Abstract
Background
Postural tachycardia syndrome (POTS) is a disorder of chronic orthostatic intolerance accompanied by excessive orthostatic tachycardia. Patients with POTS commonly have comorbid conditions such as attention deficit hyperactivity disorder, depression, or fibromyalgia that are treated with medications that inhibit the norepinephrine reuptake transporter (NRI). NRI medications can increase sympathetic nervous system tone, which may increase heart rate (HR) and worsen symptoms in POTS patients. We sought to determine whether NRI with atomoxetine increases standing tachycardia or worsens the symptom burden in POTS patients.
Methods and Results
Patients with POTS (n=27) underwent an acute drug trial of atomoxetine 40 mg and placebo on separate mornings in a randomized, crossover design. Blood pressure (BP), HR, and symptoms were assessed while seated and after standing prior to and hourly for 4 hours following study drug administration. Atomoxetine significantly increased standing HR compared with placebo (121±17 beats per minute versus 105±15 beats per minute; P=0.001) in POTS patients, with a trend toward higher standing systolic BP (P=0.072). Symptom scores worsened with atomoxetine compared to placebo (+4.2 au versus −3.5 au; P=0.028) from baseline to 2 hours after study drug administration.
Conclusion
Norepinephrine reuptake inhibition with atomoxetine acutely increased standing HR and symptom burden in patients with POTS.
Clinical Trials Registration
URL: http://clinicaltrials.gov. Unique identifier: NCT00262470.
Keywords: atomoxetine, autonomic nervous system, drugs, orthostatic intolerance, sympathetic nervous system, tachycardia
Introduction
Postural tachycardia syndrome (POTS) is one of the most common forms of chronic orthostatic intolerance, affecting an estimated 500 000 Americans.1–2 It commonly affects women of child‐bearing age and often results in significant functional disability.3–5 The hallmark of POTS is exaggerated orthostatic tachycardia in the absence of orthostatic hypotension. Posture‐related symptoms include mental clouding, blurred vision, shortness of breath, rapid heartbeat, tremulousness, chest discomfort, headache, lightheadedness, and nausea. The pathophysiology of POTS is unknown and likely heterogeneous (including partial autonomic neuropathy2 and hypovolemia6–7) but many patients have elevated sympathetic tone and norepinephrine levels upon standing.3,8–9
Atomoxetine is a norepinephrine reuptake transporter (NET) inhibitor (NRI) that is commonly used to treat attention deficit hyperactivity disorder (ADHD) in children and adolescents.10–11 Although there are no prior clinical trials of NRI in POTS, drugs that inhibit NET are sometimes prescribed by physicians seeking a clinically beneficial peripheral vasoconstriction for POTS patients.12–13 NET inhibition has been shown to increase heart rate (HR) and blood pressure (BP) in normal volunteers14 and in patients being treated for ADHD.15 These effects could be potentially deleterious in the POTS population since they experience excessive tachycardia on standing. We hypothesized that because it potentiates noradrenergic pathways, atomoxetine would increase standing HR and worsen the symptom burden in patients with POTS.
Methods
Subjects
Patients with POTS who were referred to the Vanderbilt Autonomic Dysfunction Center between May 2004 and March 2012 were candidates for inclusion in this study. All patients met criteria for POTS by developing symptoms of orthostatic intolerance, accompanied by HR rise ≥30 beats per minute (bpm) within 10 minutes of standing in the absence of orthostatic hypotension (fall in BP ≥20/10 mm Hg).6,9,16 All had symptoms for at least 6 months in the absence of additional chronic disorders known to cause orthostatic intolerance, and all were ≥18 years old. The Vanderbilt University Investigational Review Board approved this study, and written informed consent was obtained from each subject prior to study initiation. The data reported are a part of “The Treatment of Orthostatic Intolerance” study, which is registered with http://www.clinicaltrials.gov (NCT00262470).
Study Diet and Baseline Characterization
All study investigations were performed in the Elliot V. Newman Clinical Research Center. Subjects were placed on a methylxanthine‐free diet with 150 mEq/day sodium and 60 to 80 mEq/day potassium for at least 3 days prior to testing. Subjects were allowed to drink water ad libitum. Long‐term medications were discontinued at least 5 half‐life periods before the study. Fludrocortisone has an elimination half‐life of 3.5 hours,17 but was discontinued at least 5 days before the study in order to avoid potentially extended hormonal effects.
