Abstract
Alcohol dependence is characterized as a multi-factorial disorder caused by a complex interaction between genetic and environmental liabilities across development. A variety of neurocognitive deficits/dysfunctions involving impairments in different brain regions and/or neural circuitries have been associated with chronic alcoholism, as well as with a predisposition to develop alcoholism. Several neurobiological and neurobehavioral approaches and methods of analyses have been used to understand the nature of these neurocognitive impairments/deficits in alcoholism. In the present review, we have examined relatively novel methods of analyses of the brain signals that are collectively referred to as event-related oscillations (EROs) and show promise to further our understanding of human brain dynamics while performing various tasks. These new measures of dynamic brain processes have exquisite temporal resolution and allow the study of neural networks underlying responses to sensory and cognitive events, thus providing a closer link to the physiology underlying them. Here, we have reviewed EROs in the study of alcoholism, their usefulness in understanding dynamical brain functions/dysfunctions associated with alcoholism as well as their utility as effective endophenotypes to identify and understand genes associated with both brain oscillations and alcoholism.
Keywords: Event-related oscillations (EROs), Event-related potentials (ERPs), Electroencephalogram (EEG), Alcoholism, Endophenotype, Genes, Cognitive function, Frontal lobes
Introduction
Brain dysfunction, especially the frontal lobe changes, associated with chronic alcoholism and a predisposition to develop alcoholism has been well documented by neurophysiological, neuroimaging, and neuropsychological studies (see Moselhy et al. [1] for a review). Over the decades, there has been compelling evidence for brain abnormalities in ‘nonamnesic’ chronic alcoholic patients as well as in their high-risk offspring from electrophysiological [2-12], neuroimaging [13-20], and a wide range of neuropsychological investigations [21-28].
Although neuroimaging methods, such as structural/functional MRI and PET have many advantages, including a higher level of spatial resolution, they offer relatively poor temporal resolution compared to electrophysiological methods, which offer excellent temporal resolution in the millisecond range [29,30]. This ability to record millisecond-by-millisecond brain signals with electrophysiological techniques is crucial for the understanding of subtle sensory/cognitive processing as they are occurring in the brain. Electrophysiological techniques have further advantages of being non-invasive, relatively inexpensive to implement, and can be used to study a large variety of cognitive processes/brain functions; recently developed electrophysiological analytical techniques and methods allow for a better study of neural communication across different brain regions in normal [31-33] as well as pathological conditions [34-38], and provide subtle measures of neurocognitive (dys) function. More recent studies increasingly use localization techniques such as standardized low resolution brain electromagnetic tomography (sLORETA) to characterize specific activity patterns and communication across brain regions three-dimensionally and these methods have been implemented in many psychiatric disorders including alcoholism [9-11,39,40].
Alcoholism is a common, complex disorder with contributions from both genetic and environmental influences and their interactions [41]. The identification of suitable quantitative biological markers that are genetically transmitted could explicate the genetic factors involved in the etiology of alcoholism [42]. One successful approach has been the use of biological markers as “endophenotypes”, or intermediate phenotypes (measurable components, traits or variables along the pathway between disease and distal genotype, that are heritable, correlated with the illness, and are already present in those at risk prior to the onset of the illness [43]). In this regard, (electrophysiological measures such as electroencephalogram (EEG), event-related potentials (ERPs) and event-related oscillations (EROs) provide a rich source of potentially useful and powerful endophenotypes for alcoholism, as they meet these criteria, are quantitative, less complex and more proximal to gene function than traditional diagnoses, and represent important correlates of human information processing and cognition; hence they provide more power to localize and characterize disease susceptibility genes (cf. [42]).
Ongoing brain functioning in the form of electrophysiological activity can be recorded non-invasively using scalp electrodes. The German physiologist and psychiatrist Hans Berger (1873-1941) first recorded the human EEG in 1924 [44]. Scalp-recorded electrophysiological activity consists of three distinct methods: 1) EEG: frequency-dependent, spontaneous and continuous neural activity during a restful or specific mental state, 2) ERPs: time-locked, trial-averaged, and task-specific electrophysiological activity during a specific sensory/motor/cognitive event, and 3) EROs: time-frequency (TF) measures of brain electrical activity during a specific sensory/motor/cognitive event.
