Abstract
In the reward circuitry of the brain, alpha-7-nicotinic acetylcholine receptors (α7nAChRs) modulate effects of delta-9-tetrahydrocannabinol (THC), marijuana’s main psychoactive ingredient. Kynurenic acid (KYNA) is an endogenous negative allosteric modulator of α7nAChRs. Here we report that the kynurenine 3-monooxygenase (KMO) inhibitor Ro 61-8048 increases brain KYNA levels and attenuates cannabinoid-induced increases in extracellular dopamine in reward-related brain areas. In the self-administration model of drug abuse, Ro 61-8048 reduced the rewarding effects of THC and the synthetic cannabinoid WIN 55,212-2 in squirrel monkeys and rats, respectively, and it also prevented relapse to drug-seeking induced by re-exposure to cannabinoids or cannabinoid-associated cues. The effects of enhancing endogenous KYNA levels with Ro 61-8048 were prevented by positive allosteric modulators of α7nAChRs. Despite a clear need, there are currently no medications approved for treatment of marijuana dependence. Modulation of KYNA provides a novel pharmacological strategy for achieving abstinence from marijuana and preventing relapse.
The number of people seeking treatment for marijuana abuse in the U.S. (1,243,000) is higher than the number seeking treatment for cocaine or heroin use (787,000 or 507,000, respectively) (NSDUH)1. Like other drugs of abuse, marijuana’s rewarding effects involve neurochemical changes in brain reward systems2,3. Specifically, THC, the main psychoactive ingredient in marijuana, activates mesolimbic dopamine circuitry by enhancing the firing of dopamine neurons in the ventral tegmental area (VTA)4,5, resulting in increased release of dopamine from nerve terminals in the shell of the nucleus accumbens (NAc)6,7.
Developing medications that modulate these effects of THC on reward signaling might provide a therapeutic approach for the treatment of marijuana dependence. Alpha-7-nicotinic acetylcholine receptors (α7nAChRs) are present in both the VTA and NAc shell, where they are localized on glutamatergic nerve terminals8. Their activation elicits the release of glutamate, which in turn acts at ionotropic glutamate receptors on dopaminergic terminals to stimulate dopamine release9,10. We previously found that reward-related behavioral and neurochemical effects of THC or the synthetic cannabinoid-receptor agonist WIN 55,212-2 could be blocked by methyllycaconitine (MLA), a selective antagonist at α7nAChRs, pointing to modulation of α7nAChR activity as a pharmacological approach for treating marijuana dependence11,12. Unfortunately, systemic use of cholinergic antagonists acting directly at α7nAChRs is associated with central and peripheral side effects that limit their therapeutic utility13,14. Medications that enhance the formation of endogenous negative allosteric modulators of α7nAChRs might be better tolerated than directly-acting cholinergic antagonists15-17. Allosteric modulators change receptor conformations in the presence of orthosteric ligands, and often have no effect on their own, acting only when physiological receptors are activated15-17.
Kynurenic acid (KYNA) is an endogenous neuroinhibitory metabolite18, which is produced by the irreversible transamination of kynurenine, the first major catabolic product of tryptophan. Formed in astrocytes19, KYNA is present in the mammalian brain in nanomolar concentrations20. Long known as a competitive antagonist of the glycine co-agonist site of the NMDA receptor21, KYNA is also a negative allosteric modulator of α7nAChRs at endogenous concentrations, and somatodendritic and preterminal/presynaptic α7nAChRs are equally sensitive to KYNA22-24. Notably, fluctuations in brain KYNA levels have neuromodulatory consequences. Thus, reductions in brain KYNA cause an increase in extracellular levels of acetylcholine, dopamine and glutamate25-27, whereas KYNA elevations reduce α7nAChR function and result in α7nAChR-dependent, but relatively modest, decreases in extracellular levels of glutamate and dopamine in the striatum, prefrontal cortex, and caudate nucleus26,28,29. It has therefore been proposed that astrocyte-derived KYNA, through this indirect action, may serve as an endogenous modulator of both physiological and pathological glutamatergic and dopaminergic signaling30.
We hypothesized that pharmacological enhancement of brain KYNA levels could selectively counteract the behavioral and neurochemical effects of THC responsible for marijuana abuse and dependence, notably the ability to support the development of persistent drug-taking behavior31, to precipitate relapse to drug-seeking behavior in abstinent subjects32, and to increase dopamine release in the nucleus accumbens shell6,7. Production of KYNA in the brain and elsewhere can be increased by inhibiting kynurenine 3-monooxygenase (KMO), a pivotal enzyme in the kynurenine pathway of tryptophan degradation33,34. In both rodents and monkeys, peripheral KMO inhibition results in elevated blood levels of KYNA’s precursor kynurenine35,36, which readily penetrates the blood-brain barrier and accumulates in astrocytes where it undergoes transamination to KYNA19,37. Newly formed KYNA is promptly released into the extracellular compartment38. Notably, no reuptake processes exist for KYNA, and extracellular KYNA is not degraded enzymatically39 but is slowly eliminated from the brain by a non-specific acid transporter20,40.
In this study, we used 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2-yl]-benzenesulfonamide (Ro 61-8048), a potent, selective, peripherally-acting KMO inhibitor41, to indirectly increase brain KYNA levels. We combined neurochemical and behavioral approaches to evaluate effects of Ro 61-8048 on: (1) KYNA levels in the VTA and NAc shell in rats; (2) elevations of extracellular dopamine in the NAc shell and VTA induced by THC or the synthetic cannabinoid WIN 55,212-2 in rats; (3) THC self-administration in squirrel monkeys and WIN 55,212-2 self-administration in rats; (4) drug-induced and cue-induced relapse to cannabinoid-seeking behavior in abstinent animals; (5) cocaine self-administration and food-rewarded behavior in monkeys to assess specificity of the effect; and (6) working memory and THC discrimination in rats and squirrel monkeys, to assess potential side effects. To further elucidate the mechanism of the observed effects, we determined whether infusing KYNA locally in the NAc shell prevents THC-induced elevations of dopamine in the NAc shell of rats.
Results
Neurochemical effects of KMO inhibition in rats
We tested whether systemic administration of the KMO inhibitor Ro 61-8048 would increase levels of KYNA in two brain regions implicated in rewarding effects of cannabinoids: the NAc shell and VTA. In-vivo microdialysis experiments in freely-moving rats showed that systemic administration of 30 and 100 mg per kg (i.p.) of Ro 61-8048 increased extracellular KYNA levels in the NAc shell by 150% and ~225%, respectively (Fig. 1a; 30 mg per kg: F11,55 = 28.59; P < 0.001; 100 mg per kg: F11,55 = 15.03; P < 0.001). In the VTA, the 30 and 100 mg per kg doses of Ro 61-8048 elevated KYNA levels to 50% and 200%, respectively (Fig. 1b; 30 mg per kg: F11,55 = 5.85; P < 0.001; 100 mg per kg: F11,55 = 24.18; P < 0.001). Peak KYNA levels in both NAc and VTA were observed 80 min after injection of 100 mg per kg Ro 61-8048 and 140 min or later after injection of 30 mg per kg Ro 61-8048.
Figure 1.
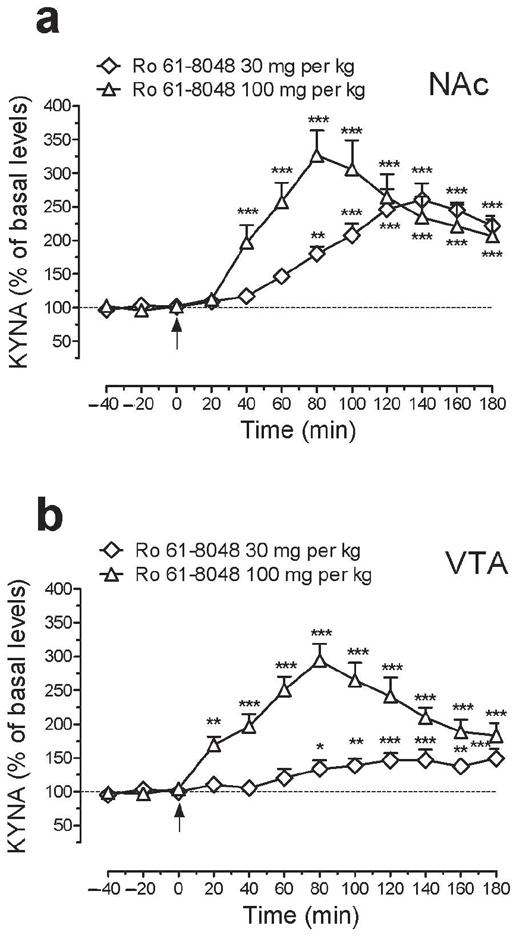
Effects of treatment with Ro 61-8048 on the extracellular concentration of kynurenic acid (KYNA) in NAc shell and VTA of freely-moving rats. (a,b) Ro 61-8048 (30 and 100 mg per kg i.p.) significantly increased extracellular KYNA levels in the NAc shell (a; basal levels: 2.29 ± 0.18 and 1.51 ± 0.1 nM, respectively) and VTA (b; basal levels: 1.61 ± 0.1 and 1.29 ± 0.1 nM, respectively). KYNA reached peak concentrations 80 min after the injection of 100 mg per kg Ro 61-8048 in both brain areas. Arrows indicate time of Ro 61-8048 injection. Results are expressed as mean (± s.e.m) percentage of basal values (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001, post-hoc vs. basal values.
