Abstract
Background
Obesity is linked with an increased risk of lymphedema, which is a serious clinical problem. Adiponectin is a circulating adipokine that is down‐regulated in obese states. We investigated the effects of adiponectin on lymphatic vessel formation in a model of lymphedema and dissected its mechanisms.
Methods and Results
A mouse model of lymphedema was created via ablation of tail surface lymphatic network. Adiponectin‐knockout mice showed the greater diameter of the injured tail compared with wild‐type mice, which was associated with lower numbers of lymphatic endothelial cells (LECs). Systemic delivery of adiponectin reduced the thickness of the injured tail and enhanced LEC formation in wild‐type and adiponectin‐knockout mice. Adiponectin administration also improved the edema of injured tails in obese KKAy mice. Treatment with adiponectin protein stimulated the differentiation of human LECs into tubelike structures and increased LEC viability. Adiponectin treatment promoted the phosphorylation of AMP‐activated protein kinase (AMPK), Akt, and endothelial nitric oxide synthase n LECs. Blockade of AMPK or Akt activity abolished adiponectin‐stimulated increase in LEC differentiation and viability and endothelial nitric oxide synthase phosphorylation. Inhibition of AMPK activation also suppressed adiponectin‐induced Akt phosphorylation in LECs. In contrast, inactivation of Akt signaling had no effects on adiponectin‐mediated AMPK phosphorylation in LECs. Furthermore, adiponectin administration did not affect the thickening of the damaged tail in endothelial nitric oxide synthase–knockout mice.
Conclusions
Adiponectin can promote lymphatic vessel formation via activation of AMPK/Akt/endothelial nitric oxide synthase signaling within LECs, thereby leading to amelioration of lymphedema.
Keywords: adiponectin, Akt, AMPK, eNOS, lymphangiogenesis
Introduction
Lymphedema is a serious clinical problem after surgical and radiation therapy for malignant diseases including breast cancer.1–2 Lymphedema in humans typically results from a destruction of lymphatic network and the failure of lymphatic fluid to drain.3 Although a number of recent reports showed that the induction of lymphangiogenesis could ameliorate lymphedema in animal models,4–7 the therapeutic options for lymphedema are currently limited.
Obesity is strongly associated with an increased risk of lymphedema. A previous study showed that overweight is associated with arm swelling after the treatment of breast cancer.8 Obesity is also linked with the development of postoperative lymphedema.9 Furthermore, an elevated body mass index is associated with an increase in lymphedema of the lower extremities.10 Conversely, weight reduction effectively improves breast cancer–related lymphedema.1 Thus, in this context, dietetic therapy is recommended as a treatment option for obese patients with lymphedema.11 However, the molecular mechanisms that are related to how obesity affects the development of lymphedema are poorly understood.
Accumulating evidence suggests that adipose tissue functions as an endocrine organ by secreting adipocytokines that can directly affect nearby or remote organs.12 Adiponectin is an adipose‐specific plasma adipokine whose concentration is down‐regulated in association with various obesity‐related diseases.13–14 Low levels of circulating adiponectin are associated with the increased prevalence of obesity‐linked vascular disorders including endothelial dysfunction and peripheral artery disease.15–16 We have demonstrated that treatment with adiponectin protein stimulates vascular endothelial cell migration, survival, and differentiation into capillary‐like structures in vitro.17–19 Adiponectin has also been shown to stimulate blood vessel growth in rabbit corneal and mouse Matrigel plug angiogenesis assays.17 Furthermore, we have shown that the disruption of adiponectin contributes to impaired revascularization in response to tissue ischemia and exacerbation of endothelial dysfunction.15,20 Thus, adiponectin exerts beneficial actions on blood vessel function by directly, at least in part, acting on vascular endothelial cells. However, nothing is known about the functional role of adiponectin in the lymphatic system.
In the present study, we investigated whether adiponectin influences lymphatic vessel formation in vivo in a mouse model of lymphedema with loss‐ and gain‐of function genetic manipulations. We also tested whether adiponectin modulates lymphatic endothelial cell (LEC) behavior in vitro and assessed its molecular mechanisms.
Methods
Materials
Recombinant human adiponectin protein obtained from mammalian cell expression system was purchased from BioVendor. Recombinant human vascular endothelial growth factor (VEGF)‐C was purchased from R&D Systems. The following primary antibodies were purchased from Cell Signaling: phospho‐Akt (Ser473) antibody, Akt antibody, phospho–endothelial nitric oxide synthase (eNOS; Ser1177) antibody, eNOS antibody, acetyl‐CoA carboxylase (ACC) antibody, hemagglutinin (HA) antibody, and β‐actin antibody. Phospho‐ACC (Ser79) and c‐Myc tag antibody were purchased from Upstate Biotechnology. Compound C and LY294002 were purchased from Calbiochem. L‐NG‐Nitroarginine Methyl Ester (L‐NAME) was purchased from Sigma Chemical Co. Adenovirus vectors containing the gene for β‐galactosidase (Ad‐β‐gal) and full‐length mouse adiponectin (Ad‐APN) were prepared as described previously.21 LECs were purchased from Lonza (HMVEC‐dLy).
Mouse Model of Tail Lymphedema
Male wild‐type (WT), adiponectin‐knockout (APN‐KO),22 and eNOS‐deficient (eNOS‐KO) mice at the ages of 8 to 10 weeks (Jackson Laboratory) in a C57/BL6 background and male diabetic KKAy mice at the ages of 10 weeks (Jackson Laboratory) were used for this study. Study protocols were approved by the Institutional Animal Care and Use Committee in Nagoya University. Mice were subjected to the ablation surgery of tail surface lymphatic network under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally) as previously described.4 In briefly, a 2‐mm‐wide circumferential annulus of the skin at 10 mm distal to the tail base, except for a 2‐mm2 dermal frap located at the ventral side, was excised from the tail using a cautery knife. In some experiments, 2×108 plaque‐forming units (pfu) of Ad‐APN or Ad‐β‐gal was systemically injected into the tail vein of mice 3 days before tail lymphatic vessel ablation.