The human scalp EEG represents the ongoing electrophysiological signals in a continuous fashion, and can be recorded during several mental states: eyes-closed relaxed state, eyes-open steady state, meditation, hypnosis, sleep, coma, and other normal/altered states of consciousness [45]. Traditionally, EEG can be decomposed into different frequency bands, most commonly: delta (0-3.5 Hz), theta (4-7.5 Hz), alpha (8-12.5 Hz), beta (13-28.5 Hz) and gamma (> 29 Hz), and each of these bands are assumed to reflect different types of brain activity. One of the robust and consistent resting EEG findings in alcoholism is that alcoholics and their high-risk offspring show increased beta band activity [46-50].
While EEG records the ongoing electrical activity of the brain, ERPs are time-locked voltage fluctuations in the brain in response to a sensory, motor, or cognitive event, and are extracted from a set of EEG trial epochs by means of filtering and signal averaging [51]. The amplitude (or the voltage measure) of an ERP component has been related to the neural resources available to process a stimulus or event [52], while the latency (or the time measure with respect to the stimulus) reflects stimulus/event processing time. Further, each ERP component has been attributed to specific functions based on the type, modality, and cognitive specificity of the task employed. The most studied and popular ERP component is the so-called P3 or P300, a large positive deflection that occurs between 300–700 ms after the stimulus onset and is related to its significance and not its physical features [53]. Across numerous ERP studies in alcoholism, the robust and consistent finding is that alcoholics and their high-risk offspring show reduced P3 amplitude across a variety of tasks and modalities (see [42,54] for reviews). Furthermore, the P3 component is highly heritable [55-59] and has proven to be useful as an endophenotypic marker of alcoholism. However, a reduction of P3 amplitude has not been found to be specific to alcoholism, but is also found in other related externalizing or disinhibitory disorders, such as substance use disorders (SUDs), conduct disorder (CD), antisocial personality disorder (ASPD), oppositional defiance disorder, (ODD), and attention deficit hyperactivity disorder (ADHD) [60,61]. In SUDs, reduced P3 amplitudes have been observed in individuals with dependence/abuse for tobacco [62], cocaine [63], cannabis [64,65], and ‘ecstacy’ or methylene-dioxy-methamphetamine (MDMA) [66]. Thus, reduced P3, while not specific to alcoholism, is a marker of alcoholism and related externalizing or disinhibitory disorders. However, the P3 component is not a unitary electrophysiological phenomenon elicited during cognitive processing; but emanates from multiple sources in the brain with contributions from frontal cortex (including anterior cingulate), parietal cortex, and hippocampus [7,67-71]; furthermore, it is primarily the outcome of event-related delta (concentrated more posteriorly) and theta (concentrated more anteriorly) oscillations [72-78]. Thus, because the EROs can analyze brain signals in terms of their constituent time-frequency components arising from various brain regions during sensory and cognitive events based on task conditions, they provide greater utility in understanding brain function than the traditional ERPs.
EROs as Measures of Cognitive Function
According to Basar et al. [79], selectively distributed delta, theta, alpha and gamma oscillatory systems act as resonant communication networks through large populations of neurons, with functional relations to cognitive and integrative functions. Relating to this view, ERP components are shown to be partially determined by the superposition of oscillatory responses of various frequencies [80-84] through phase synchronization or ‘partial phase resetting’ in response to specific stimulus event(s) [85-88]. According to the phase-resetting model, ERP components do not simply emerge from evoked, fixed latency-fixed polarity responses that are additive to and independent of ongoing EEG (i.e., ‘additive model of ERP generation’), but instead, ERPs are generated by a superposition of ongoing EEG oscillations that reset their phases in response to sensory input (see Sauseng et al. [89] for a critical discussion of this debate). Generally, EROs are broadly categorized into either ‘evoked’ or ‘induced’. Phase-aligned ERO signals (during the trials of cognitive events) are called ‘evoked’ or ‘phase-locked’ oscillations, while the (remaining) signals that become ‘out-of-phase’ across trials are termed ‘induced’ or ‘non-phase-locked’ oscillations [90]. The combination of both evoked and induced oscillatory power has been referred to as ‘total’ power [78]. Further, specific frequency bands within oscillatory responses have been attributed to underlie various cognitive processes [91-95], although the interpretation is often task-specific. For example, delta oscillations are thought to mediate signal detection and decision making (e.g., [91,96,97], while theta frequencies are linked with different cognitive processes, such as conscious awareness, recognition memory, episodic retrieval, and frontal inhibitory control [83,92,94,96,98,99]. The slow alpha rhythm (8-10 Hz) has been reported to modulate as a function of attentional demands [100-102], and fast alpha activity (10-12 Hz) has been shown to mediate semantic memory processes as well as stimulus-related aspects [92,101,103,104]. Further, it has been shown that oscillatory gamma responses are involved in visual perception, cognitive integrative function such as “binding,” and frontal input during sensory processing (top-down processing) [93,96,105-107]. Furthermore, these event-related oscillatory rhythms have been shown to mediate neural communication; higher frequency oscillations are involved in more localized neural networks, whereas the slower oscillations govern the long-range communication networks (e.g., posterior to anterior sites) [108-112].
