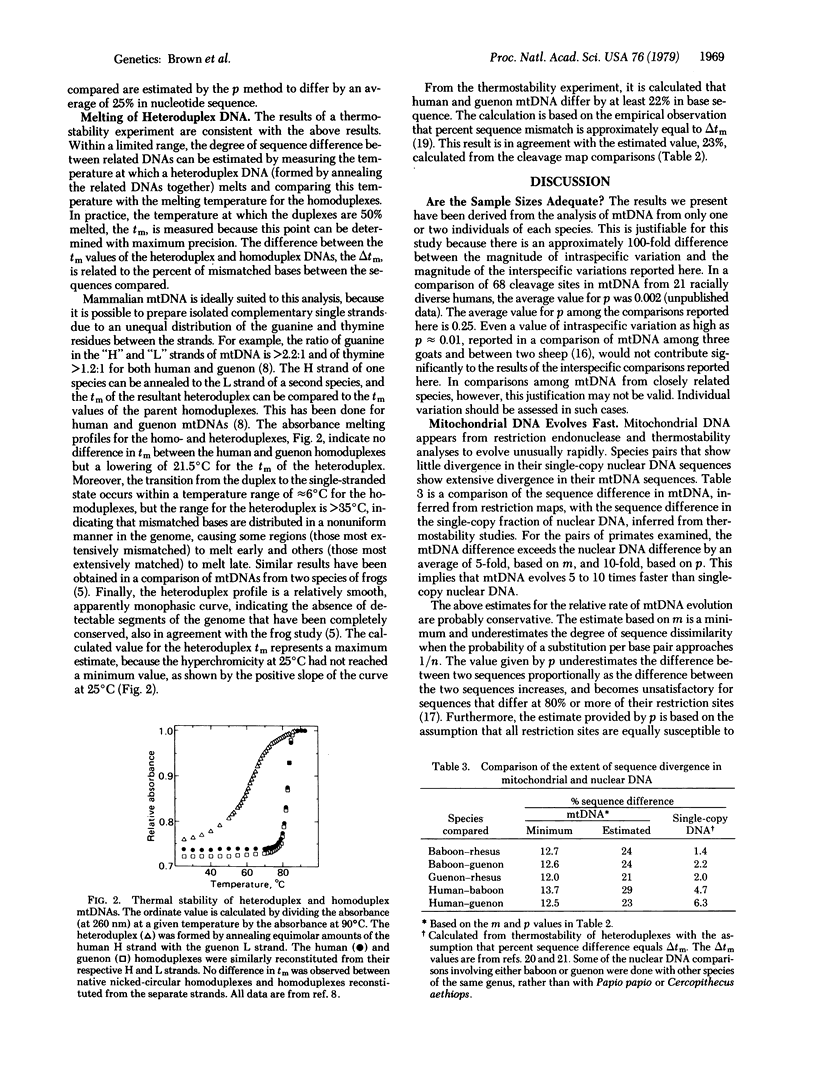
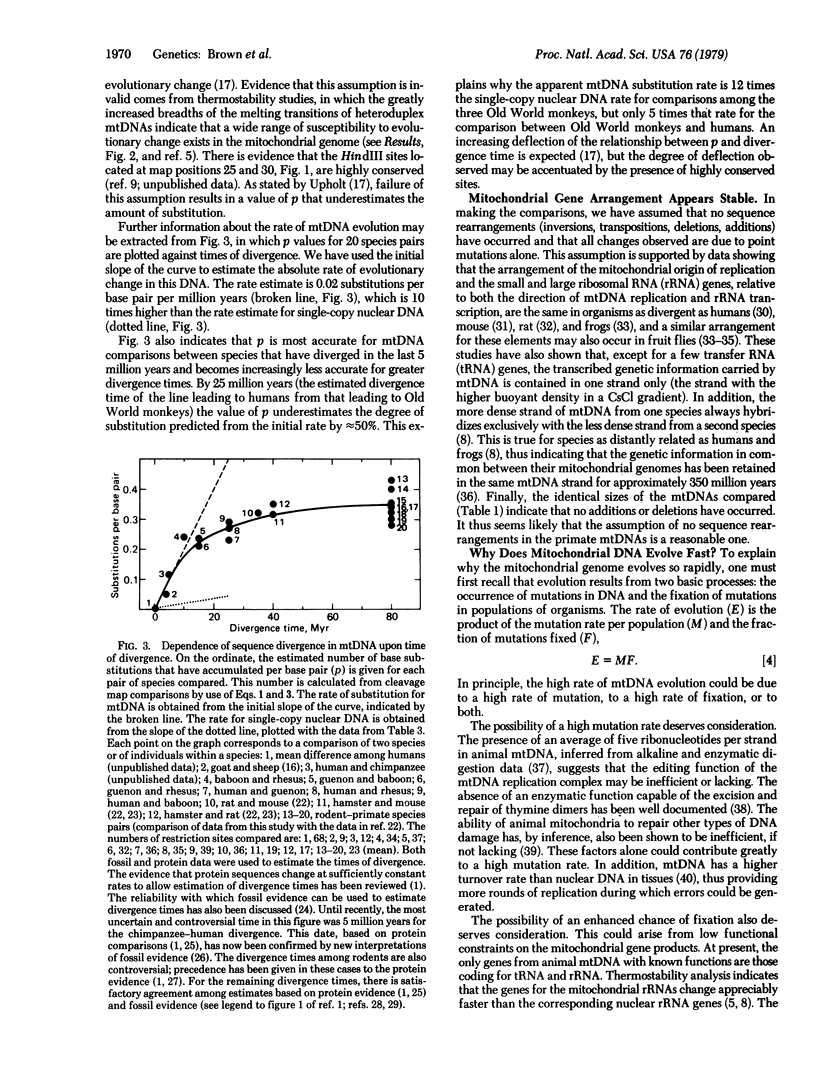
Abstract
Mitochondrial DNA was purified from four species of higher primates (Guinea baboon, rhesus macaque, guenon, and human) and digested with 11 restriction endonucleases. A cleavage map was constructed for the mitochondrial DNA of each species. Comparison of the maps, aligned with respect to the origin and direction of DNA replication, revealed that the species differ from one another at most of the cleavage sites. The degree of divergence in nucleotide sequence at these sites was calculated from the fraction of cleavage sites shared by each pair of species. By plotting the degree of divergence in mitochondrial DNA against time of divergence, the rate of base substitution could be calculated from the initial slope of the curve. The value obtained, 0.02 substitutions per base pair per million years, was compared with the value for single-copy nuclear DNA. The rate of evolution of the mitochondrial genome appears to exceed that of the single-copy fraction of the nuclear genome by a factor of about 10. This high rate may be due, in part, to an elevated rate of mutation in mitochondrial DNA. Because of the high rate of evolution, mitochondrial DNA is likely to be an extremely useful molecule to employ for high-resolution analysis of the evolutionary process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Brack C., Eberle H., Bickle T. A., Yuan R. A map of the sites on bacteriophage PM2 DNA for the restriction endonucleases HindIII and HpaII. J Mol Biol. 1976 Jun 14;104(1):305–309. doi: 10.1016/0022-2836(76)90016-4. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Watson R. M., Vinograd J., Tait K. M., Boyer H. W., Goodman H. M. The structures and fidelity of replication of mouse mitochondrial DNA-pSC 101 EcoRI recombinant plasmids grown in E. coli K12. Cell. 1976 Apr;7(4):517–530. doi: 10.1016/0092-8674(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Carlson S. S., Wilson A. C., Maxson R. D. Do albumin clocks run on time? Science. 1978 Jun 9;200(4346):1183–1185. doi: 10.1126/science.200.4346.1183. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B. Evolution of mitochondrial DNA sequences in Xenopus. Dev Biol. 1972 Oct;29(2):139–151. doi: 10.1016/0012-1606(72)90051-6. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. The structures of cytochrome c and the rates of molecular evolution. J Mol Evol. 1971;1(1):26–45. doi: 10.1007/BF01659392. [DOI] [PubMed] [Google Scholar]