Posture Study
A “posture study” was performed on a separate day from either atomoxetine or placebo evaluation for the purpose of patient diagnosis and baseline characterization. HR, systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and plasma catecholamines were measured after overnight rest with the patient in the supine position and again after standing, as tolerated, for up to 30 minutes. Hemodynamic measures were assessed using an automated oscillometric vital signs monitor (Dinamap, Critikon Corp). For catecholamine measurements, blood was collected in plastic syringes and transferred immediately to chilled heparinized vacuum tubes (BD) on ice. Plasma was centrifuged at −4°C and stored at −80°C in collection tubes with 6% reduced glutathione (Sigma‐Aldrich). Concentrations of norepinephrine and epinephrine were measured by batch alumina extraction followed by high‐performance liquid chromatography for separation with electrochemical detection and quantification.18
Medication Trials
All medication trials were started in the morning at least 2 hours after an early, light breakfast (to avoid acute hemodynamic effects from eating) in a postvoid state. In this trial, patients with POTS were given atomoxetine 40 mg (Eli Lilly Co.) or placebo (“Cebocaps,” Forest Pharmaceuticals), the standard starting dose for atomoxetine in adults, in a randomized crossover fashion on separate days. One coinvestigator (BKB) determined the order of intervention using a random number generator in a 1:1 fashion and then ordered the appropriate study drug, but was not involved in any outcome assessments. All subjects underwent both drug interventions, although not all completed the symptoms score at each time interval. The patient was blind to the intervention. Except during prescribed periods of standing, the patients were seated in a chair during data collection. Brachial oscillometric cuff BP and HR were measured with an automated vital signs monitor (Dinamap, Critikon Corp) and digitally acquired into a custom‐designed database (Microsoft Access, Microsoft Corporation). Immediately before study drug administration, and hourly for 4 hours after study drug administration, each patient was asked to stand from a seated position for 10 minutes while standing HR and BP were recorded. Although this posture change does not increase orthostatic stress as much as standing from a supine position, it does provide a response that is clinically relevant and reproducible.
Symptoms
Patients were asked to rate their symptom burden immediately before and at 2 and 4 hours after study drug administration using the Vanderbilt Orthostatic Symptom Score (VOSS).19 Using a scale of 0 to 10 (0 reflects absence of symptoms), the patients were asked to rate the severity of 9 symptoms. The sum of the scores at each time point was used as a measure of symptom burden (lower score reflects reduced symptom burden). The 9 symptoms were mental clouding, blurred vision, shortness of breath, rapid heartbeat, tremulousness, chest discomfort, headache, lightheadedness, and nausea. These symptoms were selected because they reflect common complaints of patients with POTS. The VOSS has been used previously in acute drug trials at our center.8,19–20
Missing Data
Individual missing hemodynamic data points (due to a failure of the automatic recording) were interpolated by using the within‐individual mean for the parameter at the data point for the hour immediately before and immediately after the missing data point. Hemodynamic data were not interpolated if more than 1 consecutive hourly data point was missing or if either the baseline or 4‐hour (final) value was missing. Only patients with paired sets of complete hemodynamic data (after interpolation) were included in these analyses. The total burden of interpolation was 0.5% of the overall hemodynamic data.
Sample Size Determination
The study was powered to detect a difference in standing heart rate of 10 bpm between groups. Assuming that the pooled standard deviation in standing heart rate was 15 (seen in prior similar analyses), a sample size of 26 would give 90% power to detect such a difference with α=0.05.21
Statistical Analysis
Our primary end point was the standing HR 2 hours after study drug administration. The 2‐hour time point was selected as the primary end point because the peak plasma concentration of atomoxetine occurs 1 to 2 hours after drug administration.22 The primary statistical analysis was a 2‐tailed paired t‐test comparing standing HR at 2 hours after study drug administration between atomoxetine and placebo. The null hypothesis was that standing HR would not be statistically different between the atomoxetine and placebo day.