Tail Thickness Measurement
The diameter of the tail simply represents a severity of the lymphedema. Tail diameter of the lymph‐edematous site was measured at the point exactly 10 mm distal to the incision site. At the end of the follow up, mice were killed, and their tails were obtained, sliced, and snap frozen with liquid nitrogen. Frozen tissue sections were sliced and stained with hematoxylin‐eosin (H&E).
To examine the extent of LEC density distal to the incision site, frozen tissue sections were subjected to immunohistochemical staining using a polyclonal antibody against lymphatic vascular endothelial hyaluronan receptor‐1 (LYVE‐1; Acris).
Cell Culture
LECs were cultured in endothelial cell growth medium (EGM)‐2MV. Before each experiment, cells were placed in endothelial cell basal medium (EBM)‐2 for 15 hours for serum starvation. Experiments were performed by the addition of the indicated amount of human recombinant adiponectin (30 μg/mL) or vehicle for the indicated lengths of time. In some experiments, LECs were pretreated with compound C (10 μmol/L), LY294002 (50 μmol/L), L‐NAME (1 mmol/L), or vehicle (DMSO) for 60 minutes before adiponectin stimulation. In some experiments, LECs were infected with an adenoviral vector encoding an HA‐tagged dominant‐negative AKT1 (Ad‐dnAkt), a c‐myc–tagged dominant‐negative mutant of AMPK (Ad‐dnAMPK), or Ad‐β‐gal as a control at a multiplicity of infection of 50 for 24 hours.
Tube Formation Assay
The formation of vessel‐like structures by LECs on growth factor–reduced Matrigel (BD Biosciences) was assessed according to the manufacturer's instructions as previously described.17 LECs were seeded onto coated plates at 5×104 cells/cm2 in EBM‐2 medium and incubated at 37°C for 18 hours. Network formation was assessed using an inverted phase contrast microscope (Nikon), and photomicrographs were taken at ×40 magnification. The degree of tube formation was quantified by measuring the length of tubes in 3 randomly chosen fields from each well using the Image‐J program. Each experiment was repeated 3 times.
Cell Viability Assay
Cell viability of LECs was determined by use of the PreMix WST‐1 Cell Proliferation Assay System (TAKARA Bio Inc). In brief, LECs were cultured with 100 μL of EBM‐2 with 0.5% fetal bovine serum that did not contain supplement onto 96‐well plates (3×104 cells in 100 μL of culture medium per well). After 24 hours of serum starvation, cells were incubated with EBM‐2 supplemented in the presence of recombinant adiponectin protein (30 μg/mL), VEGF‐C (50 ng/mL), or vehicle for 48 hours. Then, WST‐1 was added to dishes according to the manufacturer's instruction and incubated for an additional 5 hours, and then the absorbance was measured.
Apoptosis Assay
Terminal deoxynucleotidyl transferase–mediated dUTP nick‐end labeling (TUNEL) staining for LECs was performed using the In Situ Cell Death detection kit (Roche) as described previously.23 Cells were treated with recombinant adiponectin protein or vehicle followed by 48 hours of incubation in serum‐free EBM‐2. TUNEL‐positive cells were counted in 5 randomly selected microscopic fields. Each experiment was repeated 3 times.
Western Blot Analysis
Cell lysates were resolved by use of SDS‐PAGE as described previously.17 The membranes were immunoblotted with the indicated antibodies at a 1:1000 dilution followed by the secondary antibody conjugated with horseradish peroxidase at a 1:1000 dilution. An ECL Western blotting Detection kit (GE Healthcare) was used for detection.
Statistical Analysis
Results are expressed as mean±SE. Statistical significance was analyzed with unpaired Student's t test for comparison between the 2 groups (Figures 3C and 4D) and with ANOVA followed by Turkey's procedure for comparison among 3 groups or more (Figures 2B, 4B, 4C, and 6A through 6C). We used 2‐way repeated measures ANOVA, when multiple time points were involved (Figures 1B, 3B, 3D, and 6D). P values <0.05 denoted statistical significance.
Figure 1.
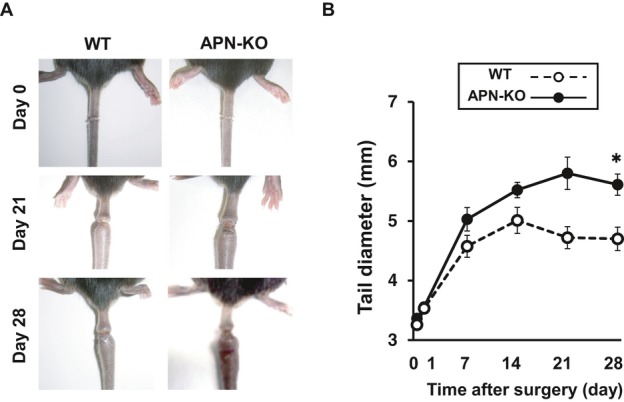
Adiponectin deficiency exacerbates lymphedema in a mouse tail model. A, Representative tails in the WT and APN‐KO mice immediately after surgery (day 0) and on postoperative days 21 and 28. B, Quantitative analysis of the tail diameter in the WT and APN‐KO mice immediately after surgery and at different time points after surgery (n=7 in each group). Results are shown as the mean±SE. *P<0.01 vs WT mice. APN indicates adiponectin; KO, knockout; WT, wild‐type.