We then determined whether systemic administration of Ro 61-8048 would block THC-induced elevations of dopamine in the NAc shell and VTA in rats. In the NAc shell, THC (3 mg per kg, i.p.) significantly increased extracellular dopamine (Fig. 2a,b; Treatment × time interaction, F30,218 = 1.99; P < 0.003; AUC: F3,22 = 6.06; P = 0.0036), but pretreatment with 30 or 100 mg per kg of Ro 61-8048 dose-dependently blocked this effect of THC (Fig. 2a,b). We saw similar effects in the VTA, where THC (3 mg per kg) also increased extracellular dopamine significantly (Fig. 2c,d; Treatment × time interaction, F30,188 = 4.25; P < 0.0001; AUC: F3,19 = 22.01; P < 0.001), and pretreatment with either 30 or 100 mg per kg of Ro 61-8048 significantly reduced this effect of THC (Fig. 2c,d). When given alone, Ro 61-8048 (100 mg per kg) did not significantly affect dopamine levels in either the NAc (Fig. 2a) or the VTA (Fig. 2c,d).
Figure 2.
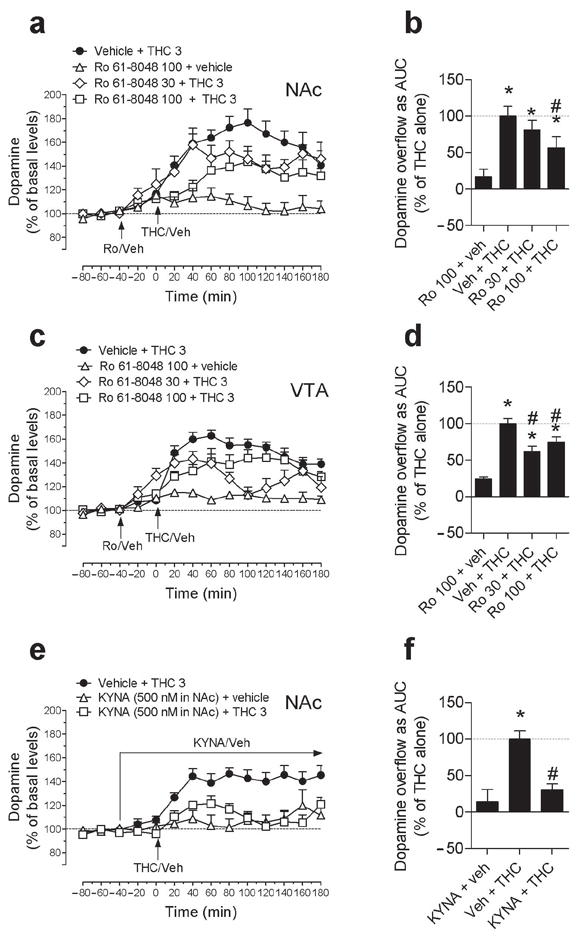
Effects of elevated brain levels of KYNA on THC-induced elevations of extracellular dopamine levels in NAc shell and VTA of freely-moving rats. (a,b,c,d) THC (3 mg per kg) increased dopamine levels compared to basal levels in both NAc shell (a; average basal levels: 37.60 ± 2.70 fmol per 10 μl; n = 7) and VTA (c; average basal levels: 26.18 ± 3.80 fmol per 10 μl; n = 7). The effects of THC on dopamine in NAc shell (a,b) were significantly attenuated by pretreatment with 100 mg per kg Ro 61-8048 (Ro; n = 6), and the effects of THC on dopamine in VTA (c,d) were significantly attenuated by pretreatment with 30 (n = 5) or 100 mg per kg Ro 61-8048 (n = 6). Ro 61-8048 alone (both n=5) did not affect extracellular levels of dopamine in either area. (e,f) Local infusion of KYNA (500 nM) (n = 5) into NAc shell significantly reduced THC-induced dopamine elevations in NAc shell (e,f; n = 5). Arrows indicate time of THC, Ro 61-8048 or vehicle injection or local infusion of KYNA. Data are presented over the course of the session as a percentage of basal levels (a,c,e), and during the first 120 min after THC or vehicle injection as area under the curve (AUC), expressed as a percentage of the mean level in the Vehicle + THC group (b,d,f). Results are expressed as means ± s.e.m. * P < 0.05 vs. baseline, #P < 0.05 vs. vehicle + THC.
This finding that systemic administration of Ro 61-8048 blocks the effects of THC on dopamine in reward-related brain areas, coupled with the finding that Ro 61-8048 increases KYNA in these areas, led us to determine whether the effects of THC on dopamine could be blocked by infusing KYNA directly into the NAc shell. We observed that THC (3 mg per kg, i.p.) significantly increased extracellular dopamine in the NAc shell of freely-moving rats when the local tissue was continually infused with vehicle, but not when the tissue was continually infused with KYNA (500 nM) (Fig. 2e,f: Treatment × time interaction, F14, 91 = 3.61; P < 0.0001; AUC: F2,13 = 13.64; P = 0.0006). In the absence of THC, local infusion of KYNA (500 nM) into the NAc shell had no effect on dopamine levels (Fig. 2e,f).
To verify that the ability of Ro 61-8048 to block THC-induced dopamine elevations in the NAc shell were due its actions at α7nAChRs, we reversed the effects of Ro 61-8048 with galantamine and PNU120596, both agonists at the allosteric potentiating site of α7nAChRs where KYNA acts23,42. In these two experiments (one with galantamine and one with PNU120596), we again found that systemic Ro 61-8048 (100 mg per kg, i.p.) significantly decreased the ability of THC (3 mg per kg, i.p.) to raise dopamine levels in the NAc shell (replicating the effect seen in Figs. 2a,b), reducing the area under the curve by about 60 to 70% (Fig. 3a; F5,27 = 8.34, P < 0.001; Fig. 3b; F4,17 = 9.87, P < 0.0003). Furthermore, we observed that pretreatment with galantamine (Fig. 3a; 3 mg per kg, i.p.) or PNU120596 (Fig. 3b; 1 mg per kg, i.p.) reversed this effect of Ro 61-8048. Neither galantamine nor PNU120596 altered dopamine levels when given alone, nor did they alter the effects of THC (Fig. 3a,b). Thus, we confirmed that α7nAChRs are involved in the ability of Ro 61-8048 to block THC-induced dopamine elevations in rats.
Figure 3.
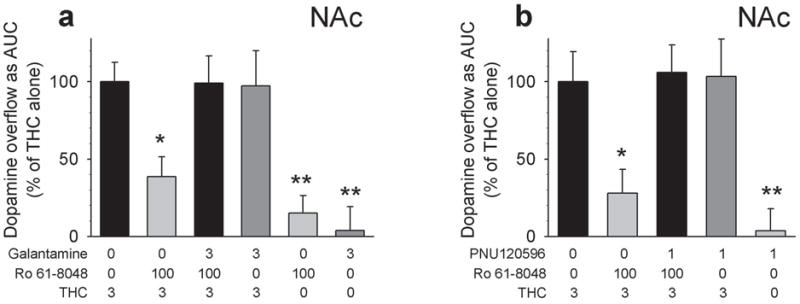
Prevention of the neurochemical effects of Ro 61-8048 by two agonists at the allosteric potentiating site of the α7nAChR, galantamine and PNU120596. (a,b) THC (3 mg per kg i.p.; a: n = 7, b: n = 4) administration elevated extracellular dopamine in NAc shell (average basal levels: 39.69 ± 5.05 fmol per 10μl) of freely-moving rats. These THC-induced increases in dopamine levels were blocked by Ro 61-8048 (100 mg per kg i.p., 40 min before THC; a: n = 6; b: n = 5), and this blockade was prevented by pretreatment with galantamine (a; 3 mg per kg i.p., 60 min before THC; n = 5) or PNU120596 (b; 1 mg/kg i.p., 60 min before THC; n = 4). Galantamine (n = 5), PNU120596 (n = 4) or Ro 61-8048 (n = 5) alone did not increase dopamine levels, and neither galantamine nor PNU120596 (both n = 5) affected THC-induced dopamine increases. Dopamine levels are expressed as area under the curve (“AUC”, relative to the mean level in the vehicle + vehicle + THC 3 condition) over the 180 min following THC or vehicle injection. Bars represent means ± s.e.m. *P < 0.05, **P < 0.01, post-hoc vs. vehicles + THC 3; Dunnet’s test. “0 mg per kg” represents vehicle.
To determine whether treatment with Ro 61-8048 alters the effects of cannabinoid CB1 agonists other than THC, we studied dopamine elevations induced by the synthetic agonist WIN 55,212-2. Like THC, WIN 55,212-2 (0.3 mg per kg, i.v.) significantly increased extracellular dopamine levels in the NAc shell (Fig. 4a; Treatment × time interaction, F28,98 = 3.28, P < 0.0001). Although Ro 61-8048 (30 or 100 mg per kg) alone did not affect dopamine levels (Fig. 4b), pretreatment with 30 or 100 mg per kg of Ro 61-8048 significantly reduced the ability of WIN 55,212-2 to increase dopamine levels in the NAc shell (Fig 4a,c; AUC: F4,14 = 3.73; P = 0.0288).
Figure 4.