EROs offer a few advantages over ERPs: (1) examining the oscillatory dynamics of EROs helps to dissect and subgroup the cognitive processes (e.g., [86,113]), especially for complex ERP components with multiple sources like P300, N400 etc. (2) correlated maps of brain regions for different cognitive processes can emerge from studying the cross frequency synchrony in EROs (e.g., [108,114,115]), (3) EROs can facilitate the understanding of how different cortical networks are integrated in response to an external stimulus and how information can be transferred between such circuits (e.g.,[87]), and (4) EROs allow for analysis of single trial epochs, which provide information about trial-by-trial variation in event-related brain dynamics, which is far superior to simple response averaging [116-119]. In a study from our lab, Chorlian et al. [120] demonstrated that single trial data enables both phase and amplitude information to be extracted and then compared across trials to determine whether amplitude or frequency modulation has occurred in bands of interest. The objective of the experiment was to produce the entrainment of alpha EEG activity by photic driving (through the use of a flashing background stimulus on which a non-flashing foreground stimulus appears), and to affect the entrainment by imposing an oddball task. Higher peak gamma amplitude was consistently observed during post-target-stimulus (300-750 ms) alpha suppression than at the time of maximum alpha activity during the immediate post-stimulus period. Further, the amplitude modulation of gamma (i.e., the amplitude envelope of the gamma activity) was at alpha frequency which was the frequency of entrainment). The authors suggested that there was an interaction between the alpha and gamma generating systems, in which gamma triggered alpha activity and was subsequently inhibited by it, thus producing the observed amplitude modulation. EROs have been used to understand not only normal cognitive processes but also neuro cognitive dysfunctions underlying psychopathological conditions and disorders that include alcoholism.
EROs and Alcoholism
As EROs represent the basic mechanisms of neural communication during cognitive tasks, they provide links to associative and integrative brain functions [121] that can be used to investigate normal brain function, as well as dysfunction in clinical and subclinical conditions. While relatively few studies have implemented EROs to study alcoholism, they have been used to examine several groups: 1) individuals affected with alcohol dependence, 2) unaffected offspring and relatives of alcoholics who are at high risk to develop alcoholism, 3) subjects under the direct effect of alcohol on the brain. Studies have examined several ERO measures including evoked, induced, and total power [122,123] in alcoholism [99,122,124-127]. Thus, EROs provide useful tool to investigate brain dysfunction in alcoholism, and to tease apart those that are a consequence of alcoholism, and those involved in a predisposition to develop alcoholism. Furthermore, they provide powerful quantitative endophenotypes that have successfully been used to identify genes.
Theta and delta EROs in alcoholics and high-risk individuals
Event-related delta activity is generated by cortico-cortical interactions [74], and is a product of the distributed network system of the brain [73,91] and involved in signal detection and decision making [73,91,96]. On the other hand, event-related theta oscillations are related to cortico-hippocampal [91,128] or frontolimbic interactions [83], and are associated with a complex set of cognitive processes including alertness, arousal or readiness [91], episodic encoding and retrieval processes [92,129], selective attention and short-term memory [73,130-132], error processing [133-135] and reward processing [136-139]. These delta and theta EROs have been derived from several cognitive paradigms, including the oddball task, Go/NoGo task, and a gambling task, to study alcoholism and related clinical conditions, as discussed below.