Results
Adiponectin Deficiency Exacerbates Lymphedema After Ablation of Tail Lymphatic Vessels
To test whether adiponectin modulates lymphedema in vivo, we subjected APN‐KO and WT mice to the ablation of tail surface lymphatic network. Figure 1A shows representative pictures of tail in the WT and APN‐KO mice immediately after surgery and on postoperative days 21 and 28. In WT mice, lymphedema was significantly induced within a few days after surgery, and the tail diameter peaked at around postoperative day 7. Lymphedema continued until at least 28 day after surgery. APN‐KO mice exhibited a significant increase in tail diameter after surgery compared with WT mice (Figure 1A and 1B).
Because inhibition of lymphangiogenesis is associated with enhancement of lymphedema, we assessed the frequency of LECs in histological sections harvested from tails in WT and APN‐KO mice by immunostaining with LEC marker LYVE‐1. Figure 2A shows representative photomicrographs of tail tissues stained with LYVE‐1. Quantitative analysis of LYVE‐1–positive cells revealed that WT mice showed a marked reduction of LEC density at day 28 after tail ablation surgery compared with sham‐operated WT mice and that APN‐KO mice exhibited a further reduction of LEC density in injured tails on postoperative day 28 compared with WT mice (Figure 2B). Immunofluorescence staining also revealed that LYVE‐1–positive cells coexpressed podoplanin but not platelet endothelial cell adhesion molecule‐1 (PECAM‐1) in the edematous tissue consistent with the previous report4 (data not shown).
Figure 2.
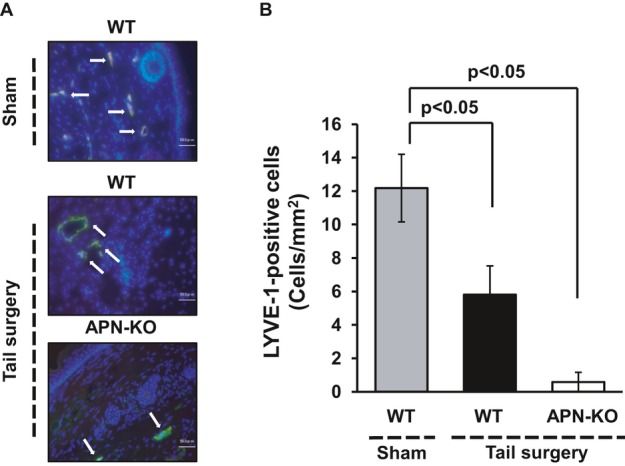
Adiponectin deficiency reduces the number of lymphatic endothelial cells in a mouse model of lymphedema. A, Fluorescence staining of tail tissues with lymphatic endothelial cell marker, LYVE‐1 (green), in WT or APN‐KO mice at day 28 after tail ablation surgery or sham operation (white arrows). Total nuclei were identified by 4′,6‐diamidino‐2‐phenylindole counterstaining (blue). B, Quantitative analysis of LYVE‐1–positive cells in the injured tails in WT or APN‐KO mice at day 28 after tail ablation surgery or sham operation (n=7 in each group). Results are shown as the mean±SE. APN indicates adiponectin; KO, knockout; LYVE‐1, lymphatic vascular endothelial hyaluronan receptor‐1; WT, wild‐type.
Adiponectin Improves Lymphedema and Enhances LEC Formation In Vivo
To test whether supplementation of adiponectin could modulate lymphedema, we delivered Ad‐APN or Ad‐β‐gal as a control via jugular vein into WT and APN‐KO mice at 3 days before tail ablation of lymphatic vessel. On day 6 after the injection, plasma adiponectin levels were 13.1±1.6 μg/mL in WT/Ad‐β‐gal, 22.8±3.4 μg/mL in WT/Ad‐APN, <0.05 μg/mL in APN‐KO/Ad‐β‐gal, and 12.5±1.3 μg/mL in APN‐KO/Ad‐APN. Ad‐APN–treated WT mice showed a significant decrease in thickness of injured tails after surgery compared with control mice that were treated with Ad‐β‐gal (Figure 3 A and 3B). Treatment with Ad‐APN reduced the diameter of edematous tails in APN‐KO mice to the levels similar to those of WT mice.
Figure 3.
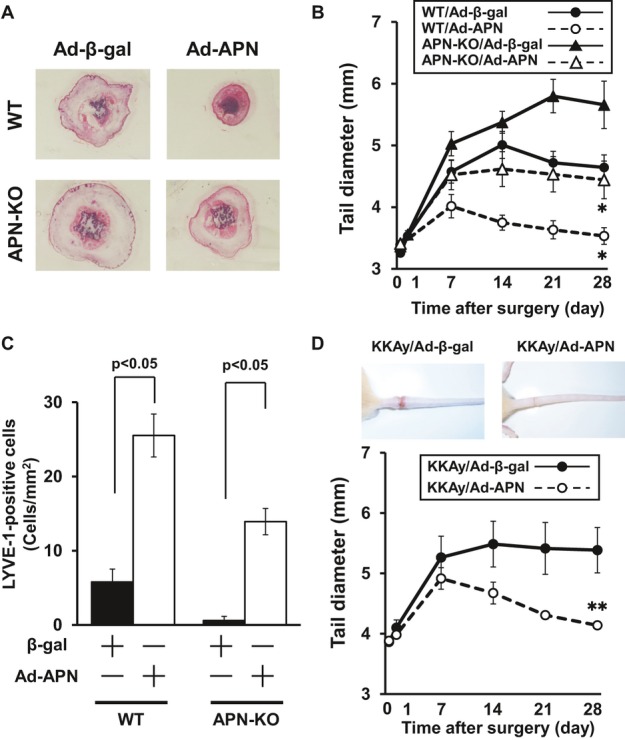
Effect of exogenous adiponectin on lymphedema and lymphatic endothelial cell formation. Ad‐APN or Ad‐β‐gal (control) was injected into the jugular vein of WT, APN‐KO or KKAy mice (2×108 pfu in each group) at 3 days prior to surgery. A, Representative histological sections of edematous tails in WT and APN‐KO mice treated with Ad‐β‐gal or Ad‐APN on postoperative day 28. B, Quantitative analysis of the tail diameter in WT and APN‐KO mice treated with Ad‐β‐gal or Ad‐APN at different time points after surgery (n=6 in each group). C, Quantitative analysis of LYVE‐1–positive cells in injured tails in WT and APN‐KO mice treated with Ad‐β‐gal or Ad‐APN on postoperative day 28 (n=6 in each group). D, Quantitative analysis of the tail diameter in KKAy mice treated with Ad‐β‐gal and Ad‐APN at different time points after surgery (n=7 in each group). Results are sown as the mean±SE. *P<0.05, **P<0.01 vs Ad‐β‐gal. Ad indicates adenovirus; APN, adiponectin; β‐gal, β‐galactosidase; KO, knockout; LYVE‐1, lymphatic vascular endothelial hyaluronan receptor‐1; WT, wild‐type.