- Grossman L. I., Watson R., Vinograd J. The presence of ribonucleotides in mature closed-circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3339–3343. doi: 10.1073/pnas.70.12.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcic S., Casey J., Rabinowitz M. Sequence homology of the mitochondrial leucyl-tRNA cistron in different organisms. Biochemistry. 1975 May 20;14(10):2037–2042. doi: 10.1021/bi00681a001. [DOI] [PubMed] [Google Scholar]
- Johanson D. C., White T. D. A systematic assessment of early African hominids. Science. 1979 Jan 26;203(4378):321–330. doi: 10.1126/science.104384. [DOI] [PubMed] [Google Scholar]
- King M. C., Wilson A. C. Evolution at two levels in humans and chimpanzees. Science. 1975 Apr 11;188(4184):107–116. doi: 10.1126/science.1090005. [DOI] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Pepe G., Bakker H., Holtrop M., Bollen J. E., Van Bruggen E. F., Cantatore P., Terpstra P., Saccone C. The restriction fragment map of rat-liver mitochondrial DNA: a reconsideration. Biochim Biophys Acta. 1977 Sep 20;478(2):128–145. doi: 10.1016/0005-2787(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Selective nicking of mammalian mitochondrial DNA in vivo: photosensitization by incorporation of 5-bromodeoxyuridine. J Mol Biol. 1975 Dec 25;99(4):761–776. doi: 10.1016/s0022-2836(75)80183-5. [DOI] [PubMed] [Google Scholar]
- Nass M. M. A restriction endonuclease cleavage map of mitochondrial DNA from transformed hamster cells. Nucleic Acids Res. 1978 Feb;5(2):403–424. doi: 10.1093/nar/5.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M. Restriction endonuclease cleavage maps of rat and mouse mitochondrial DNAs. Nucleic Acids Res. 1977;4(5):1291–1299. doi: 10.1093/nar/4.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E., Hutchison C. A., 3rd, Edgell M. H. Specific cleavage analysis of mammalian mitochondrial DNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4496–4500. doi: 10.1073/pnas.72.11.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz M., Swift H. Mitochondrial nucleic acids and their relation to the biogenesis of mitochondria. Physiol Rev. 1970 Jul;50(3):376–427. doi: 10.1152/physrev.1970.50.3.376. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sarich V. M. Generation time and albumin evolution. Biochem Genet. 1972 Dec;7(3):205–212. doi: 10.1007/BF00484818. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E. Evolutionary processes and evolutionary noise at the molecular level. I. Functional density in proteins. J Mol Evol. 1976 Apr 9;7(3):167–183. doi: 10.1007/BF01731487. [DOI] [PubMed] [Google Scholar]