Visual oddball task
The most commonly used paradigm to elicit the P3 component of the ERP is the oddball paradigm [140], in which a random sequence of stimuli is presented. The stimuli can be classified into one of two (or more) categories, and the task is to classify the stimuli, either by counting or by pressing a button to members of one category (usually called the ‘target’ stimuli) while ignoring the other category (referred as ‘non-target’ stimuli). The first study to investigate EROs in alcoholism using a visual oddball paradigm was reported by Jones et al. [78] from our laboratory. The authors used a time-frequency signal analysis method called the “S-transform” to decompose the event-related signals in an age-matched sample of control (N=100) and alcoholic male subjects (N=100). The results indicated that the P3 waveform, commonly elicited using infrequent salient stimuli, was primarily composed of frontal theta and posterior delta band powers. Additionally, each ERO band activity contributed unique information to discriminate between the groups. In order to investigate whether these deficits in theta and delta oscillations antecede the development of alcoholism, Rangaswamy et al. [141] investigated adolescent offspring of alcoholics (14-17 years) with age-matched normal controls in the large Collaborative Study on the Genetics of Alcoholism (COGA), using the same paradigm. They found that similar to the alcoholics, the adolescent offspring of alcoholics had reduced delta and theta band ERO amplitude while processing the target stimuli compared to controls. The differences were most prominent centroparietally for theta, and parietally for delta. On the basis of the finding that stronger differences between groups were observed for the ERO oscillations than for the P3 amplitude, the authors suggested that the ERO measures appeared to be superior to P3 amplitude in differentiating between high-risk and low-risk offspring, and may serve as more stable and useful endophenotypes in the study of alcoholism and related disorders. Hence, the results of these two studies [78,141] indicate that decreased theta and delta EROs to target stimuli may prove to be a strong phenotypic marker in the development of alcoholism, which may be useful for genetic studies.
In a later study, Andrew and Fein [142] reported significantly lower evoked delta ERO power as well as total delta and theta ERO power in long-term abstinent alcoholics (LTAAs) compared to the nonalcoholic controls (NACs), replicating Jones et al. [78] results. However, on the basis of the similar magnitude of effect sizes for the ERO power and P3 amplitude in their study, in contrast to Jones et al. [78] and Rangaswamy et al. [141], these authors concluded that ERO power does not provide a more powerful group discriminator than P3 amplitude. In another study using the same sample, Andrew and Fein [143] reported significantly higher induced theta power in LTAAs as compared to NACs. Based on this finding, the authors concluded that induced (non-phase locked) theta oscillations are more powerful and independent group discriminators than the P3 amplitude.
Recently, using a principal component analysis (PCA) based time-frequency (TF) analysis, Gilmore et al. [144] examined the relationship between the P3, its associated ERO components in a spectrum of externalizing disorders, such as substance use disorders (including alcoholism), ADHD, and conduct disorder in a community-based sample of adolescent males. It was found that 1) P3 and each TF-PCA derived component could successfully discriminate diagnostic groups from controls, and 2) delta components in specific time ranges accounted for variance beyond that accounted for by P3. Further, one delta component was associated with all diagnostic groups, suggesting that ERO delta may represent a more parsimonious endophenotype for externalizing disorders than P3. In another similar study using data from a large twin sample (634 MZ and 335 DZ, male and female same-sex pairs), Gilmore et al. [145], analyzed several electrophysiological measures (the P3, delta and theta ERO components, and resting state beta frequency band) collectively in externalizing disorders. They found these measures (1) were heritable, (2) showed significant phenotypic and genetic correlation with a general vulnerability to externalizing disorders, (3) showed modest phenotypic and genetic correlation with each other, and (4) were sensitive to genetic effects that differed as a function of gender. These relationships suggested that these endophenotypes were likely tapping into electrophysiological processes and genes that were both common across them and unique to each. All these electrophysiological endophenotypes were highly relevant to a biological vulnerability to externalizing psychopathology. These two studies further indicated that delta and theta EROs are useful endophenotypes not only specifically for alcoholism but also for the spectrum of related externalizing disorders that includes alcoholism.
Go/NoGo task
The Go/NoGo task has been most widely used to assess response inhibition, in which the subject withholds his/her response for one set of stimuli (“NoGo” trials) while responding to another set of stimuli (“Go” trials). The task can be either a type of ‘reverse oddball’, with more trials for the Go condition, or have equal trials in both Go and NoGo conditions [146]. Only a few studies have analyzed EROs using the Go/NoGo paradigm to investigate alcoholism. Using an equal probability visual Go/NoGo task, Kamarajan et al. [99] reported deficient delta and theta oscillatory activity in abstinent alcoholics. This reduction in delta and theta EROs in alcoholic subjects was found in the frontal region, particularly during NoGo trials [99]. To examine whether or not these deficits anteceded the development of alcoholism, the authors studied offspring of alcoholic parents and found that these high-risk subjects manifested significantly decreased ERO activity in delta (1-3 Hz), theta (4-7 Hz), and low alpha (8-9 Hz) bands during the NoGo condition, as well as reduced delta and theta activity during the Go condition. This study provided further evidence that these deficient oscillatory responses antecede the development of alcoholism [147] and perhaps could serve as effective endophenotypes in the study of alcoholism.