To examine whether increased levels of adiponectin affect lymphangiogenesis in vivo, we assessed the number of LECs in edematous tails in WT and APN‐KO mice treated with Ad‐APN or Ad‐β‐gal. Quantitative analysis of LYVE‐1–positive cells revealed that Ad‐APN treatment increased the LEC density in both WT and APN‐KO mice (Figure 3C).
Furthermore, we examined whether adiponectin affects lymphedema in obese states. KKAy mice were treated systemically with Ad‐APN or Ad‐β‐gal at 3 days before surgery. Plasma adiponectin levels were 8.5±0.5 μg/mL in KKAy/Ad‐β‐gal and 15.7±0.4 μg/mL in KKAy/Ad‐APN on day 6 after the injection. Ad‐APN treatment decreased the thickness of damaged tails in KKAy mice (Figure 3D).
Adiponectin Promotes LEC Differentiation and Viability In Vitro
We next examined whether adiponectin modulates LEC differentiation into capillary‐like structures when LECs were plated onto a Matrigel matrix. LECs were treated with recombinant human adiponectin protein or vehicle. Treatment with a physiological concentration of adiponectin promoted the formation of capillary‐like tubes (Figure 4A). Quantitative analysis of the LEC network revealed that treatment with adiponectin significantly increased the tube length relative to control cultures (Figure 4B). To test whether adiponectin modulates the LEC viability, a WST‐1–based assay was performed under serum‐deprived conditions. Adiponectin significantly stimulated LEC viability in serum‐starved cultures (Figure 4C). The effects of adiponectin on LEC differentiation and viability were comparable with those of VEGF‐C, which is reported to act as a potent lymphatic growth factor (Figure 4A through C). To corroborate these observations, apoptotic LECs were measured by use of TUNEL staining. Adiponectin significantly suppressed the number of TUNEL‐positive cells under serum‐deprived conditions (Figure 4D). Thus, adiponectin promotes prolymphangiogenic cellular responses in vitro.
Figure 4.
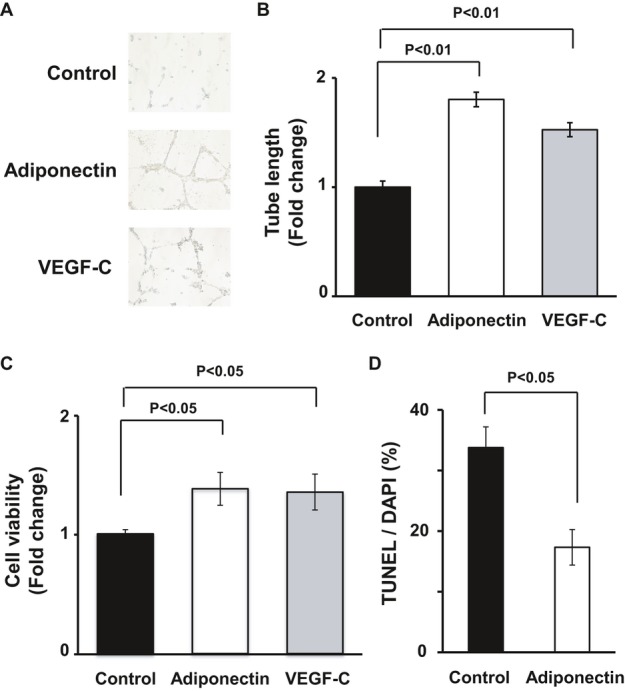
Adiponectin promotes lymphatic endothelial cell differentiation and survival in vitro. A and B, Representative photomicrographs of network formation of LECs (A) and quantitative analyses of network tube length (B) (n=3 in each group). After 18 hours of serum deprivation, LECs were cultured in Matrigel‐coated dishes in the presence of recombinant adiponectin protein (30 μg/mL), VEGF‐C (50 ng/mL), or vehicle. C, WST‐1–based assay for evaluating cell viability was performed. LECs were treated with adiponectin (30 μg/mL), VEGF‐C (50 ng/mL), or vehicle in serum‐starved media for 48 hours (n=4 in each group). Results are expressed relative to the values compared with control. D, TUNEL assay for detecting apoptotic cells was performed. LECs were treated with adiponectin (30 μg/mL) or vehicle in serum‐starved media for 48 hours (n=5 in each group). Results are shown as the mean±SE. DAPI indicates 4′,6‐diamidino‐2‐phenylindole; LEC, lymphatic endothelial cell; TUNEL, transferase–mediated dUTP nick‐end labeling.