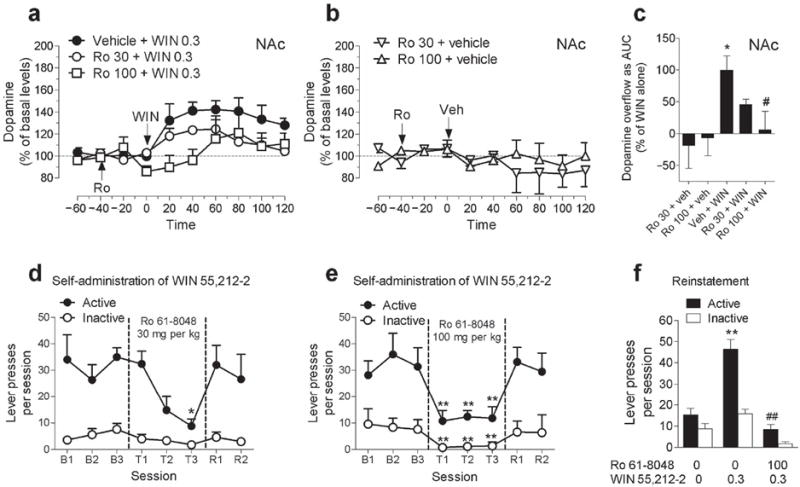
Effects of treatment with Ro 61-8048 on abuse-related effects of the synthetic CB1 agonist WIN 55,212-2 in rats. (a,b) Ro 61-8048 (Ro; 30 or 100 mg per kg) reduced the increases in extracellular dopamine levels produced by WIN 55,212-2 (WIN; 0.3 mg per kg) in NAc shell (a). Ro 61-8048 alone did not significantly affect dopamine levels in NAc shell (b). Results expressed as a percentage of basal values over time (all groups n = 4). Arrows indicate time of injection. (c) Dopamine levels expressed as area under the curve (“AUC”, relative to mean level in vehicle + WIN condition) over the 120 min following WIN or vehicle injection. *P < 0.05 vs. baseline, #P < 0.05 vs. Veh + WIN. (d,e) Ro 61-8048 (sessions T1-T3), but not its vehicle (sessions B1-B3; recovery sessions R1-R2), dose-dependently decreased the number of WIN injections (12.5 μg per kg; 1 injection per active-lever response) self-administered over 2-h sessions. Presses on active and inactive levers per session are shown (n = 5). *P < 0.05,**P < 0.01, post-hoc vs. average of sessions B1-B3, Tukey test. (f) Ro 61-8048 (100 mg per kg) blocked reinstatement of extinguished drug-seeking induced by injection of WIN (0.3 mg per kg) (n = 5). Presses on active and inactive levers per session are shown. **P < 0.01, post-hoc vs. vehicles; ## P < 0.01, post-hoc vs. vehicle + WIN 0.3 condition, Tukey test. All points or bars represent the means ± s.e.m. The symbol “0” represents vehicle.
Behavioral effects of KMO inhibition in rats
Having determined that the KMO inhibitor Ro 61-8048 can block the effects of cannabinoid CB1 agonists in reward-related brain areas, we tested the effects of this treatment in behavioral models of cannabinoid abuse. We first turned to a rodent model of cannabinoid reinforcement in which rats intravenously self-administer WIN 55,212-2 (12.5 μg per kg per injection). This synthetic cannabinoid had a clear reinforcing effect, causing rats to respond significantly more on the lever that delivered the drug than on an inactive control lever (Fig. 4d,e; F1,4 = 23.95, P = 0.008). Treatment with 30 or 100 mg per kg Ro 61-8048 40 min before each session significantly decreased self-administration of WIN 55,212-2 (Fig. 4d; 30 mg per kg: F3,12 = 19.5, P < 0.001; Fig. 4e; 100 mg per kg: F3,12 = 6.92, P = 0.006). The 100 mg per kg dose of Ro 61-8048 decreased self-administration responding over all three days of testing, but it also significantly affected responding on the inactive lever (Fig. 4e; F3,12 = 18.35, P < 0.001). This effect on inactive-lever responding was not seen with 30 mg per kg Ro 61-8048 (Fig. 4d; F3,12 = 1.76, P = 0.21), yet this lower dose was effective in blocking the self-administration of WIN 55,212-2. Self-administration quickly recovered to baseline levels when Ro 61-8048 treatment was discontinued.
Since relapse to drug use after long periods of abstinence represents one of the greatest challenges for the treatment of addiction, we also investigated whether Ro 61-8048 would block reinstatement of drug seeking by abstinent rats in an animal model of relapse. When WIN 55,212-2 delivery was discontinued, rats’ drug-seeking behavior decreased to low levels (Fig. 4f). A non-contingent priming injection of WIN 55,212-2 (0.3 mg per kg., i.p., 10 min before the session) reinstated drug-seeking behavior, but this relapse-like effect was completely blocked by pretreatment with 100 mg per kg Ro 61-8048 (Fig. 4f; F5,20 = 231.13, P < 0.001). None of these treatments significantly affected responding on the inactive lever (Fig. 4f). Thus, Ro 61-8048 prevented the relapse-like effect induced by re-exposure to cannabinoids in rats.
KMO inhibition and THC reward in squirrel monkeys
Since THC self-administration in squirrel monkeys provides the most congruent animal model of human cannabinoid abuse31, we used this model to examine the effects of Ro 61-8048. We also tested the effects of Ro 61-8048 in monkeys trained to self-administer food and cocaine under the same schedule of reinforcement (fixed ratio 10, FR10) to determine whether the effects of Ro 61-8048 are specific to cannabinoid reward.
At the peak of the THC self-administration dose-effect curve (4 μg per kg per injection THC; Fig. 5a), squirrel monkeys self-administered an average of 50.80 ± 1.90 injections per session and lever-pressed at an average rate of 1.20 ± 0.25 responses per second in the presence of a green light signaling THC availability. Self-administration of this THC dose was investigated in monkeys over three consecutive days of treatment with Ro 61-8048 (10 or 20 mg per kg, 40 min before each session). Ro 61-8048 significantly and dose-dependently reduced THC self-administration during all three sessions (Fig. 5a; 10 mg per kg Ro 61-8048: F3,12 = 4.07; P = 0.033; 20 mg per kg Ro 61-8048: F3,12 = 30.93; P < 0.001). Self-administration behavior returned to baseline levels when Ro 61-8048 treatment ended.
Figure 5.
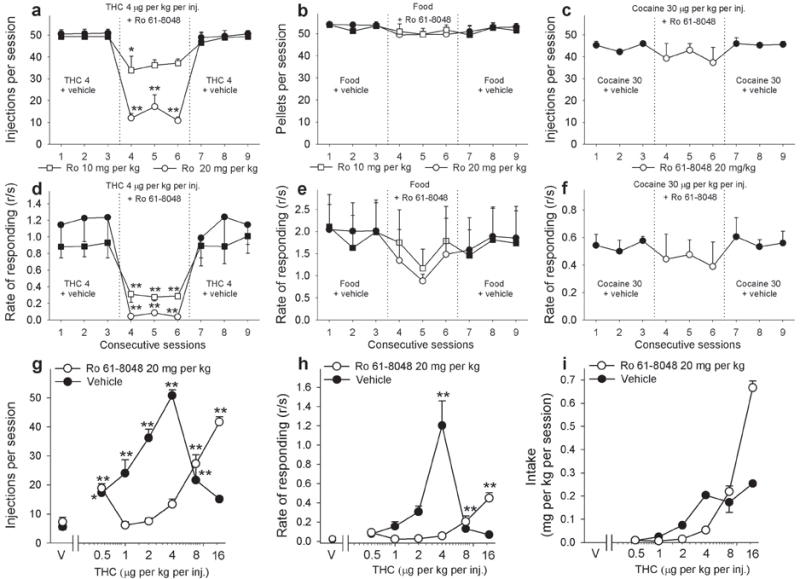
Effects of Ro 61-8048 on THC, food, and cocaine self-administration in squirrel monkeys. (a-f) Ro 61-8048 (10 and 20 mg per kg i.m.) significantly decreased the number of THC injections self-administered during one-h sessions (a) and decreased overall response rates (d) by squirrel monkeys under a fixed-ratio ten (FR10) schedule at a THC dose of 4 μg per kg per injection (n = 5, means ± s.e.m). Ro 61-8048 did not significantly affect food-reinforced behavior (b, e) or cocaine (30 μg per kg per injection) self-administration behavior (c, f) in monkeys under conditions that paralleled the THC self-administration procedure (n = 4 for food, n = 3 for cocaine, means ± s.e.m). Ro 61-8048’s vehicle was given 40 min before each baseline session. (g-i) Pretreatment with Ro 61-8048 (20 mg per kg) caused a significant (P < 0.001) rightward shift of the THC dose-response curves compared to vehicle pretreatment. Number of THC injections per session (g), overall response rates in the presence of the green light signaling THC availability (h) and total THC intake per session (i) are shown as a function of the THC dose. For the dose-response curves (g-i), each data point represents the mean ± s.e.m of the last three sessions under each THC condition and under vehicle conditions (n = 3-5). *P < 0.05, **P < 0.01, post-hoc vs. vs. the last session with vehicle pretreatment (session 3) (a-f) or vehicle conditions (g,h), Bonferroni test. The symbol “V” represents vehicle.
In the food self-administration model (Fig. 5b), monkeys self-administered 53.46±1.29 food pellets per session on average, with a response rate of 1.97 ± 0.66 responses per second in the presence of a green light signaling food availability. During three daily sessions with Ro 61-8048 pretreatment, food-reinforced responding in monkeys was not affected by either 10 or 20 mg per kg of Ro 61-8048 under testing conditions that paralleled those used to evaluate THC self-administration (Fig. 5b; 10 mg per kg Ro 61-8048: F3,9 = 1.76, P = 0.22; 20 mg per kg Ro 61-8048: F3,9 = 1.77, P = 0.22). Thus, Ro 61-8048 did not produce a non-specific disruption of behavior.