Gambling task
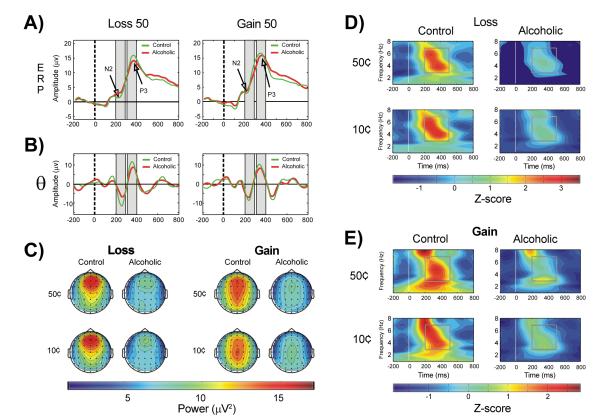
A typical gambling task involves ‘choice’ stimuli (for which the subjects have to choose one of two or more choices to bet with) and ‘outcome’ or ‘feedback’ stimuli which show the resultant outcome (e.g., loss or gain) for the choice made during a particular trial [148]. Alcoholics have been reported to have deficient ERP components obtained from gambling tasks [11,149]. However, to our knowledge, only one ERO study has used a gambling paradigm in alcoholics; Kamarajan et al. [150] examined the relationship among the factors of ERO measures, impulsivity, and alcoholism. In this study, ERO theta power in the time window of 200-500 ms (wherein the ‘outcome-related’ ERP negativity and positivity occur) was compared across alcoholic and control groups (Figure 1). The subjects in the study 1) were asked to select one of two amounts (10¢ or 50¢) to bet with, and 2) received feedback/outcome about the selected amount (i.e., Loss 10, Loss 50, Gain 10, and Gain 50). The alcoholic group showed significantly decreased theta power during reward/outcome processing compared to controls. A strong association between reduced anterior theta power and impulsive task performance was also observed. Furthermore, alcoholics exhibited increased impulsivity and risk-taking on the behavioral measures. Similar studies in high-risk offspring of alcoholics are underway to further evaluate this ERO measure (anterior theta power) and determine whether it antecedes the development of alcoholism, and can serve as a potential endophenotype.
Figure 1.
A) The grand-averaged ERP waveforms for Loss 50 and Gain 50 conditions; B) Theta bands during Loss 50 at FZ electrode and Gain 50 at CZ electrode are shown. The region of ORN (N2) and ORP (P3) peaks and the corresponding theta activity during the time window are shaded in gray color. There is a partial phase-alignment of the theta activity corresponding to ORN (200-300 ms) and ORP (300-400 ms) components. Alcoholics showed decreased amplitude in theta (3–7 Hz) band more anteriorly (frontal) for the loss condition and more posteriorly (centro-parietal) for the gain condition. The dashed vertical line (at 0 ms) represents the onset of an outcome stimulus; C) Topographic maps of theta (3-7 Hz) power in control and alcoholic groups illustrating that the loss condition had an anterior topography and the gain condition had a posterior topography. Decreased theta band power in alcoholics in both conditions is illustrated; D) Time-frequency (TF) plots during the loss condition at the Fz electrode in the alcoholic and control groups; and E) TF plots during the gain condition at the Cz electrode. The square box inside TF plots marks the time-frequency region of interest, namely the time interval of 200–500 ms across the theta frequency range 3-7 Hz for analysis. The alcoholic group showed a significant reduction in theta power during each outcome condition. The color scale (in TF plots) represents the theta power in terms of Z-scores, which were computed from the overall data (representing all groups and conditions) and hence are comparable among the TF plots.