Adiponectin Stimulates the Phosphorylation of eNOS Through Activation of AMPK‐Akt Signaling
AMPK signaling is associated with the regulation of angiogenesis under pathological states.17,20,24 Therefore, to test whether adiponectin induces AMPK signaling in LECs, cultured LECs were incubated with adiponectin protein or vehicle, and AMPK phosphorylation at Thr172 was assessed by the use of Western blot analysis. Treatment with adiponectin stimulated the phosphorylation of AMPK in a time‐dependent manner with maximal AMPK phosphorylation occurring at 30 minutes (Figure 5A). Akt also plays important roles in the angiogenic response to several growth factors.25 Therefore, the effect of adiponectin on the activating phosphorylation of Akt at Ser473 in LECs was assessed. Adiponectin treatment led to a time‐dependent increase in Akt phosphorylation (Figure 5A). Because AMPK and Akt can phosphorylate and activate eNOS at Ser1179 in endothelial cells,26–27 eNOS phosphorylation was examined in these cultures. Adiponectin stimulated the phosphorylation of eNOS, with maximal levels occurring at 60 minutes (Figure 5A). Adiponectin had no effects on AMPK, Akt, and eNOS protein levels.
Figure 5.
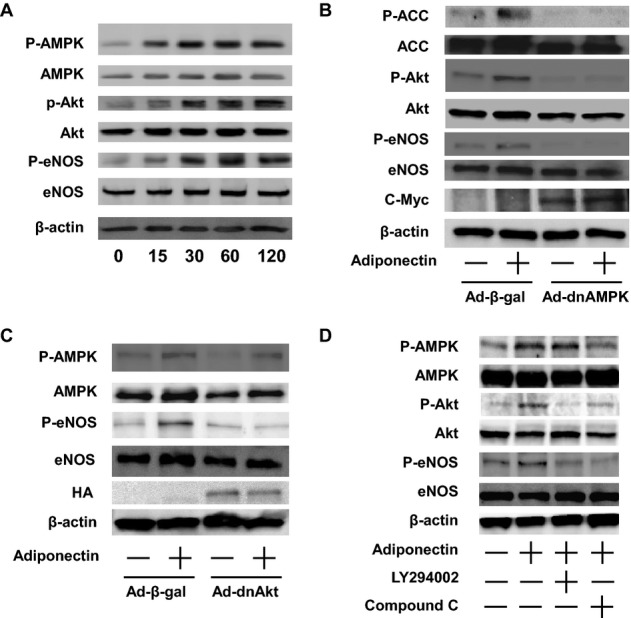
Adiponectin stimulates the phosphorylation of AMPK, Akt, and eNOS in LECs. A, Time‐dependent changes in the phosphorylation of AMPK, Akt, and eNOS signaling in LECs following stimulation with adiponectin. The phosphorylation of AMPK (P‐AMPK), Akt (P‐Akt), and eNOS (P‐eNOS) was determined by Western blot analysis. Representative blots are shown from 4 independent experiments. B, Role of AMPK in regulation of adiponectin‐induced Akt and eNOS signaling. LECs were transduced with c‐myc–tagged Ad‐dnAMPK or Ad‐β‐gal for 24 hours. After 16 hours of serum starvation, cells were treated with adiponectin (30 μg/mL) or vehicle for 60 minutes. P‐ACC, P‐Akt, and P‐eNOS were determined by Western blot analysis. Representative blots are shown from 4 independent experiments. C, Role of Akt in regulation of adiponectin‐induced eNOS signaling. LECs were transduced with HA‐tagged Ad‐dnAkt or Ad‐β‐gal for 24 hours. After 16 hours of serum starvation, cells were treated with adiponectin (30 μg/mL) of vehicle for 60 minutes. P‐AMPK and P‐eNOS were determined by Western blot analysis. Representative blots are shown from 4 independent experiments. D, Effect of compound C and LY294002 on adiponectin‐stimulated phosphorylation of AMPK, Akt, and eNOS. LECs were pretreated with compound C (10 μmol/L), LY294002 (50 μmol/L), or vehicle (DMSO) for 60 minutes and treated with adiponectin (30 μg/mL) or vehicle for 60 minutes. P‐AMPK, P‐Akt, and P‐eNOS were determined by Western blot analysis. Representative blots are shown from 4 independent experiments. Ad indicates adenovirus; AMPK, AMP‐activated protein kinase; β‐gal, β‐galactosidase; DMSO, dimethyl sulfoxide; eNOS, endothelial nitric oxide synthase; HA, hemagglutinin; LEC, lymphatic endothelial cell.
To examine the relative contribution of AMPK and Akt to the regulation of adiponectin‐induced phosphorylation of eNOS, LECs were transduced with Ad‐dnAMPK, Ad‐dnAkt, or control Ad‐β‐gal followed by treatment with adiponectin protein. Transduction with Ad‐dnAMPK suppressed adiponectin‐induced phosphorylation of ACC, which is downstream of AMPK (Figure 5B). Transduction with Ad‐dnAMPK also attenuated adiponectin‐stimulated phosphorylation of Akt and eNOS in LECs. Moreover, transduction with Ad‐dnAkt suppressed the adiponectin‐induced phosphorylation of eNOS without altering that of AMPK (Figure 5C). To corroborate these findings, LECs were treated with the AMPK inhibitor compound C, the phosphoinositide‐3 kinase inhibitor LY294002, or vehicle. Pretreatment with compound C blocked adiponectin‐stimulated phosphorylation of AMPK, Akt, and eNOS (Figure 5D). LY294002 treatment reduced adiponectin‐induced phosphorylation of Akt and eNOS, while LY294002 had no effect on AMPK phosphorylation (Figure 5D). These data suggest that adiponectin stimulates eNOS phosphorylation in LECs via activation of both AMPK‐ and Akt‐dependent signaling.