Moreover, Ro 61-8048 (20 mg per kg i.m., 40 min before the session) reversed the disruptive effects of THC (0.56 mg per kg i.v., immediately before the session) on food-maintained self-administration behavior (Supplementary Fig. 1a; pellets per session: F3,6 = 23.29, P = 0.001, post-hoc analysis - P = 0.003; Supplementary Fig. 1b rate of responding: F3,6 = 15.37, P = 0.003, post-hoc analysis, THC 0.56 vs. Ro 61-8048 20 + THC 0.56 - P = 0.018). Treatment with 0.56 mg per kg THC alone significantly reduced both the rate of food-maintained responding (from 0.90 ± 0.17 to 0.09 ± 0.03 responses per second) and the number of food pellets per session (from 49.5 ± 1.76 to 19.00 ± 4.51) compared to levels observed after vehicle treatment (post-hoc analysis, vehicle vs. THC 0.56: rate of responding - P = 0.003; pellets per session - P = 0.002). Pretreatment with Ro 61-8048 had no effect by itself (post-hoc analysis, vehicle vs. Ro 61-8048 20: rate - P = 0.28, pellets - P = 0.93), but completely reversed the effects of THC on rates of responding (to 0.62 ± 0.08 responses per second; post-hoc analysis, vehicle vs. Ro 61-8048 20 + THC 0.56 - P = 0.22) and food intake (to 46.67 ± 1.45 pellets per session; post-hoc analysis - P = 0.91).
In monkeys trained to self-administer cocaine (Fig. 5c), the dose of Ro 61-8048 (20 mg per kg) that effectively decreased THC self-administration did not alter cocaine self-administration (30 μg per kg per injection). Monkeys averaged 44.56 ± 0.62 injections per session with a mean response rate of 0.54 ± 0.04 responses per second in the presence of a green light signaling cocaine availability. Pretreatment with Ro 61-8048 40 min before each of three daily sessions did not significantly affect cocaine self-administration during those sessions (Fig. 5c; F3,6 = 1.33; P = 0.35).
Rates of lever responding were significantly affected during Ro 61-8048 treatment only in the group self-administering THC (Fig. 5d; 10 mg per kg Ro 61-8048: F3,12 = 9.82; P = 0.001; 20 mg per kg Ro 61-8048: F3,12 = 15.92; P < 0.001). In monkeys self-administering food or cocaine, response rates were not affected by treatment with Ro 61-8048 (Fig. 5e; food self-administration; 10 mg per kg Ro 61-8048: F3,9 = 1.1; P = 0.40; 20 mg per kg Ro 61-8048: F3,9 = 1.61, P = 0.26; Fig. 5f; cocaine self-administration: F3,6 = 0.69; P = 0.59).
To further characterize the nature of the effects of Ro 61-8048 on THC self-administration, we varied the dose of THC and obtained classic inverted U-shaped dose-effect curves (Fig. 5g,h). THC maintained significantly more self-injections (Fig. 5g; F6,22 = 29.34, P < 0.001) than vehicle at 0.5, 1, 2, 4 and 8 μg per kg per injection, and significantly higher rates of responding (Fig. 5h; F6,22 = 36.76, P < 0.001) than vehicle at 4 μg per kg per injection. We found that pretreatment with 20 mg per kg of Ro 61-8048 significantly shifted the THC dose-response curve for injections per session down and to the right (Fig. 5g; interaction of THC and Ro 61-8048; F5,17 = 35.45, P < 0.001), consistent with a decrease in THC’s rewarding effects. This Ro 61-8048 dose also produced a significant downward-rightward shift for response rates (Fig. 5h; interaction of THC and Ro 61-8048; F5,17 = 16.10, P < 0.001). Post-hoc pairwise comparisons revealed significant differences in the effects of 1, 2, 4, and 16 μg THC per kg per injection after Ro 61-8048 pretreatment on the number of self-administered injections per session (all P < 0.001), and significant differences in effects of 2, 4 and 16 μg per kg THC per injection after Ro 61-8048 pretreatment on response rates (dose 2: P = 0.04; dose 4: P < 0.001; dose 16: P = 0.012). The total amount of THC received during the session was significantly decreased by Ro 61-8048 across most of the dose-effect function (Fig. 5i; F5,17 = 59.4, P < 0.001), but increased at the highest dose per injection.
We then asked whether positive allosteric modulators of α7nAChRs (i.e., galantamine and PNU120596) would prevent the effects of Ro 61-8048 on THC self-administration in monkeys. Galantamine (0.3–3 mg per kg, i.m.) dose-dependently prevented the effects of Ro 61-8048 (20 mg per kg, i.m.) on THC self-administration in monkeys (Fig. 6a; F4,12 = 36.48, P < 0.0001), but galantamine alone (0.3–3 mg per kg, i.m) had no significant effect (Fig. 6b; F3,9 = 3.41, P = 0.067). Like galantamine, PNU120596 also dose-dependently (0.3–3 mg per kg, i.m.) prevented the effects of Ro 61-8048 (20 mg per kg, i.m.) on THC self-administration in monkeys (Fig. 6c; F4,12 = 35.71, P < 0.0001) but had no significant effect when given alone (0.3–3 mg per kg, i.m) (Fig. 6d: F3,9 = 1.98, P = 0.19). Thus, we confirmed that α7nAChRs are involved in the ability of Ro 61-8048 to block the reinforcing effects of THC in nonhuman primates.
Figure 6.
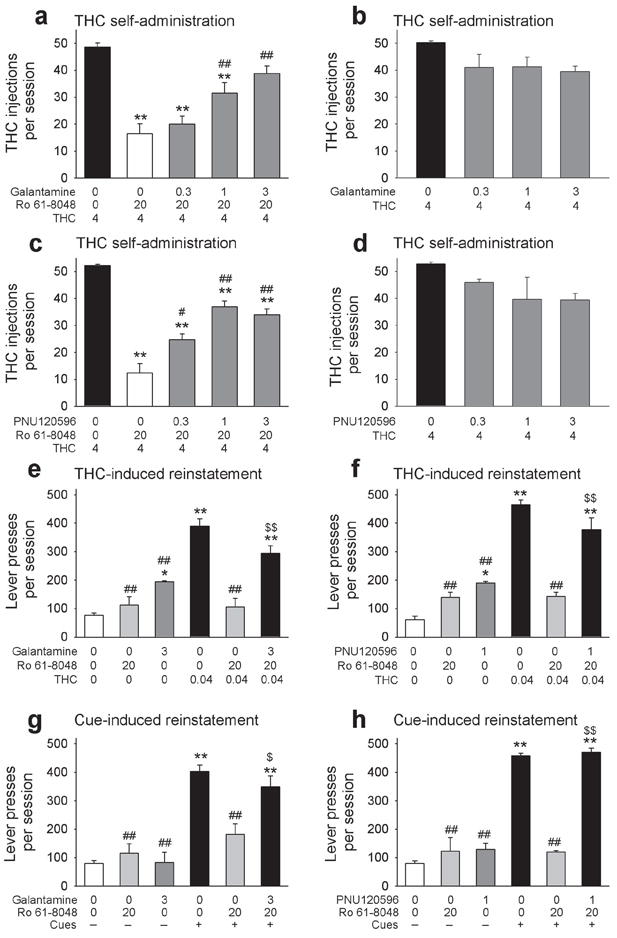
Reversal of behavioral effects of Ro 61-8048 by positive allosteric modulators of α7nAChRs. Galantamine (a) or PNU120596 (c) dose-dependently reversed the blockade of THC (4 μg per kg per injection) self-administration caused by pretreatment with Ro 61-8048 (20 mg per kg). Galantamine (b) and PNU120596 (d) alone had no significant effect. **P < 0.01, post-hoc vs. vehicles + THC 4; ##P < 0.01, post-hoc vs. vehicle + Ro 20 + THC 4, Tukey test. (e,f) Treatment with Ro 61-8048 blocked the reinstatement of extinguished THC-seeking responses produced by a priming injection of THC (0.04 mg per kg i.v.), and this effect was prevented by pretreatment with galantamine (e) or PNU120596 (f). **P < 0.01, post-hoc vs. vehicles; ##P < 0.01, post-hoc vs. vehicles + THC 0.04, $$P < 0.01, post-hoc vs. vehicle + Ro 20 + THC 0.04, Tukey test. (g,h) Treatment with Ro 61-8048 also blocked the reinstatement of extinguished THC-seeking responses induced by reintroduction of cues previously associated with THC, and this effect of Ro 61-8048 was reversed by pretreatment with galantamine (g) or PNU120596 (h). *P < 0.05, **P < 0.01, post-hoc vs. vehicles + no cues; ##P < 0.01, post-hoc vs. vehicles + cues, $$P < 0.01, post-hoc vs. vehicle + Ro 20 + cues, Tukey test. N=4 for all conditions, except n=3 in panel h. Bars represent means ± s.e.m. “0” represents vehicle in all panels. All doses expressed in mg per kg, except THC in panels a-d (μg per kg per injection).
KMO inhibition and relapse in squirrel monkeys
To further study the effects of KMO inhibition in animal models of relapse to THC seeking, we determined whether Ro 61-8048 blocked reinstatement induced by re-exposure to THC or THC-associated cues, in squirrel monkeys, and whether α7nAChRs were involved in this blockade, When lever-press responding for THC had been extinguished by discontinuing THC delivery, administration of a non-contingent priming injection of THC (0.04 mg per kg, i.v.) before the session reinstated drug-seeking (Fig. 6e: F5,14 = 34.37, P < 0.001; Fig. 6f: F5,13 = 77.81, P < 0.001). Treatment with Ro 61-8048 (20 mg per kg, i.m.) blocked this THC-induced reinstatement (Fig. 6e,f: both P < 0.001 vs. THC), and pretreatment with either galantamine (3 mg per kg) or PNU120596 (1 mg per kg) prevented this blockade (Fig. 6e,f). Ro 61-8048 alone did not reinstate drug-seeking behavior (Fig. 6e,f). Both galantamine and PNU120596 produced a low level of reinstatement of drug-seeking behavior (Fig 6e: P = 0.033vs. vehicles, Fig 6g: P = 0.012 vs. vehicles), but this effect was significantly smaller than the reinstatement produced by a priming injection of THC (Fig. 6e: P < 0.001 vs. THC; Fig. 6f: P < 0.001 vs. THC).