In a simulated gambling task, Bernat et al. [151] examined the relationship between externalizing proneness and the feedback-related negativity (FRN), a brain response that indexes performance monitoring related to exogenous cues. They found delta-P300 amplitude was reduced among individuals high in externalizing proneness whereas theta-FRN response was unrelated to externalizing. The authors suggested that in contrast to previously reported deficits in endogenously based performance monitoring [152] as indexed by the error-related negativity (ERN), individuals prone to externalizing problems show intact monitoring of exogenous cues. The results of Bernat et al. [151] appear to be in contrast with the findings of Kamarajan et al. [150], who reported deficient theta EROs in alcoholics compared to normal controls for the outcome related components. However, there are important differences in the methodologies of these studies in terms of the paradigms used, analysis methods, and subject samples under study. Kamarajan et al. [150] used an abstinent alcoholic sample (DSM-IV) whereas Bernat et al. [151] used a sample of undergraduate students who were classified into high and low proneness to externalizing. Secondly, Kamarajan et al. [150] used an S-Transform analysis to decompose TF signals where a broader time window of 200-500 ms was used that encompasses both outcome-related negativity and positivity (ORN/ORP). On the other hand, Bernat et al. [151] used a PCA based TF approach and included the components that accounted for most of the variance; i.e., they separated contributions of the delta (P3) and theta (FRN) time windows. It has been reported that P3 is primarily composed of delta and theta oscillations [72-78] and N2 is primarily composed of theta oscillations (e.g., [133,134,136,137,153]). Therefore, the differences in study samples and methodological approaches in these two studies may explain the contrasting results, but further studies will be required to resolve these issues.
Gamma ERO
The early phase-locked gamma has been considered to represent an important processing stage related to the selection and identification of target stimuli, indicative of top-down mechanisms involved in selective attention [105,106,154]. This phase-locked or evoked gamma is larger for attended stimuli compared to unattended stimuli, particularly over frontal regions [79,155], while the induced gamma oscillations have been associated with a wide range of cognitive processes, object representation, and learning [106,156,157]. Although many studies have examined the gamma band ERO activity in healthy individuals, such studies are very rare in clinical disorders including alcoholism.
Padmanabhapillai et al. [158] investigated early evoked gamma activity (during the first 150 ms poststimulus activity) using a visual oddball task. They found that alcoholic subjects showed significantly decreased evoked gamma activity at frontal regions during target processing compared to nonalcoholic controls. The authors suggested that this reduction in early evoked gamma response at frontal locations may be due to frontal lobe dysfunction associated with deficient top-down processing mechanisms in alcoholics. The authors further conducted a similar study in children of alcoholics (14-17 years) compared to an age-matched nonalcoholic control group [159]. They found that offspring of alcoholics, similar to alcoholics, showed significant reductions in early evoked gamma activity in frontal as well as parietal regions for the target condition, with the maximum difference in the parietal region. Additionally, high-risk offspring exhibited less differentiation between target and non-target conditions in terms of level of gamma activity, indicating a further deficiency in stimulus discrimination processes. It is likely that a dysfunctional frontoparietal attentional network may underlie this deficit as shown in an fMRI study during a visual oddball task in high-risk subjects, reported by Rangaswamy et al. [15].
Alcohol-Induced ERO alterations
There are a number of studies showing the changes in resting EEG and ERPs due to the direct effects of alcohol ingestion [160-162]. However, studies on alcohol-induced alterations in EROs are quite rare. Krause et al. [163] examined the effects of alcohol on event-related desynchronization (ERD) and event-related synchronization (ERS) during an auditory memory task. It was found that the administration of alcohol decreased the early-appearing ERS responses during auditory encoding and increased the later-appearing ERD responses during retrieval; these effects were significant in the slow theta (4-6 Hz), fast theta (6-8 Hz), and slow alpha (8-10 Hz) frequency bands. The authors concluded that alcohol intake has a direct effect on the brain and cognitive functioning by disorganizing the brain oscillatory systems in the theta and lower alpha frequency range during stimulus/task processing. In another study during a selective attention task, Jaaskelainen et al. [164] investigated the dose-related impact of alcohol on auditory transient evoked 40-Hz responses and found that evoked 40-Hz response amplitude was suppressed by alcohol during both attended and non-attended conditions, starting at the blood alcohol concentration (BAC) level of about 0.05%. This study further demonstrated that cognitive processing associated with gamma band activity is affected by alcohol administration.