Secondary analyses were performed using paired t‐tests to compare standing HR at other time points after drug administration as well as seated HR, ΔHR (standing minus seated), standing, seated, and ΔSBP, standing and seated DBP, standing and seated MAP, and VOSS for each time point. Repeated‐measures analysis of variance (ANOVA) were used to compare HR (standing, seated and Δ) and SBP (standing, seated, and Δ) over time on both the atomoxetine and placebo days; the Greenhouse‐Geisser correction to the degrees of freedom from these analyses was used to adjust for departures of the variance‐covariance matrix from the sphericity assumption. ANOVA P values were generated for the effects over time (PTime), the effects of the drug (PDrug) and the interaction of the drugs over time (PInt).
Values are reported as means and standard deviations unless otherwise noted. Probability values ≤0.05 were considered statistically significant for the ANOVA. A threshold of ≤0.0125 was used for posthoc individual paired tests for hemodynamic data due to the multiple comparisons. All tests were 2‐tailed. Statistical analyses were performed with SPSS for Windows (version 21.0, IBM Corporation). Prism for Windows 5 (version 5.02, GraphPad Software Inc.) was used for graphical presentation.
Results
Baseline Characteristics
Patients with POTS (n=27; 25 female, 34±9 years) underwent paired administration of atomoxetine and placebo on different days.
Baseline “posture study” data are presented in Table 1. Supine HR was 73±12 bpm, and BP was 105±10/67±10 mm Hg. The supine plasma norepinephrine (1.33±0.89 nmol/L) and epinephrine (0.078±0.069 nmol/L) values were within the normal range (norepinephrine <2.81 nmol/L and epinephrine <0.41 nmol/L) for each subject, with the exception of 3 subjects with elevated norepinephrine. On standing, there was a significant increase in HR (120±25 bpm; P<0.001), norepinephrine (5.17±2.86 nmol/L; P<0.001), and epinephrine (0.38±0.38 nmol/L; P=0.001).
Table 1.
Postural Vital Signs and Catecholamine Values of the Subjects With Postural Tachycardia Syndrome (n=24)
| Supine | Standing | P Value | |
|---|---|---|---|
| Heart rate, bpm | 73±12 | 120±25 | <0.001 |
| Systolic blood pressure, mm Hg | 105±01 | 100±26 | 0.311 |
| Diastolic blood pressure, mm Hg | 67±10 | 69±18 | 0.542 |
| Norepinephrine, nmol/L | 1.33±0.89 | 4.77±2.64 | <0.001 |
| Epinephrine, nmol/L | 0.33±0.074 | 0.38±0.377 | 0.001 |
Data are presented as the mean±standard deviation. Reported P values are for paired t‐tests comparing supine and upright parameters. bpm indicates beats per minute.
Heart Rate Effects
Baseline seated HR was not significantly different between atomoxetine (86±10 bpm) and placebo (84±12 bpm, P=0.334). Atomoxetine increased seated HR compared with placebo over the 4 hours following drug administration (PDrug=0.002). This effect was seen starting at 1 hour (P<0.002) and continuing at 2 hours (P<0.001), and 4 hours (P<0.001) following study drug administration (Figure 1; Table 2).
Figure 1.
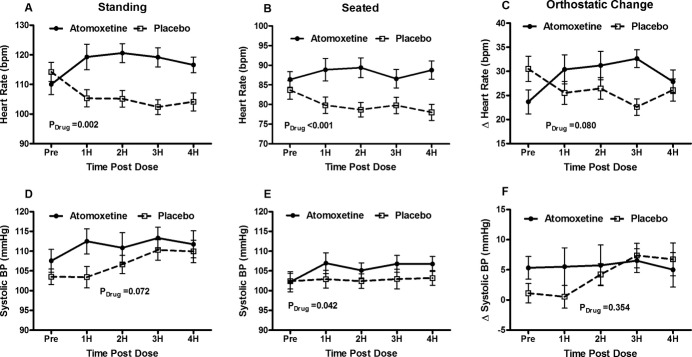
Changes in heart rate (HR) and systolic blood pressure (SBP) before and after atomoxetine vs placebo. HR and SBP data are presented immediately before (pre), and hourly for 4 hours (4H) following study drug administration for the atomoxetine 40 mg day (solid circles) and the placebo day (open squares). Peak HR after standing for a maximum of 10 minutes (A), seated HR immediately before standing (B) and the orthostatic changes in HR (sit to stand; C) are shown. Standing SBP (D), seated SBP (E) and the orthostatic changes in SBP (sit to stand; F) are shown. The error bars represent the standard error of the mean. The ANOVA P values are presented for the overall interaction effect between the study drug and time. ANOVA indicates analysis of variance; bpm, beats per minute.