Since relapse can be triggered by re-exposure to drug-related environmental cues, we looked at cue-induced reinstatement of THC seeking. When both THC delivery and presentation of cues signaling delivery of THC were discontinued, THC seeking by the monkeys decreased to very low levels (Fig. 6g,h). When visual cue presentation was restored and i.v. vehicle was delivered contingent on responding, THC-seeking behavior was reinstated (Fig. 6g: F5,13 = 21.16, P < 0.001; Fig. 6h: F5,10 = 57.87, P < 0.001). This cue-induced reinstatement was significantly decreased by Ro 61-8048 (20 mg per kg) (Fig. 6g, h). Pretreatment with either galantamine (3 mg per kg) or PNU120596 (1 mg per kg) prevented these effects of Ro 61-8048 (Fig. 6g,h). When Ro 61-8048, galantamine or PNU120596 were given with THC’s vehicle without presentation of cues, THC-seeking behavior was not reinstated (Fig. 6g; post-hoc analysis vs. vehicle + no cues: Ro 61-8048 P = 0.95; galantamine P = 1.00; Fig 6h; Ro 61-8048 P = 0.79; PNU120596 P = 0.69). These results suggest that treatment with a KMO inhibitor could prevent relapse caused by re-exposure to THC or to THC-associated cues, and that this effect of KMO inhibition occurs via an α7nAChR-mediated mechanism.
Effects of KMO inhibition and THC on working memory
Since excessive levels of KYNA may be associated with cognitive impairment43,44, and since THC is well known to impair memory, effects of Ro 61-8048 on working memory were tested in rats trained with a delayed nonmatching-to-position procedure and in squirrel monkeys trained with a delayed matching-to-sample procedure. In rats, THC (3 or 5.6 mg per kg) and Ro 61-8048 (100 mg per kg) were administered alone and in combination. Ro 61-8048 had no effect on memory when given alone (Fig. 7a), but THC decreased accuracy in a delay-dependent manner, consistent with a selective impairment of working memory (Fig. 7b). Ro 61-8048 did not alter the effects of THC (Fig. 7c,d). The main effects of THC (F2,13 = 13.56; P < 0.0001) and delay (F4,28 = 47.34; P < 0.0001) were both significant, but the effects of Ro 61-8048 were not (P=0.85). Paired comparisons indicated that both doses of THC significantly impaired working memory (P < 0.0035).
Figure 7.
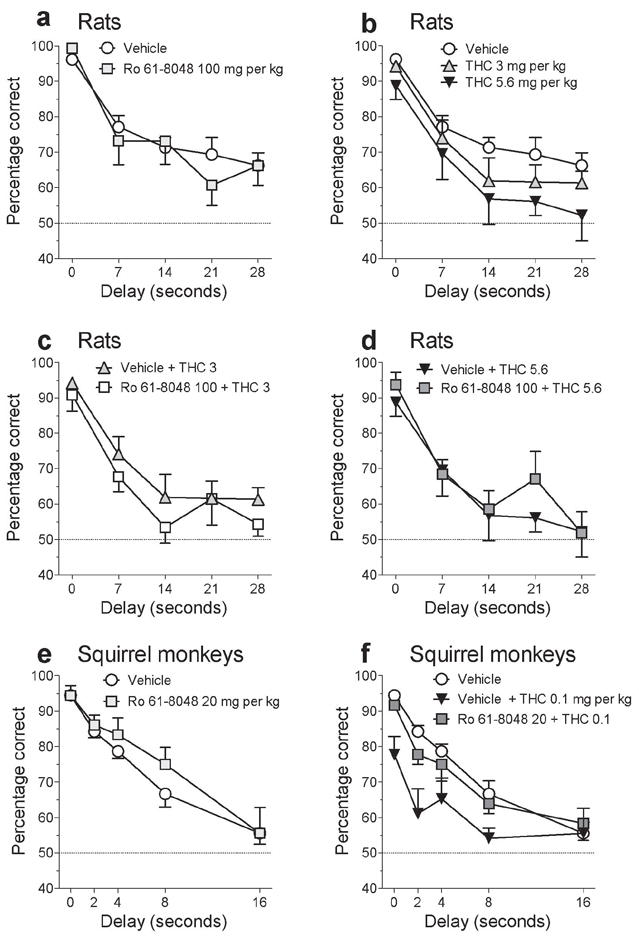
Effects of Ro 61-8048 and THC on working memory in rats and squirrel monkeys. (a-d) The 100 mg per kg dose of Ro 61-8048, which was effective in blocking the effects of THC in reward-related brain areas in rats, did not have deleterious effects on short-term memory in rats when given alone (a) or in combination with THC (3 or 5.6 mg per kg i.p.) (c,d) in a delayed nonmatching-to-position model of working memory. Both doses of THC significantly decreased accuracy (b; P’s < 0.007), but this was not exacerbated by Ro 61-8048 (c,d). (e,f) The 20 mg per kg dose of Ro 61-8048, which was effective in blocking the effects of THC in reward-related brain areas in monkeys, did not have deleterious effects on short-term memory in monkeys when given alone (e) or in combination with THC (f) in a delayed matching-to-sample model of working memory. THC (0.1 mg per kg i.m.) significantly decreased accuracy (f), and this was reversed by Ro 61-8048 (f). Accuracy (percentage of trials with a correct response) is shown (means ± s.e.m.; rats - n = 8; monkeys - n = 3) as a function of delay and of drug treatment.
In squirrel monkeys, working memory was also impaired by THC alone (0.1 mg per kg) but not by Ro 61-8048 (20 mg per kg) alone (Fig. 7e,f). Accuracy in monkeys was also decreased slightly by THC at short delay values, suggesting that impairments might have been due in part to nonselective disruption by THC, similar to the disruptions in food-maintained behavior described above. THC-induced impairments in monkeys were reversed by Ro 61-8048 (Fig. 7f: main effect of Ro 61-8048: F1,2 = 20.46; P < 0.05; main effect of THC: F1,2 = 42.42; P < 0.05; main effect of delay: F4,8 = 59.65; P < 0.0001).
KMO inhibition and discriminative-stimulus effects of THC
To determine whether Ro 61-8048 can affect not only the reinforcing effects of THC, but also its subjective effects, we studied effects of Ro 61-8048 in rodent and primate models of cannabinoid discrimination. In rats trained to detect whether they had been injected with THC or its vehicle, lever selection was dose-dependent, with maximal selection of the drug lever (99.66%) at the 3 mg per kg training dose of THC (Fig. 8a; F5,40 = 28.03; P < 0.001). Notably, the THC dose-effect curve was not significantly shifted by treatment with either 30 or 100 mg per kg of Ro 61-8048 (Fig. 8a; F2,45 = 2.66; P = 0.1), indicating that Ro 61-8048 does not block all of the subjective effects of cannabinoid CB1 agonists in rats. Also in this procedure, Ro 61-8048 did not disrupt food-reinforced behavior when given alone (Fig. 8b; F2,16 = 0.53; P = 0.59) or in combination with different doses of THC (Fig. 8b; F2,45 = 0.24; P = 0.79), indicating that decreases in WIN 55,212-2 self-administration produced by Ro 61-8048 in rats (Fig. 4d,e) were not due to nonspecific behavioral disruption.
Figure 8.
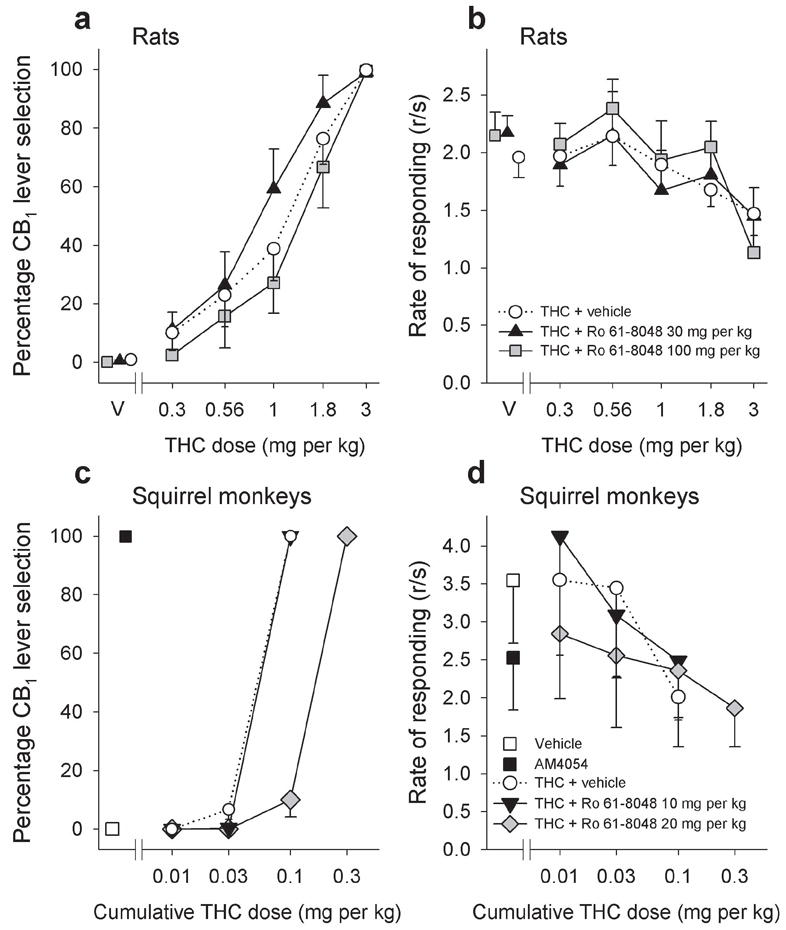
Effects of Ro 61-8048 on discriminative-stimulus effects of THC in rats and squirrel monkeys. (a,b) Rats trained to discriminate THC (3 mg per kg, i.p.) from vehicle (V) under a fixed-ratio (FR10) schedule of food delivery were tested with various doses of THC, and the percentage of responses on the CB1-appropriate lever was a monotonically increasing function of dose. Treatment with Ro 61-8048 (30 or 100 mg per kg i.p.) did not significantly alter this curve (a) and Ro 61-8048 did not significantly alter the rate of food-reinforced responding after THC or vehicle administration (b). Abscissae, dose, log scale; ordinate, percent of responses on the THC-associated lever (a), or response rate (b). (c,d) When monkeys, trained under a stimulus-shock termination schedule to discriminate injection of the selective cannabinoid CB1 agonist AM4054 (0.1 mg per kg i.m.) from vehicle, were injected i.m. with various doses of THC, the percentage of responses on the CB1-appropriate lever was a monotonically increasing function of cumulative dose (c). Ro 61-8048 (20 mg per kg i.m.) reduced the monkey’s ability to detect interoceptive effects of THC in the cannabinoid CB1 discrimination procedure (c). Ro 61-8048 did not significantly affect response rates after THC administration in this procedure (d). Abscissae, cumulative THC dose, log scale; ordinate, percent of responses on the AM4054-associated lever (c), or response rate (d). Symbols left of the abscissae break indicate performance during vehicle and AM4054 control sessions. All data are presented as means ± s.e.m (rats n=9, monkeys n=3).