Role of eNOS Signaling in Lymphangiogenic Responses to Adiponectin In Vitro and In Vivo
To test whether AMPK signaling is involved in adiponectin‐mediated LEC function, LECs were pretreated with compound C or vehicle. Treatment with compound C cancelled adiponectin‐stimulated differentiation of LECs into capillary‐like tubes (Figure 6A). Treatment with LY294002 also abolished the stimulatory actions of adiponectin on LEC differentiation into capillary‐like structures. To test the potential involvement of eNOS in LEC responses to adiponectin, LECs were treated with the NOS inhibitor L‐NAME or vehicle. Treatment with L‐NAME significantly reduced adiponectin‐stimulated formation of capillary‐like structures (Figure 6A). In addition, treatment with compound C or LY294002 blocked adiponectin‐stimulated viability of LECs as determined by WST‐1–based assay (Figure 6B). L‐NAME treatment also reduced adiponectin‐induced viability of LECs. Moreover, adiponectin‐mediated inhibition of the frequency of TUNEL‐positive LECs was reversed by treatment with compound C, LY294002, or L‐NAME (Figure 6C). Collectively, these results indicate that AMPK‐Akt‐eNOS signaling is required for LEC responses to adiponectin in vitro.
Figure 6.
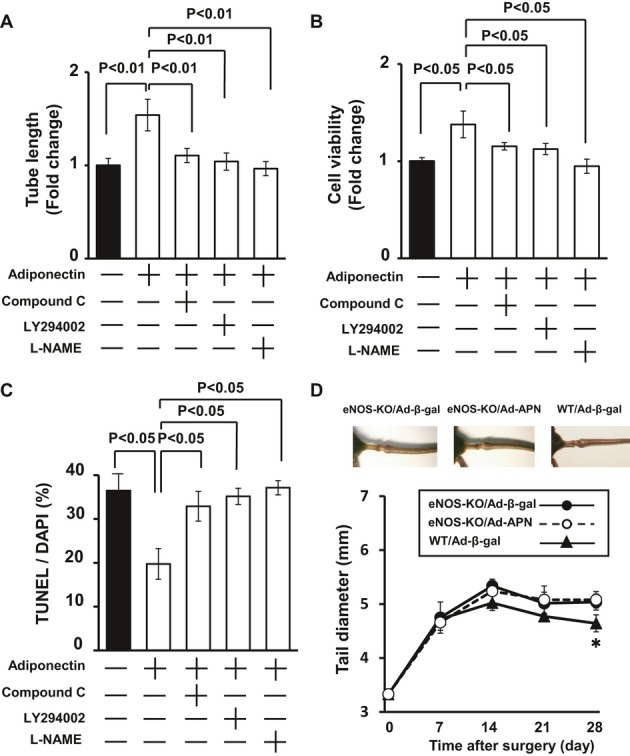
eNOS signaling participates in lymphatic vessel responses to adiponectin. A through C, Effect of compound C, LY294002, and L‐NAME on adiponectin‐mediated LEC differentiation (A), viability (B), and apoptosis (C). LECs were pretreated with compound C (10 μmol/L), LY294002 (50 μmol/L), L‐NAME (1 mmol/L), or vehicle (DMSO) for 60 minutes and treated with adiponectin (30 μg/mL) or vehicle. Matrigel assay was performed, and quantitative analyses of the network tube length are shown (A) (n=5 in each group). WST‐1–based assay was performed to assess cell viability (B) (n=4 in each group). TUNEL assay was performed for assessing apoptotic cells (C) (n=4 in each group). D, Quantitative analysis of the tail diameter in eNOS‐KO and WT mice treated with Ad‐β‐gal or Ad‐APN at different time points after surgery (n=5 in each group). Results are shown as the mean±SE. *P<0.05 vs eNOS‐KO/Ad‐β‐gal. Ad indicates adenovirus; AMPK, AMP‐activated protein kinase; APN, adiponectin; β‐gal, β‐galactosidase; eNOS, endothelial nitric oxide synthase; HA, hemagglutinin; KO, knockout; LEC, lymphatic endothelial cell; L‐NAME, L‐NG‐Nitroarginine Methyl Ester; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling assay; WT, wild‐type.
Finally, we examined whether eNOS is required for the actions of adiponectin on lymphedema in vivo, we systemically injected Ad‐APN or control Ad‐β‐gal into eNOS‐KO mice 3 days before surgery. As shown in Figure 6D, eNOS‐KO mice treated with Ad‐β‐gal exhibited a significant increase in tail diameter after surgery compared with WT mice treated with Ad‐β‐gal on postoperative days 21 and 28. On day 6 after injection, Ad‐APN increased plasma adiponectin levels of eNOS‐KO mice to 21.2±4.3 μg/mL, which was similar to those in Ad‐APN–treated WT mice. In contrast to WT mice (Figure 1B), systemic delivery of Ad‐APN did not affect the thickness of injured tails of eNOS‐KO mice on postoperative days 0, 7, 14, 21, and 28 (Figure 6D). Therefore, the in vivo effect of adiponectin on lymphedema is dependent on its ability to modulate eNOS activity.
Discussion
The present study provides the first evidence that adiponectin promotes the lymphatic vessel function and ameliorates the development of lymphatic edema in a mouse tail model. Adiponectin deficiency exacerbates lymphedema in damaged tails, which was accompanied by the reduction of lymphangiogenesis. Systemic administration of adiponectin to WT and APN‐KO resulted in improvement of lymphedema and enhancement of LEC formation in edematous tails. Adiponectin treatment also ameliorated the lymphedema in obese mice. Treatment with adiponectin led to enhancement of LEC differentiation and viability in vitro. These data suggest that adiponectin acts as an endogenous modulator of lymphatic vessel responses to tissue injury and exerts a salutary action on the lymphatic vasculature.