Table 2.
Orthostatic Hemodynamics and Symptoms With Atomoxetine and Placebo in Patients With Postural Tachycardia Syndrome (n=27)
| Pre | 2 Hours Post | 4 Hours Post | RM ANOVA | |
|---|---|---|---|---|
| P Drug | ||||
| Standing HR, bpm | ||||
| Atomoxetine | 110±18 | 121±17 | 117±14 | |
| Placebo | 114±17 | 105±15.0 | 104±16 | |
| P Value (between drugs) | 0.204 | 0.001 | 0.001 | 0.002 |
| Seated HR, bpm | ||||
| Atomoxetine | 86±10 | 89±13 | 89±12 | |
| Placebo | 84±12 | 79±10 | 78±11 | |
| P Value (between drugs) | 0.334 | <0.001 | <0.001 | <0.001 |
| Δ HR (standing–seated), bpm | ||||
| Atomoxetine | 24±13 | 31±15 | 28±13 | |
| Placebo | 31±14 | 26±12 | 26±12 | |
| P Value (between drugs) | 0.010 | 0.119 | 0.508 | 0.080 |
| Standing SBP, mm Hg | ||||
| Atomoxetine | 108±15 | 111±20 | 112±18 | |
| Placebo | 104±10 | 107±12 | 110±15 | |
| P Value (between drugs) | 0.113 | 0.239 | 0.501 | 0.072 |
| Sitting SBP, mm Hg | ||||
| Atomoxetine | 102±13 | 105±10 | 107±10 | |
| Placebo | 102±10 | 102±10 | 103±10 | |
| P Value (between drugs) | 0.918 | 0.128 | 0.040 | 0.042 |
| HR SBP (standing–seated), mm Hg | ||||
| Atomoxetine | 5±10 | 6±18 | −5±15 | |
| Placebo | 1±8 | 4±9 | 7±14 | |
| P Value (between drugs) | 0.053 | 0.657 | 0.570 | 0.251 |
| Symptom score, au | ||||
| Atomoxetine | 14±10 | 19±15 | 16±15 | |
| Placebo | 18±16 | 15±14 | 14±12 | |
| P Value (between drugs) | 0.054 | 0.250 | 0.622 | 0.038 |
Repeated measures analysis of variance (RM ANOVA) was used to determine the P Value for the overall change between study drug and placebo and paired comparisons were made with the Wilcoxon Signed Rank test for paired data. Data are presented as mean±standard deviation. P<0.05 was considered significant for ANOVA and P<0.0125 was considered significant for the post‐hoc hemodynamic t‐tests. au indicates arbitrary units; bpm, beats per minute; HR, heart rate; NS, not significant; SBP, systolic blood pressure.
Prior to study drug administration, there was no significant difference in standing HR between atomoxetine (110±18 bpm) and placebo (114±17 bpm, P=0.204). Following study drug administration, standing HR increased with atomoxetine and decreased with placebo (PDrug<0.001). Atomoxetine significantly increased HR compared with placebo at 1 hour (P=0.004), 2 hours (121±17 bpm versus 105±15 bpm; P=0.001; primary study endpoint), 3 hours (P<0.001), and 4 hours (P=0.001).
Overall, there was not a statistically significant increase in ΔHR over time with atomoxetine compared with placebo (PDrug=0.080).
Blood Pressure Effects
There was no significant difference in baseline seated (P=0.918) or standing (P=0.113) SBP between groups. Overall, atomoxetine was associated with significantly higher seated SBP (PDrug=0.042) and a trend toward higher standing SBP (PDrug=0.072) (Figure 1).