In squirrel monkeys trained to discriminate the cannabinoid CB1 agonist AM4054 (0.01 mg per kg) from vehicle, THC generalized to the cannabinoid CB1 training stimulus in a dose-dependent manner (Fig 8c; F3,6 = 864.0; P < 0.001). This dose-effect curve was significantly shifted to the right by treatment with 20 mg per kg of Ro 61-8048 (Fig. 8c; F2,15 = 152.32; P < 0.001), but not by 10 mg per kg of Ro 61-8048 (Fig. 8c). Ro 61-8048 did not affect rates of responding in this task (Fig. 8b; F2,11 = 4.54; P = 0.09).
Discussion
The present results indicate that pharmacological modulation of brain KYNA levels by KMO inhibitors could provide an effective approach for the treatment of marijuana dependence. It is well-established that THC, like other drugs of abuse, elevates extracellular levels of dopamine in the NAc shell2,7,11, an effect that is mediated by cannabinoid CB1 receptors and presumably underlies the rewarding and dependence-inducing effects of marijuana. Systemic administration of the KMO inhibitor Ro 61-8048 in rats increased extracellular KYNA levels in the VTA and NAc shell and substantially reduced the ability of THC or the synthetic cannabinoid WIN 55,212-2 to stimulate dopamine release in these areas. This blockade of THC’s effects appears to be due to actions of KYNA in the NAc shell, since, like systemic administration, local infusion of KYNA into the shell also prevented THC from elevating extracellular dopamine levels.
Ro 61-8048 also produced highly promising results in behavioral models of drug abuse. In rats, it markedly reduced self-administration of the synthetic cannabinoid WIN 55,212-2. In monkeys, Ro 61-8048 decreased the rewarding effects of THC, as demonstrated by a shift of the self-administration dose-response curves of THC down and to the right. After pretreatment with Ro 61-8048, THC intake was reduced over a wide range of THC doses and increased only at the highest THC dose. This increase is consistent with a reduced rewarding effect of high THC doses, and may also be due to the reversal by Ro 61-8048 of rate-depressing effects of THC such as we observed in monkeys self-administering food.
Relapse to drug use (as opposed to initial achievement of abstinence) is typically the main obstacle to successful cessation of drug use. In abstinent monkeys with extensive histories of THC self-administration, Ro 61-8048 prevented relapse-like THC-seeking behavior induced by re-exposure to THC. Parallel effects were obtained in rats, where Ro 61-8048 prevented drug-induced seeking of WIN 55,212-2. Moreover, Ro 61-8048 was able to block the relapse-inducing effects of THC-associated cues in monkeys, suggesting it might reduce drug craving in humans.
The ability of the KMO inhibitor Ro 61-8048 to reduce neurochemical and behavioral effects of THC in rats and monkeys was prevented by galantamine, an agonist at the allosteric potentiating site of α7nAChRs that overlaps with the site where KYNA acts as an antagonist24. Since galantamine is also a weak cholinesterase inhibitor, we confirmed prevention of the effects of Ro 61-8048 using PNU120596, a selective positive allosteric modulator of α7nAChRs that does not inhibit cholinesterase42. These results indicate that the anti-abuse actions of KMO inhibition are due to KYNA-induced negative allosteric modulation of α7nAChRs.
Although further experimentation will be required to fully elucidate the circuitry and mechanisms involved in KYNA’s ability to block cannabinoid reward, the available evidence supports the following hypothesis. THC and WIN 55,212-2 facilitate dopamine release in the NAc shell5,6,45, and this is believed to be due at least in part to activation of excitatory glutamatergic pyramidal neurons that project from the prefrontal cortex to the VTA and NAc shell46,47. Since α7nAChRs are localized on the terminals of these glutamatergic cells8, negative allosteric modulation of α7nAChRs by KYNA could reduce the release of glutamate by these cells and thereby reduce glutamate-induced dopamine release in the VTA and NAc shell9,10,45. Since elevated levels of dopamine in the NAc shell are considered central to the rewarding effects of cannabinoid drugs2, and as local infusion of KYNA directly into the NAc shell was sufficient to completely block THC-induced dopamine elevations, it is likely that effects of KYNA in the shell of the NAc are a main factor underlying the ability of Ro 61-8048 to reduce the rewarding effects of cannabinoids.
The safety of KMO inhibitors in humans will have to be considered in future translational studies. Although high levels of KYNA have been associated with cognitive deficits43,44, Ro 61-8048 has neuroprotective and anticonvulsant effects in animal models30. In our experiments, we found that the effects of Ro 61-8048 were specific to cannabinoid reward and were not associated with adverse side effects. Of special relevance, the modest increase in brain KYNA produced by Ro 61-8048 did not adversely affect working memory in rats or squirrel monkeys in tests highly sensitive to impairments induced by THC and other amnesic agents48. Moreover, in rats, KMO inhibition by itself neither produced THC-like subjective effects nor altered the effects of THC itself, which are most likely comprised of both reward-related and non-reward components. However, in squirrel monkeys, KMO inhibition attenuated the discriminative-stimulus effects of THC. The reason(s) for this species-specific effect may be related to differences in cannabinoid mechanisms between rodents and primates31. Notably, moderate KMO inhibition did not affect baseline levels of dopamine in the NAc shell or VTA in the present study, and is known not to affect brain levels of the neurotoxic kynurenine pathway metabolites 3-hydroxykynurenine and quinolinic acid36.
The decreases in cannabinoid self-administration observed here were not due to nonspecific suppression of operant behavior. Although responses on an inactive lever were decreased along with those on the active lever in the WIN 55,212-2 self-administration experiment when rats received 100 mg per kg Ro 61-8048, the 30 mg per kg dose decreased WIN 55,212-2 self-administration without affecting inactive-lever responses. Moreover, the higher dose of Ro 61-8048 did not alter food-maintained behavior in rats. In monkeys, Ro 61-8048 did not affect food or cocaine self-administration behavior, and, in fact, reversed rate-depressant effects of THC on food self-administration.
Taken together, our results suggest that KMO inhibitors could be safe and effective, decreasing cannabinoid reward and relapse at doses devoid of adverse behavioral or neurotoxic effects. Since enhancing endogenous KYNA levels counteracts the abuse-related effects of THC through negative allosteric modulation of α7nAChRs, rather than by direct interference with CB1 receptor function, drugs such as Ro 61-8048 might be better tolerated than orthosteric inverse agonists or antagonists of CB1 receptors, which can have adverse side effects due to actions at CB1 receptors not directly related to THC abuse49.
A medication that would safely and effectively assist in the treatment of marijuana dependence would be an important step forward in dealing with cannabis-use disorders. In the present study, KMO inhibition selectively blocked cannabinoid reward and also had the highly-promising effect of counteracting the ability of drugs and drug-related cues to trigger relapse to cannabinoid seeking. As in rodent or non-human primate models of neurological diseases, where KMO inhibition provides marked benefits ranging from behavioral remediation to neuroprotection35,36,50, pharmacological elevation of brain KYNA offers an attractive novel strategy for treating human marijuana dependence.
Online methods
Animals
Male squirrel monkeys (Saimiri sciureus) weighing 0.8-1.1 kg, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) and male Long-Evans rats (Charles River Laboratories, Wilmington, MA) weighing 300-350 g were singly housed and maintained in temperature- and humidity-controlled facilities fully accredited by AAALAC. Animals were housed on a regular 12 h:12 h light/dark cycle (lights on from 7:00 am), in temperature- and humidity-controlled facilities. Experiments were conducted in light phase. Only for the WIN 55,212-2 experiments, male Lister Hooded rats (Harlan–Nossan, Italy) weighing 250–275 g were housed 4 per cage on a reversed 12 h:12 h light/dark cycle (lights on from 7:00 pm). Rats started the experiments at age 8 weeks. Ages of monkeys ranged from 8 – 15 years. Monkeys had a previous self-administration history.
Experiments were approved by the Animal Care Committees of NIDA IRP, Harvard Medical School/Mclean Hospital and the University of Cagliari and were carried out in strict accordance with the 2003 National Research Council guidelines or the E.C. Regulations for Animal Use in Research (CEE No. 86/609).