Recently, Boha et al. [165] studied the acute effects of alcohol on task-related EEG during the performance of a mental arithmetic task that involved working memory. While no detrimental alcohol effect was seen on behavioral indices of task performance, alcohol decreased the usually occurring task-related frontally dominant theta increase corresponding to the working memory demand. Further analysis using complexity measures by the same group of authors [166] suggested that task-related decrease of the omega complexity (OC, reflecting the lesser level of complexity or higher spatial correlation between different electrodes) and increase of the synchronization likelihood (SL, reflecting the higher strength of coupling between EEG-channels) was found in the theta frequency band, indicating increased synchrony in the event-related theta band. Further, high dose of alcohol intake increased the OC values and decreased the anterior SL values under working memory condition, indicating decreased synchrony in ERO theta band.
Apart from these studies on the direct effect of alcohol, there has been an attempt to examine EROs in the groups of social drinkers. For example, De Bruin et al. [167] conducted a task-related EEG study on heavy social drinkers with 24 hours abstinence before the recording session. Functional connectivity was assessed with the SL method. It was found that heavy drinking students had more synchronization in the theta (4-8 Hz) and gamma (30-45 Hz) band than light drinking students during a mental-rehearsal task, which the authors attributed to possible changes in hippocampal-neocortical connectivity.
Animal Studies of EROs related to Alcoholism
Animal models have been developed to simulate several relevant human phenotypic markers or traits associated with alcohol use and dependence. In the models relating to electrophysiological measures, differences in EEG (e.g., [168]), ERP (e.g., [168,169]), and ERO [170,171] responses have been identified to distinguish high alcohol preferring (HAP) from low alcohol preferring (LAP) mice. It was confirmed that these electrophysiological changes in high versus low alcohol preferring mammals closely resemble differences seen in human studies of individuals with high and low risk for alcohol dependence. Ehlers and Criado [172] first demonstrated that EROs can be generated in cortical sites in mice in the delta, theta, alpha/beta frequency ranges in response to auditory stimuli. They observed that oscillations in the 7.5-40 Hz frequencies were significantly affected in the 0-50 ms time window in response to differences in tone frequency, whereas, changes in tone loudness produced changes in oscillations in the 7.5-40 Hz frequencies in the 350-800 ms time window.
In their first ERO study of animal models of alcoholism, Criado and Ehlers [170] investigated the utility of EROs as effective risk markers by using genetic mouse models of high alcohol preference. The authors measured delta, theta and alpha/beta ERO energy and the degree of phase variation in groups of high and low alcohol preferring mice and found that the decreased P3 amplitudes previously shown in B6 mice (with a low level of withdrawal severity), compared to D2 mice (with extreme withdrawal severity) [169], was related to reductions in evoked delta ERO energy and delta and theta phase locking. In contrast, the increase in P1 amplitudes reported in HAP mice, compared to LAP mice, is associated with increases in evoked theta ERO energy. Further, these differences in delta and theta ERO measures in mice mirrored the changes observed in human subjects between high and low risk for alcoholism where changes in EROs were found to be more significant than group differences in P3 amplitudes. Based on these findings, the authors suggested that ERO these measures are more stable endophenotypes in the study of alcohol dependence than P3.
In another recent study by Criado and Ehlers [171], EROs in selectively bred adult alcohol-preferring (P) and alcohol-non preferring (NP) rats were compared. While the previous electrophysiological studies have shown P rats exhibited more EEG fast frequency activity and reduced P3 amplitude in the parietal cortex than NP rats [173], findings that are more common in alcohol-dependent individuals, the authors tested the possibility that EROs could be excellent endophenotypes associated with ethanol dependence using similar animal. The findings confirmed that the decrease in P3 amplitudes previously shown in P rats were significantly associated with decreases in evoked delta and alpha/beta phase locking. These findings in animal models of alcoholism further confirmed the findings of human studies that that ERO measures provide good endophenotypes in the study of alcoholism.
In summary, studies of EROs during visual oddball, Go/NoGo, and gambling paradigms indicate that delta, theta, and gamma power is reduced in alcoholics. As these findings are also obtained in offspring at high risk, it suggests that these ERO differences antecede the development of alcoholism. Hence, not only is the traditional finding of P3 amplitude reduced in alcoholics and subjects at risk, but the underlying delta and theta oscillations are also reduced. Animal studies utilizing high and low alcohol preferring model appear to confirm these findings in alcoholic human subjects and their offspring. As will be seen in the next section, these ERO markers of risk provide excellent endophenotypes that have been successfully implemented in genetic studies.