Symptoms
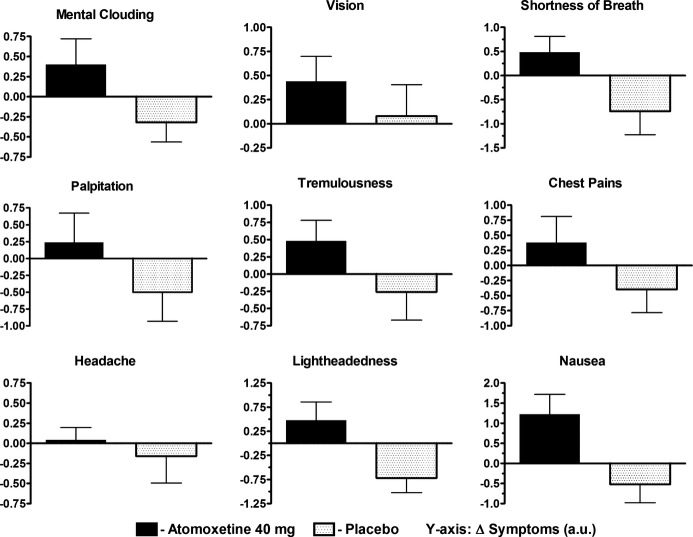
Baseline symptom scores were similar between groups (P=0.054). Over time, atomoxetine worsened the symptoms score compared with placebo (PInt=0.038; Figure 2A). From baseline to 2 hours (time of primary end point), symptom scores significantly increased with atomoxetine (worse) but decreased (improved) with placebo (+4.2 au versus −3.5 au; P=0.028; Figure 2B). While the changes in individual symptoms were not large enough to meet statistical significance, all symptoms, worsened from baseline to 2 hours compared to placebo (Figure 3).
Figure 2.
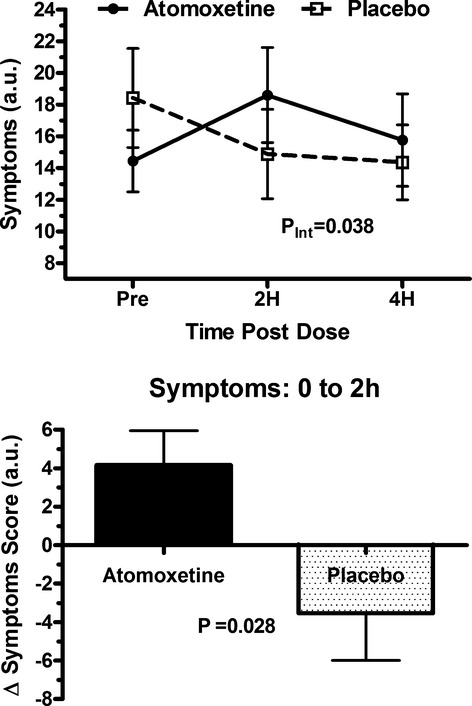
Changes in symptom score with atomoxetine and placebo. Top—Total Vanderbilt Orthostatic Symptoms Score ratings are presented immediately before (pre), at 2 hours (2H) and 4 hours (4H) following study drug administration for the atomoxetine 40 mg day (solid circles) and the placebo day (open squares). The ANOVA P values are presented for the overall interaction effect between the study drug and time. Bottom—The changes in the total Vanderbilt Orthostatic Symptom Score are presented from immediately before to 2 hours after study drug administration for atomoxetine 40 mg (solid black) and placebo (black dots). A negative score reflects a reduction in symptom burden. The error bars represent standard error of the mean. au indicates arbitrary units; PInt, ANOVA P values generated for the interaction of the drugs over time. ANOVA indicates analysis of variance.
Figure 3.
Changes in individual symptoms with atomoxetine and placebo. The changes in the 9 individual components of the Vanderbilt Orthostatic Symptom Score are presented from immediately before to 2 hours after study drug administration for atomoxetine 40 mg (solid black) and placebo (black dots). A negative number represents an improvement in symptoms. The error bars represent standard error of the mean. au indicates arbitrary units.
Discussion
This report is the first placebo‐controlled trial of norepinephrine reuptake inhibition in patients with POTS. We found that (1) oral atomoxetine 40 mg produced a statistically significant increase in standing HR and seated HR compared to placebo; and (2) atomoxetine significantly increased the self‐reported symptom burden in patients with POTS.