Clinically, obese status contributes to the development of lymphedema,8–10 and it leads to the reduced levels of adiponectin in blood stream.28–29 Thus, the findings reported here suggest that low levels of circulating adiponectin may be responsible for the progression of lymphedema in obese subjects. It has been shown that body weight reduction results in a significant increase in circulating adiponectin level in obese subjects.30 Furthermore, a dietetic therapy could be valuable for treatment of lymphedema in obese patients.11 Collectively, the approaches to restore or increase plasma adiponectin levels may be useful for the prevention or treatment of obesity‐associated lymphedema.
It is well established that eNOS is a key regulator of cardiovascular function, blood vessel maturation, and angiogenesis.31–32 Accumulating evidence indicates that adiponectin protects against the development of vascular disorders via activation of eNOS signaling pathways. Adiponectin stimulates eNOS phosphorylation and NO production in vascular endothelial cells, thereby leading to improvement of endothelial cell function.17,33–34 We have demonstrated that adiponectin promotes ischemia‐induced revascularization, at least in part, through modulation of eNOS signaling.35 We have also shown that adiponectin prevents cerebrovascular injury via activation of eNOS.36 Therefore, these results suggest that eNOS is indispensible for the effects of adiponectin on the blood vessel diseases. Furthermore, it has been shown that eNOS is expressed in the lymphatic system37 and plays an important role in regulation of lymphangiogenesis and lymphatic metastasis.38 This study showed that adiponectin promotes the phosphorylation of eNOS in cultured LECs in vitro and that treatment with NOS inhibitor reverses the effect of adiponectin on LEC response. In addition, the suppressive effects of adiponectin on lymphedema were abolished in eNOS‐KO mice in vivo. Thus, it is conceivable that eNOS is essential for the lymphatic vascular responses to adiponectin. Taken together, these observations suggest that eNOS mediates the protective actions of adiponectin on the blood and lymphatic vessel disorders.
Adiponectin functions as an AMPK activator in different types of cells.17,21,39–40 We have previously reported that adiponectin promotes the phosphorylation of Akt and eNOS and angiogenic phenotypes in vascular endothelial cells through activation of AMPK.17 Adiponectin‐mediated activation of AMPK also participates in angiogenic repairs of ischemic muscle in vivo.20,35 Here, it is shown that adiponectin stimulates the activating phosphorylation of AMPK and Akt in LECs and that AMPK inactivation blocks adiponectin‐induced stimulation of Akt and eNOS activity and LEC function. Furthermore, blockade of Akt activation abolished adiponectin‐induced eNOS phosphorylation and LEC responses without affecting AMPK phosphorylation. Thus, adiponectin can promote Akt‐eNOS signaling pathways and subsequent lymphangiogenic cellular responses via activation of AMPK within LECs.
It has been recognized that lymphedema may contribute to adipose expansion and accumulation.41–43 Chy mice having a heterozygous inactivating mutation in vascular endothelial growth factor receptor 3 (VEGFR3) exhibit an increased accumulation of subcutaneous fat tissue as a result of hypoplastic cutaneous lymphatic vessels and lymphatic edema.44 It has been shown that Prox1 haploinsufficiency contributes to leakage of lymph from abnormally formed lymphatic vessels, thereby resulting in adult‐onset obesity.45 Lack of Prox1 in LECs also causes adult‐onset obesity.45 Moreover, the addition of lymph to the culture media is reported to promote adipocyte differentiation in vitro.45–46 Therefore, lymphatic dysfunction within adipose tissue appears to participate in the development of obesity, at least in part, via modulation of adipogenesis.
In conclusion, our observations indicate that adiponectin functions as a novel modulator of lymphatic vessel responses to injury by directly activating the lymphangiogenic signaling in LECs. The ability of adiponectin on ameliorate lymphedema is dependent, at least in part, on its ability to promote lymphatic vessel function. The dysfunction of lymphatic vessels participates in a variety of pathological conditions including lymphedema and inflammation.47 Thus, adiponectin may represent a target molecule for a therapeutic option for the prevention and/or treatment of lymphatic vessel diseases.
Acknowledgements
We gratefully acknowledge the technical assistance of Yoko Inoue.
Sources of Funding
This work was supported by Grant‐in‐Aid for Scientific Research, Grant‐in‐Aid for Challenging Exploratory Research, and grants from Takeda Science Foundation, the Uehara Memorial Foundation, Daiichi‐Sankyo Foundation of Life Science, AstraZeneca Research & Development Grant, and SENSHIN Medical Research Foundation to Dr Ouchi. Dr Shibata was supported with the Grant‐in‐Aid for Young Scientists B, Banyu Life Science Foundation International, Kanae Foundation, and Kowa Life Science Foundation.
Disclosures
None.