Drugs
Δ9-tetrahydrocannabinol (THC; NIDA Research Resources Drug Supply Program, Bethesda, MD, USA) was dissolved in a vehicle containing 1% ethanol and 1% Tween 80 in saline for monkeys and 40% cyclodextrin in saline for rats. (R)-(+)-WIN 55,212-2 mesylate salt (WIN 55,212-2, Sigma-Aldrich, Italy) was dissolved in one drop of Tween 80, and diluted in heparinized (1%) sterile saline solution (volume of injection: 0.1 mL). 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2-yl]-benzenesulfonamide (Ro 61-8048, Sai Advantium Pharma, Hyderabad, India) was dissolved in a vehicle containing 5-6% Tween 80 in saline and injected typically 40 min before the session (b.s.; behavioral experiments) or before THC or WIN 55,212-2 (microdialysis experiments) unless noted otherwise. Galantamine (Sigma-Aldrich, St. Louis, MO) was dissolved in saline. PNU120596 (Sigma-Aldrich, St. Louis, MO) was dissolved in a vehicle containing 5% ethanol and 5% cremophor EL in saline. Both galantamine and PNU120596 were always injected 50 min b.s. in behavioral experiments or 60 min before THC or vehicles in microdialysis experiments.
In-vivo microdialysis in freely-moving rats
Experiments with THC
The general procedure was described previously11,26. Dialysis (perfusion rate: 1 μl per min) was performed in Sprague-Dawley rats 20-24 h after implantation of probes aimed at the NAc shell (2.0 mm anterior and 1.1 mm lateral from bregma, 8.0 mm below the dura) or the VTA (5.3 mm posterior to, and 0.9 mm lateral from, bregma, 8.4 mm below the dura)51. Samples (20 μl) were collected every 20 min. Dopamine levels were immediately analyzed by HPLC coupled to electrochemical detection while samples for KYNA determination were frozen and analyzed later. Test drugs or vehicles were injected after stable dopamine levels (≤15% variation) were obtained in three consecutive samples. Ro 61-8048 (30 or 100 mg per kg i.p.) was injected 40 min before THC (3 mg per kg i.p.) injection. Galantamine (3 mg/kg) or PNU120596 (1 mg/kg) were injected i.p. 60 min before THC or vehicles. KYNA was dissolved in Ringer solution and delivered by reverse dialysis at a constant flow rate of 1 ul per l through a concentric probe implanted in the NAc shell and following the coordinates previously described. Ringer+vehicle or Ringer+KYNA were infused after stable dopamine levels (≤15% variation) obtained in three consecutive samples. Perfusion with KYNA or vehicle continued until the end of the experiment. THC (3 mg per kg, i.p.) was injected 40 minutes after KYNA infusion started (500 nM), and dopamine samples were collected every 20 min for 3 h.
Experiments with WIN 55,212-2
Apparatus and procedure were the same as described previously52. Male Lister Hooded rats were surgically implanted with a dialysis probe aimed at the shell of the nucleus accumbens (1.6 mm anterior and 1.1 mm lateral from bregma, 7.9 mm below dura)51, and dialysate samples were collected every 20 min and immediately analyzed by an HPLC system coupled to electrochemical detection. Ro 61-8048 (30 and 100 mg per kg i.p.) or its vehicle were injected 40 min before WIN 55,212-2 (0.3 mg per kg i.v.; average basal levels of dopamine: 67.26 ± 10.80 fmol per 10 μl) or vehicle. Only rats with correct probe placement were included in the study.
Intravenous self-administration of WIN 55,212-2 by rats
The general procedure was the same as described previously53. Briefly, under deep anesthesia, rats were surgically implanted with a catheter in the right jugular vein and left to recover for 6–7 d before starting self administration training. In this study, animals were trained to press a lever for a response-contingent injection of WIN 55,212-2 (12.5 μg per kg per injection) under a continuous fixed-ratio one (FR1) schedule of reinforcement during 2-h daily sessions. There was a 10-s timeout period after each injection. After stabilization of daily intake (no more than 15% variation over 3 sessions), Ro 61-8048 pretreatment (vehicle, 30 and 100 mg per kg i.p.) was tested for three sessions.
WIN 55,212-2-induced reinstatement of extinguished drug-seeking behavior in rats
Self-administration of WIN 55,212-2 was extinguished by replacing WIN 55,212-2 with vehicle (1% Tween 80 in saline), leaving all other experimental parameters unchanged. Once extinction criteria were reached (mean number of active-lever presses decreased by 85% or more for at least five sessions), rats were randomly divided into groups and were given a priming injection of either saline, WIN 55,212-2 (0.3 mg per kg i.p., 10 min b.s.), or 0.3 mg per kg WIN 55,212-2 plus 100 mg per kg Ro 61-8048 in a counterbalanced within-subject design. Lever pressing was then monitored during a 2-h reinstatement test session in which responding resulted in intravenous injections of saline, as before.
Intravenous self administration of THC by monkeys
Monkeys self-administered THC (4 μg per kg per injection; FR10; 60-s timeout; paired visual stimulus; 1-h sessions)31,54. When responses showed <15% variability for at least 5 consecutive sessions, Ro 61-8048 pretreatment (vehicle, 10 and 20 mg per kg, i.m.) was tested for three sessions. Ro 61-8048 (10 and 20 mg per kg) was also examined under parallel conditions in separate groups of monkeys self-administering 190-mg food pellets or intravenous cocaine (30 μg per kg per injection). Effects of Ro 61-8048 (20 mg per kg) on the THC dose-response curve (0.5, 1, 2, 4, 8, and 16 μg per kg per injection) were also assessed, with each dose combination tested for three consecutive sessions, preceded and followed by 3-4 sessions with vehicle pretreatment. Single-session pre-treatment with galantamine or PNU120596 (both 0.3, 1, 3 mg per kg i.m.) was tested to determine whether it altered the effects of Ro 61-8048 (20 mg per kg) or vehicle on THC self-administration (4 μg per kg per injection). Galantamine and PNU120596 doses were tested in ascending order in two monkeys and in descending order in the other two.
In a group of monkeys self-administering food, we also tested effects of different doses of THC (0.04–0.56 mg per kg i.v., immediately b.s.) to find a dose that disrupts food self-administration. We then administered this dose (0.56 mg per kg) after pretreatment with Ro 61-8048 (20 mg per kg i.m.) to assess whether the disruption of the food-maintained behavior can be reversed by KMO inhibition.
THC-induced reinstatement of extinguished drug-seeking behavior in monkeys
Monkeys that self-administered THC (4 μg per kg per injection) were placed under extinction by substituting vehicle for THC. When responses reached a low, stable level, priming injections of THC (vehicle or 0.04 mg per kg i.v., immediately b.s.) were tested with vehicles or 20 mg per kg Ro 61-8048 pretreatment (i.m.). Galantamine (3 mg per kg i.m.) or PNU120596 (1 mg per kg i.m.) were given alone or in combination with THC priming and 20 mg per kg Ro 61-8048 pretreatment. Ro 61-8048 pretreatment was also tested with combination of vehicles. Each test was preceded and followed by one or two extinction sessions. Pretreatment with combination of vehicles was given prior to extinction sessions.
Cue-induced reinstatement of THC seeking in monkeys
After the completion of THC-induced reinstatement testing, monkeys were returned to baseline THC self-administration for several weeks. Then, presentation of THC-associated visual cues and intravenous injections were discontinued for 3 extinction sessions. Cue-induced reinstatement tests (with 1-2 extinction sessions before each test) were then conducted by reinstituting response-contingent cue presentations and delivering saline injections on the FR10 schedule, after pretreatment with vehicles or Ro 61-8048 (20 mg per kg i.m.) combined with vehicle, galantamine (3 mg per kg i.m.) or PNU120596 (1 mg per kg i.m.). Pretreatments with combination of vehicles, vehicles plus Ro 61-8048 (20 mg per kg), vehicles plus galantamine or vehicles plus PNU120596 were also given prior to selected extinction sessions.
Delayed nonmatching-to-position procedure in rats
The procedure was described previously55. Briefly, male Long-Evans rats were trained in a chamber with a horizontal array of three apertures. During each trial, the house light was extinguished, and one of the two side apertures (left or right) was lit from within as a sample. After two responses in the sample aperture, the aperture light was extinguished, and the delay period began (0, 7, 14, 21, or 28 s, in pseudo-random order over trials). The first response in the center aperture after the designated delay lit both side apertures. If the rat responded correctly (i.e., in the nonmatching aperture, opposite to the sample), it received a 45-mg food pellet. The next trial began after a 15-s timeout period with the house light on. Sessions lasted until 100 pellets were delivered or after 90 min. THC (3 or 5.6 mg per kg) was given i.p. 40 min b.s., and Ro 61-8048 (100 mg per kg) was given i.p. 100 min b.s.
Delayed matching-to-sample procedure in squirrel monkeys
The procedure was described in detail previously56. Briefly, male squirrel monkeys were trained in a customized touch-screen chamber57. Trials began with presentation of a 7×7cm digital photograph (sample stimulus). After twenty touch responses to the sample, the stimulus was terminated and the delay period began (0, 2, 4, 8, or 16 s, in pseudorandom order over trials). Following the delay, 2 comparison stimuli were presented left and right of the midline. A touch response to the stimulus that matched the previously presented sample resulted in delivery of 0.15 ml of the sweetened condensed milk reinforcer followed by a 10-s timeout period, whereas a mismatch immediately initiated the timeout. Daily sessions were comprised of 60 trials (12 trials of each delay). THC (0.1 mg per kg) was given i.m. 30 min b.s., and Ro 61-8048 (20 mg per kg) was given i.m. 70 min b.s.
THC discrimination in rats
Rats were trained under a discrete-trials schedule of food reinforcement (10 responses per pellet – FR10, 45-s timeout) in which responses on one lever produced food when an injection of THC (3 mg per kg, i.p., 30 min b.s.) was given, and responses on the other lever produced food when a vehicle injection was given58. Sessions lasted for 20 pellets or 30 min. Ro 61-8048 (vehicle, 30 or 100 mg per kg i.p., 70 min b.s.) and THC (vehicle, 0.3, 0.56, 1, 1.8 or 3 mg per kg i.p., 30 min b.s.) were given before test sessions, up to two times per week. During test sessions, food was delivered whenever there were 10 consecutive responses on either lever.