Atomoxetine and NET
Atomoxetine is an inhibitor of catecholamine reuptake that possesses a higher affinity for NET than the dopamine or serotonin transporters.23–24 NET is the primary mechanism of norepinephrine synaptic clearance. Inhibition of NET leads to an increased synaptic concentration of norepinephrine and increased activation of pre‐ and postsynaptic adrenoreceptors. While the precise mechanism of action is unclear, it is thought that modulation of noradrenergic signaling in the prefrontal cortex is responsible for atomoxetine's efficacy in the treatment of ADHD. This constitutes its primary FDA‐approved clinical use.
The potentiation of noradrenergic pathways also has effects on the cardiovascular system, resulting in significant increases in HR and BP in patients with ADHD.15 The global effect of atomoxetine on the cardiovascular system is the result of 2 opposing actions. In peripheral sympathetic neurons, atomoxetine increases HR and BP, but the central effect of atomoxetine is a clonidine‐like α‐2 mediated sympatholytic effect that results in decreased supine venous norepinephrine.16,25–28
Atomoxetine Increases HR in POTS
In this study, atomoxetine significantly increased seated HR and standing HR compared with placebo in patients with POTS. The ΔHR was not significantly increased with atomoxetine, likely because both standing and seated HR increased comparably with atomoxetine. The increases in HR and BP observed in this study indicate that, in patients with POTS, peripheral potentiation of noradrenergic signaling by atomoxetine likely predominated over its central sympatholytic effects. This effect is consistent with the finding that the overall effect of oral atomoxetine in patients with ADHD was an increase in HR and BP. Given that orthostatic tachycardia is a characteristic of patients with POTS, medications like atomoxetine that increase standing HR should likely be avoided due to their potential to exacerbate this core feature of their disease. Unfortunately, the alternative medications for ADHD are stimulants,29 which are likely to also be poorly tolerated in POTS for similar reasons.
Symptoms
Atomoxetine significantly increased symptom burden compared with placebo. Interestingly, this contrasted sharply with a decreased symptom burden at 2 hours for the placebo group. Given that atomoxetine increased standing HR compared with placebo, it is not surprising that symptoms worsened. Several placebo‐controlled medication trials in POTS that reported a decrease in symptom burden also reported a decrease in standing HR.8,19–20 Interestingly, there was a nonsignificant increase in symptom score for each of the 9 symptoms from baseline to 2 hours for the atomoxetine group, suggesting that atomoxetine consistently worsened all the core symptoms of POTS. As symptom control is the mainstay of POTS treatment, the increase in symptom‐burden and HR suggest that NRI medications are unlikely to be tolerated in POTS patients.
Response to Placebo
As has been seen in prior acute, placebo‐controlled medication trials in POTS,8,19–20 the standing HR decreased over time on the placebo day (Table 2). This was associated with a small reduction in symptoms score with placebo, likely driven by the reduction in standing HR. The reasons underlying this HR reduction with placebo are not clear. Possibilities include diurnal variability in standing HR,30 a “training effect” from repeated standing in the morning of the study, or a psychological benefit from expectation of beneficial therapy. Importantly, other therapies8,19–20 showed a reduction in HR and symptoms score greater than placebo while atomoxetine behaved in the opposite manner (increasing both HR and symptoms scores).
Norepinephrine and POTS
Despite the heterogeneous pathophysiology of POTS, increased sympathetic activity seems to be a common final pathway, and thus an area of focus in POTS research. There are two possible mechanisms for increased synaptic concentrations of norepinephrine: an increase in synaptic norepinephrine release or a decrease in synaptic norepinephrine clearance. Synaptic norepinephrine clearance is accomplished in 2 ways: 80% to 90% is cleared by presynaptic neuronal reuptake of norepinephrine via NET and 10% to 20% is cleared by diffusion out of the synaptic cleft and into the circulation or extraneuronal tissues.31
Altered NET Expression in POTS
The first indication that altered NET activity was implicated in the hyperadrenergic state observed in POTS came from the study of a 33‐year‐old female with a 20‐year history of orthostatic tachycardia (among other symptoms of orthostatic intolerance).32 In response to upright posture, she experienced a 4‐fold increase in plasma norepinephrine, but only a doubling of muscle sympathetic nerve activity, indicating an electrochemical dissociation in the sympathetic neuron. A point mutation in the coding region of the NET gene (SLC6A2) was identified that encoded a dysfunctional protein with dramatically reduced norepinephrine reuptake compared to wild‐type NET. While neither this mutation, nor single nucleotide polymorphisms (SNPs) in the NET gene have been found in other unrelated POTS patients, Lambert et al33 have found that some POTS patients have decreased NET protein expression when compared with healthy subjects. This may be attributable to altered posttranscriptional modification.34 This suggests that reduced NET expression may be more globally involved in the pathophysiology of POTS.