References
- 1.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer‐related lymphedema. Cancer. 2007; 110:1868-1874 [DOI] [PubMed] [Google Scholar]
- 2.Meric F, Buchholz TA, Mirza NQ, Vlastos G, Ames FC, Ross MI, Pollock RE, Singletary SE, Feig BW, Kuerer HM, Newman LA, Perkins GH, Strom EA, McNeese MD, Hortobagyi GN, Hunt KK. Long‐term complications associated with breast‐conservation surgery and radiotherapy. Ann Surg Oncol. 2002; 9:543-549 [DOI] [PubMed] [Google Scholar]
- 3.Ji RC. Lymphatic endothelial cells, lymphedematous lymphangiogenesis, and molecular control of edema formation. Lymphat Res Biol. 2008; 6:123-137 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y, Shibata R, Shintani S, Ishii M, Murohara T. Therapeutic lymphangiogenesis with implantation of adipose‐derived regenerative cells. J Am Heart Assoc. 2012; 1:e000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF‐C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003; 111:717-725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang LK, Garcia‐Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose‐dependent response of FGF‐2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004; 101:11658-11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos FL, Caunt M, Peterson‐Maduro J, Planas‐Paz L, Kowalski J, Karpanen T, van Impel A, Tong R, Ernst JA, Korving J, van Es JH, Lammert E, Duckers HJ, Schulte‐Merker S. CCBE1 is essential for mammalian lymphatic vascular development and enhances the lymphangiogenic effect of vascular endothelial growth factor‐C in vivo. Circ Res. 2011; 109:486-491 [DOI] [PubMed] [Google Scholar]
- 8.Segerstrom K, Bjerle P, Graffman S, Nystrom A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1992; 26:223-227 [DOI] [PubMed] [Google Scholar]
- 9.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010; 16:48-54 [DOI] [PubMed] [Google Scholar]
- 10.Greene AK, Grant FD, Slavin SA. Lower‐extremity lymphedema and elevated body‐mass index. N Engl J Med. 2012; 366:2136-2137 [DOI] [PubMed] [Google Scholar]
- 11.Clinical‐Resource‐Efficiency‐Support‐Team Guidelines for the diagnosis, assessment and management of lymphoedema. 2008Belfast: CREST [Google Scholar]
- 12.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11:85-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003; 14:561-566 [DOI] [PubMed] [Google Scholar]
- 14.Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009; 73:608-614 [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003; 42:231-234 [DOI] [PubMed] [Google Scholar]
- 16.Iwashima Y, Horio T, Suzuki Y, Kihara S, Rakugi H, Kangawa K, Funahashi T, Ogihara T, Kawano Y. Adiponectin and inflammatory markers in peripheral arterial occlusive disease. Atherosclerosis. 2006; 188:384-390 [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross‐talk between AMP‐activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004; 279:1304-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata R, Skurk C, Ouchi N, Galasso G, Kondo K, Ohashi T, Shimano M, Kihara S, Murohara T, Walsh K. Adiponectin promotes endothelial progenitor cell number and function. FEBS Lett. 2008; 582:1607-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004; 94:e27-e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of AMP‐activated protein kinase signaling. J Biol Chem. 2004; 279:28670-28674 [DOI] [PubMed] [Google Scholar]
- 21.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin‐mediated modulation of hypertrophic signals in the heart. Nat Med. 2004; 10:1384-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet‐induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002; 8:731-737 [DOI] [PubMed] [Google Scholar]
- 23.Maruyama S, Shibata R, Kikuchi R, Izumiya Y, Rokutanda T, Araki S, Kataoka Y, Ohashi K, Daida H, Kihara S, Ogawa H, Murohara T, Ouchi N. Fat‐derived factor omentin stimulates endothelial cell function and ischemia‐induced revascularization via endothelial nitric oxide synthase‐dependent mechanism. J Biol Chem. 2012; 287:408-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouchi N, Shibata R, Walsh K. AMP‐activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005; 96:838-846 [DOI] [PubMed] [Google Scholar]
- 25.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002; 90:1243-1250 [DOI] [PubMed] [Google Scholar]
- 26.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez‐Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. Amp‐activated protein kinase phosphorylation of endothelial no synthase. FEBS Lett. 1999; 443:285-289 [DOI] [PubMed] [Google Scholar]
- 27.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium‐derived nitric oxide production by the protein kinase Akt. Nature. 1999; 399:597-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose‐specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257:79-83 [DOI] [PubMed] [Google Scholar]
- 29.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003; 46:459-469 [DOI] [PubMed] [Google Scholar]
- 30.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose‐derived anti‐inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001; 86:3815-3819 [DOI] [PubMed] [Google Scholar]
- 31.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006; 6:521-534 [DOI] [PubMed] [Google Scholar]
- 32.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998; 101:2567-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003; 278:45021-45026 [DOI] [PubMed] [Google Scholar]
- 34.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin‐induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007; 56:1387-1394 [DOI] [PubMed] [Google Scholar]
- 35.Kondo M, Shibata R, Miura R, Shimano M, Kondo K, Li P, Ohashi T, Kihara S, Maeda N, Walsh K, Ouchi N, Murohara T. Caloric restriction stimulates revascularization in response to ischemia via adiponectin‐mediated activation of endothelial nitric‐oxide synthase. J Biol Chem. 2009; 284:1718-1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008; 117:216-223 [DOI] [PubMed] [Google Scholar]
- 37.Marchetti C, Casasco A, Di Nucci A, Reguzzoni M, Rosso S, Piovella F, Calligaro A, Polak JM. Endothelin and nitric oxide synthase in lymphatic endothelial cells: immunolocalization in vivo and in vitro. Anat Rec. 1997; 248:490-497 [DOI] [PubMed] [Google Scholar]
- 38.Lahdenranta J, Hagendoorn J, Padera TP, Hoshida T, Nelson G, Kashiwagi S, Jain RK, Fukumura D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009; 69:2801-2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty‐acid oxidation by activating AMP‐activated protein kinase. Nat Med. 2002; 8:1288-1295 [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of amp‐activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003; 52:1355-1363 [DOI] [PubMed] [Google Scholar]
- 41.Schneider M, Conway EM, Carmeliet P. Lymph makes you fat. Nat Genet. 2005; 37:1023-1024 [DOI] [PubMed] [Google Scholar]
- 42.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro‐ and microcirculation of adipose tissue. FEBS J. 2009; 276:5738-5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010; 24:2115-2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, Yla‐Herttuala S, Finegold DN, Ferrell RE, Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001; 98:12677-12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by prox1 haploinsufficiency cause adult‐onset obesity. Nat Genet. 2005; 37:1072-1081 [DOI] [PubMed] [Google Scholar]
- 46.Nougues J, Reyne Y, Dulor JP. Differentiation of rabbit adipocyte precursors in primary culture. Int J Obes. 1988; 12:321-333 [PubMed] [Google Scholar]
- 47.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010; 140:460-476 [DOI] [PubMed] [Google Scholar]