THC discrimination in squirrel monkeys
Squirrel monkeys responded under a 10-response fixed ratio (FR10) schedule of stimulus-shock termination to identify injection of the cannabinoid CB1 agonist AM4054 (0.01 mg per kg i.m., 50 min b.s.) from vehicle in a two-lever drug discrimination procedure59. The two levers were designated as the drug (AM4054) and saline levers, with assignment remaining the same for a subject throughout the study. Brief, low-intensity shock was scheduled for delivery every 10 s until either the FR 10 was completed on the correct lever or 30 s elapsed, whichever came first. Training sessions ended upon completion of 20 trials. The test session consisted of four components of 10 trials, each component beginning with a 10-min timeout period. No shock deliveries were scheduled during test sessions. Cumulative dosing procedures were used to establish dose-response relationships for the discriminative-stimulus effects of THC (0.01-0.3 mg per kg i.m., 30 min b.s.) administered i.m. at the onset of sequential 10-min timeout periods. Modification of the discriminative stimulus effects of THC by Ro 61-8048 (vehicle, 10 or 20 mg per kg i.m., 70 min b.s.) was studied by determining how pretreatment with Ro 61-8048 altered the position and per or slope of the THC dose-effect function.
Statistical analysis
All data are presented as means ± s.e.m. The sample sizes were chosen based on our previous experience with the used procedures and they are adequate to detect meaningful differences between conditions. All data met the assumptions of the test with regard to the normality, skew and homogeneity of variance. All tests were two-tailed. Rats were randomly assigned to the groups for between-groups experiments in microdialysis experiments. Counterbalanced assignment of treatment order for within-subject design was used in behavioral experiments. Experimenters were not blind to the treatment assignment.
Microdialysis data were expressed as a percentage of basal KYNA and dopamine values; basal values were the mean of three consecutive samples (differing from each other by ≤15%) taken immediately before the first injection of test compound or vehicle. Microdialysis and behavioral data were analyzed using one-way or two-way repeated measures (RM) analysis of variance (ANOVA) and Tukey paired comparisons. To compare the effects of treatments in dialysis experiments, area under the curve (AUC) was calculated and expressed for each condition as a percentage of the AUC for the group receiving THC alone or WIN alone; simultaneous confidence intervals were used to determine whether the condition differed from 0% (thereby indicating a significant change from baseline) or 100% (thereby indicating a significant change vs. the THC-alone or WIN-alone group). Response rates in self-administration experiments did not include responses or time elapsed during timeout. Self-administration responding after Ro 61-8048 treatment was compared to the previous 1-3 consecutive sessions of vehicle treatment; for dose-effect curves, the last three sessions under each condition were averaged. Reinstatement data (extinction baseline and reinstatement test) represent the mean of 1-3 sessions under each condition. Working memory data (arcsine-transformed percentage of trials with a correct response) were analyzed with delay, THC dose, and Ro 61-8048 dose as factors.
Supplementary Material
Acknowledgments
We thank Eric Thorndike for his excellent technical assistance during the rodent studies. We thank Dr. Ira Baum, Stephen Stevens, and Phil White for their excellent veterinary assistance during the primate studies.
This study was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, NIDA Residential Research Support Services Contract N01 DA59909 (Kelly PI), by the Italian Ministry of University and Scientific Research, by DA023142 (Bergman) and DA035794 (Kangas) and by the Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine. Tamara Zara was supported by a Grant from Regione Autonoma della Sardegna, and the European Social Fund (ESF), LR7 2007.
Footnotes
Author contributions
Z.J. was involved in the design of the study, supervised and analyzed the primate self-administration and reinstatement experiments, rodent drug discrimination experiments and wrote the first draft of the manuscript. P.M. was involved in design of the study, conducted microdialysis experiments including collection of all samples, performed dopamine assay, analyzed microdialysis data, and helped prepare the final draft of the manuscript. H.Q.W. analyzed KYNA levels in microdialysis samples. M.Sec. conducted and analyzed microdialysis experiments with local KYNA infusion and PNU120596. G.H.R. conducted the primate self-administration and reinstatement experiments. L.V.P designed and analyzed the experiments with delayed nonmatching to position and was involved in discussions of the data. C.B. conducted rodent drug discrimination experiments and was involved in discussions of the data. T.Z. conducted the rat self-administration and microdialysis experiments with WIN 55.212-2. M.Sch. supervised the rat self-administration and microdialysis experiments with WIN 55,212-2, analyzed the data and was involved in the discussions of the data. W.F. was involved in the discussions of the data. A.P. assisted in conducting the microdialysis experiments with THC. J.B. and B.D.K. designed, conducted and analyzed the data from the primate discrimination and memory experiments. S.F., M.Sol., M.P. and G.T. were involved in the design of the study and discussions of the data. R.S. and S.R.G. conceptualized, designed and supervised the study and edited the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Summary of National Findings. Volume I. Rockville, MD: 2010. [Google Scholar]
- 2.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol. 2008;154:369–383. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10:2825–2830. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- 5.French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 6.Chen JP, et al. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 7.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 8.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 9.Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- 10.Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 11.Solinas M, et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Curr Pharm Des. 2011;17:1351–1358. doi: 10.2174/138161211796150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arias HR, et al. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int J Biochem Cell Biol. 2009;41:1441–1451. doi: 10.1016/j.biocel.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Roegge CS, Levin ED. Nicotinic receptor antagonists in rats. In: Levin ED, Buccafusco JJ, editors. Animal models of cognitive impairment. CRC Press; Boca Raton, FL: 2006. [PubMed] [Google Scholar]
- 15.Arias HR. Positive and negative modulation of nicotinic receptors. Adv Protein Chem Struct Biol. 2010;80:153–203. doi: 10.1016/B978-0-12-381264-3.00005-9. [DOI] [PubMed] [Google Scholar]
- 16.Bertrand D, et al. Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2-M3 segment. Mol Pharmacol. 2008;74:1407–1416. doi: 10.1124/mol.107.042820. [DOI] [PubMed] [Google Scholar]
- 17.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 18.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 19.Kiss C, et al. Kynurenate production by cultured human astrocytes. J Neural Transm. 2003;110:1–14. doi: 10.1007/s00702-002-0770-z. [DOI] [PubMed] [Google Scholar]
- 20.Moroni F, Russi P, Lombardi G, Beni M, Carla V. Presence of kynurenic acid in the mammalian brain. J Neurochem. 1988;51:177–180. doi: 10.1111/j.1471-4159.1988.tb04852.x. [DOI] [PubMed] [Google Scholar]
- 21.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52:1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 22.Alkondon M, et al. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilmas C, et al. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes C, et al. Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at alpha7* nicotinic receptors. J Pharmacol Exp Ther. 2007;322:48–58. doi: 10.1124/jpet.107.123109. [DOI] [PubMed] [Google Scholar]
- 25.Amori L, et al. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu HQ, et al. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40:204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zmarowski A, et al. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci. 2009;29:529–538. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 28.Carpenedo R, et al. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 29.Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–765. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 32.Justinova Z, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaral M, et al. Structural basis of kynurenine 3-monooxygenase inhibition. Nature. 2013;496:382–385. doi: 10.1038/nature12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 35.Gregoire L, et al. Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behav Brain Res. 2008;186:161–167. doi: 10.1016/j.bbr.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Zwilling D, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 38.Turski WA, Gramsbergen JB, Traitler H, Schwarcz R. Rat brain slices produce and liberate kynurenic acid upon exposure to L-kynurenine. J Neurochem. 1989;52:1629–1636. doi: 10.1111/j.1471-4159.1989.tb09218.x. [DOI] [PubMed] [Google Scholar]
- 39.Turski WA, Schwarcz R. On the disposition of intrahippocampally injected kynurenic acid in the rat. Exp Brain Res. 1988;71:563–567. doi: 10.1007/BF00248748. [DOI] [PubMed] [Google Scholar]
- 40.Uwai Y, Honjo H, Iwamoto K. Interaction and transport of kynurenic acid via human organic anion transporters hOAT1 and hOAT3. Pharmacol Res. 2012;65:254–260. doi: 10.1016/j.phrs.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Rover S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. J Med Chem. 1997;40:4378–4385. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- 42.Hurst RS, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pocivavsek A, et al. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 46.Pistis M, Porcu G, Melis M, Diana M, Gessa GL. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci. 2001;14:96–102. doi: 10.1046/j.0953-816x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- 47.Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur J Neurosci. 2002;15:1795–1802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 48.Panlilio LV, et al. Combined effects of THC and caffeine on working memory in rats. Br J Pharmacol. 2012;165:2529–2538. doi: 10.1111/j.1476-5381.2011.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3,4-dimethoxy-[-N-4-(nitrophenyl)thiazol-2yl]-benzenesulfonamide (Ro 61-8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999;19:771–777. doi: 10.1097/00004647-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- 52.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 53.Fattore L, et al. Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci. 2007;25:2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- 55.Panlilio LV, Yasar S, Thorndike EB, Goldberg SR, Schindler CW. Automatic recording of mediating behavior in delayed matching- and nonmatching-to-position procedures in rats. Psychopharmacology (Berl) 2011;214:495–504. doi: 10.1007/s00213-010-2057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kangas BD, Berry MS, Branch MN. On the development and mechanics of delayed matching-to-sample performance. J Exp Anal Behav. 2011;95:221–236. doi: 10.1901/jeab.2011.95-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kangas BD, Bergman J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Methods. 2012;209:331–336. doi: 10.1016/j.jneumeth.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- 59.Kangas BD, et al. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther. 2013;344:561–567. doi: 10.1124/jpet.112.201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