Altered NET Activity and Atomoxetine
The increased HR in response to atomoxetine seen in this study is consistent with the growing evidence that decreased expression or activity of NET is involved in the pathophysiology of POTS.33–34 If reduced NET activity is present in some patients with POTS, then a further decrease in NET activity (such as with NRI medications) could exacerbate the signs and symptoms of POTS. This model aligns with our study findings of a significant increase in both HR and symptom burden with atomoxetine compared with placebo. There are also potential safety concerns with NRI medications. The SCOUT (Sibutramine Cardiovascular OUTcomes) study found that long‐term use of sibutramine in patients with known cardiovascular disease resulted in an increased risk of nonfatal myocardial infarction and nonfatal stroke.35 NRI medications also have complex effects on cognition, with increasing cognitive impairment at higher levels. This might limit tolerability in some POTS patients given their altered NET expression.36
Study Limitations
Detailed sympathetic nervous system assessments were not performed before and after atomoxetine administration in this study. Assessments of sympathetic nerve traffic and plasma norepinephrine levels might help to better understand the physiological responses observed in this trial. Further, this was an acute study, and longer‐term studies are needed to assess chronic tolerability and clinical utility of NRIs in POTS.
Conclusions
NET inhibition with atomoxetine acutely increased standing HR and worsened symptom burden in patients with POTS. This suggests that NRIs are poorly tolerated in patients with POTS and should be administered with caution.
Sources of Funding
Supported in part by NIH grants R01 HL102387, U54 NS065736, P01 HL56693, K23 HL103976 and UL1 TR000445 (Clinical and Translational Science Award). Dr Shibao is also supported by the PhRMA foundation (Washington, DC).
Disclosures
None.
Acknowledgments
We would like to thank our patients who participated in this study and to recognize the highly professional care provided by the staff of the Elliot V. Newman Clinical Research Center at Vanderbilt University.
References
- 1.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993; 43:132-137 [DOI] [PubMed] [Google Scholar]
- 2.Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999; 317:88-101 [DOI] [PubMed] [Google Scholar]
- 3.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007; 69:790-798 [DOI] [PubMed] [Google Scholar]
- 4.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, Robertson D, Raj SR. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011; 7:204-210 [PMC free article] [PubMed] [Google Scholar]
- 5.Benrud‐Larson LM, Sandroni P, Haythornthwaite JA, Rummans TA, Low PA. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross‐sectional findings. Health Psychol. 2003; 22:643-648 [DOI] [PubMed] [Google Scholar]
- 6.Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci. 2007; 334:57-60 [DOI] [PubMed] [Google Scholar]
- 7.Fouad FM, Tadena‐Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986; 104:298-303 [DOI] [PubMed] [Google Scholar]
- 8.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009; 120:725-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raj SR. Postural Tachycardia Syndrome (POTS). Circulation. 2013; 127:2336-2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childress AC, Berry SA. Pharmacotherapy of attention‐deficit hyperactivity disorder in adolescents. Drugs. 2012; 72:309-325 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan G, Newcorn JH. Pharmacotherapy for child and adolescent attention‐deficit hyperactivity disorder. Pediatr Clin North Am. 2011; 58:99-120 [DOI] [PubMed] [Google Scholar]
- 12.Grubb BP. Postural tachycardia syndrome. Circulation. 2008; 117:2814-2817 [DOI] [PubMed] [Google Scholar]
- 13.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Use of methylphenidate in the treatment of patients suffering from refractory postural tachycardia syndrome. Am J Ther. 2012; 19:2-6 [DOI] [PubMed] [Google Scholar]
- 14.Kelly RP, Yeo KP, Teng CH, Smith BP, Lowe S, Soon D, Read HA, Wise SD. Hemodynamic effects of acute administration of atomoxetine and methylphenidate. J Clin Pharmacol. 2005; 45:851-855 [DOI] [PubMed] [Google Scholar]
- 15.Wernicke JF, Faries D, Girod D, Brown J, Gao H, Kelsey D, Quintana H, Lipetz R, Michelson D, Heiligenstein J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003; 26:729-740 [DOI] [PubMed] [Google Scholar]
- 16.Schroeder C, Birkenfeld AL, Mayer AF, Tank J, Diedrich A, Luft FC, Jordan J. Norepinephrine transporter inhibition prevents tilt‐induced pre‐syncope. J Am Coll Cardiol. 2006; 48:516-522 [DOI] [PubMed] [Google Scholar]
- 17.Monarch Pharmaceuticals I. Florinef acetate – fludrocortisone acetate tablet product label. Daily Med NIH Gov 2011. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=71912 [Google Scholar]
- 18.Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda‐Garcia R, Robertson RM, Robertson D. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation. 1997; 96:575-580 [DOI] [PubMed] [Google Scholar]
- 19.Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005; 111:2734-2740 [DOI] [PubMed] [Google Scholar]
- 20.Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, Dupont WD, Robertson D, Raj SR. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 2012; 9:1484-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990; 11:116-128 [DOI] [PubMed] [Google Scholar]
- 22.Witcher JW, Long A, Smith B, Sauer JM, Heilgenstein J, Wilens T, Spencer T, Biederman J. Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2003; 13:53-63 [DOI] [PubMed] [Google Scholar]
- 23.Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther. 1982; 222:61-65 [PubMed] [Google Scholar]
- 24.Bolden‐Watson C, Richelson E. Blockade by newly‐developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993; 52:1023-1029 [DOI] [PubMed] [Google Scholar]
- 25.Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol. 1991; 260:R817-R823 [DOI] [PubMed] [Google Scholar]
- 26.Birkenfeld AL, Schroeder C, Boschmann M, Tank J, Franke G, Luft FC, Biaggioni I, Sharma AM, Jordan J. Paradoxical effect of sibutramine on autonomic cardiovascular regulation. Circulation. 2002; 106:2459-2465 [DOI] [PubMed] [Google Scholar]
- 27.Eisenhofer G, Saigusa T, Esler MD, Cox HS, Angus JA, Dorward PK. Central sympathoinhibition and peripheral neuronal uptake blockade after desipramine in rabbits. Am J Physiol. 1991; 260:R824-R832 [DOI] [PubMed] [Google Scholar]
- 28.Tank J, Schroeder C, Diedrich A, Szczech E, Haertter S, Sharma AM, Luft FC, Jordan J. Selective impairment in sympathetic vasomotor control with norepinephrine transporter inhibition. Circulation. 2003; 107:2949-2954 [DOI] [PubMed] [Google Scholar]
- 29.Ramos‐Quiroga JA, Montoya A, Kutzelnigg A, Deberdt W, Sobanski E. Attention deficit hyperactivity disorder in the European adult population: prevalence, disease awareness, and treatment guidelines. Curr Med Res Opin. 2013; 29:1093-1104 [DOI] [PubMed] [Google Scholar]
- 30.Plash WB, Diedrich A, Biaggioni I, Garland EM, Paranjape SY, Black BK, Dupont WD, Raj SR. Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond). 2013; 124:109-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990; 70:963-985 [DOI] [PubMed] [Google Scholar]
- 32.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency. N Engl J Med. 2000; 342:541-549 [DOI] [PubMed] [Google Scholar]
- 33.Lambert E, Eikelis N, Esler M, Dawood T, Schlaich M, Bayles R, Socratous F, Agrotis A, Jennings G, Lambert G, Vaddadi G. Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ Arrhythm Electrophysiol. 2008; 1:103-109 [DOI] [PubMed] [Google Scholar]
- 34.Bayles R, Harikrishnan KN, Lambert E, Baker EK, Agrotis A, Guo L, Jowett JB, Esler M, Lambert G, El‐Osta A. Epigenetic modification of the norepinephrine transporter gene in postural tachycardia syndrome. Arterioscler Thromb Vasc Biol. 2012; 32:1910-1916 [DOI] [PubMed] [Google Scholar]
- 35.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp‐Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010; 363:905-917 [DOI] [PubMed] [Google Scholar]
- 36.Del CN, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention‐deficit/hyperactivity disorder. Biol Psychiatry. 2011; 69:e145-e157 [DOI] [PubMed] [Google Scholar]