EROs as Endophenotypes
Alcoholism is a common, complex (non-Mendelian) disorder with contributions from both genetic and environmental influences and their interactions [41]. As psychiatric diagnosis is dichotomous (either affected or unaffected), it is difficult to use diagnosis as the sole phenotype when studying the genetics of complex disorders, such as alcoholism. It has been suggested that, ideally, molecular genetic studies should not be performed on psychiatric diagnoses alone, which reflect distal and variable effects of genes, but on quantitative neurobiological measures or markers that reflect more proximal effects of genes involved in the genetic predisposition for developing psychiatric disorders [174]. These quantitative biological markers (endophenotypes or intermediate phenotypes) serve as covariates that correlate with the main trait of interest (diagnosis) and serve to better define that trait or its underlying genetic mechanism [43,175,176]. The advantages of using quantitative neurobiological measures of risk as endophenotypes in the search for genes involved in complex disorders are that they are closer to gene action involved in the predisposition for the disorder, they are genetically simpler than clinical endpoints, and quantitative traits provide more power to localize and characterize disease susceptibility genes [177].
In order to be considered as an endophenotype, several criteria must be met [43]: 1) the endophenotype must be associated with illness in the population, 2) the endophenotype must be heritable, 3) the endophenotype must be primarily state-independent (manifests in an individual whether or not illness is active), 4) within families, endophenotype and illness co-segregate, and 5) the endophenotype must be found in affected family members, and must also be found in non-affected family members at a higher rate than in the general population. Endophenotype-based strategies have successfully identified genes associated with many psychiatric disorders.
Brain function is likely to be involved in a genetic predisposition to develop alcoholism and other psychiatric disorders, and neuroelectric events may serve as excellent biological markers or endophenotypes. Understanding genetic control of brain electrical activity may provide clues about cerebral function, and may shed light on pathogenic mechanisms involved in neurological and psychiatric disorders, where impairment in brain electrical activity is apparent. Brain oscillations provide a rich source of potentially useful endophenotypes for psychiatric genetics, as they represent important correlates of human information processing and cognition.
In order for genetic studies using the endophenotype approach to be successful, it is extremely important to select well characterized (endo) phenotypes that meet the criteria of Gottesman and Gould [43]. Neuroelectric phenotypes (brain oscillations, such as EEG and those EROs underlying ERPs, including the P3 component) meet these criteria for endophenotypes for alcoholism, as they are heritable, and characterize not only those who are affected, but also offspring at risk [42]. The data on the heritability of EEG frequencies are quite compelling. High concordance rates in the spectral characteristics of resting eyes-closed EEG have been reported from monozygotic twin pairs compared to dizygotic twin pairs. A large twin study indicates that power in all frequency bands of the resting EEG is highly heritable: delta 76%, theta 89%, alpha 89%, and beta 86% [57,58]. The power estimates of frequency bands may be more heritable than ERP components giving them a slight edge as endophenotypes [178]. As reviewed in the previous sections, event-related oscillations (EROs) meet the criteria of endophenotypes for alcoholism, as reduced delta, theta and gamma oscillations not only characterize affected alcoholics, but also their relatives, particularly offspring at risk prior to the onset of the illness. Therefore, the use of quantitative ERO endophenotypes provides a powerful strategy for identifying underlying susceptibility genes for developing alcoholism and related disinhibitory disorders.
One ERO endophenotype for genetic studies, event-related theta power, has been examined in densely affected alcoholic families in the large Collaborative Study on the Genetics of Alcoholism (COGA). The densely affected families consisted of a proband and two additional first-degree relatives affected with alcohol dependence (DSM-IV), and control families that were randomly ascertained from the general population. These studies reported significant linkage and association between the CHRM2 (cholinergic muscarinic receptor M2) gene on chromosome 7 and frontal theta oscillations to target stimuli in a visual oddball task. Association analyses using both population based tests (Measured Genotype) and pedigree based tests (Quantitative Pedigree Disequilibrium Test, QPDT) indicated significant association of the frontal theta band ERO phenotype with several single-nucleotide polymorphisms (SNPs) surrounding exon 4 of CHRM2. Further, an examination of the slower frequency parietal delta band ERO revealed significant association with several SNPs surrounding the coding region of the CHRM2 gene [179,180].
These findings implicate the possible role of CHRM2 in the generation and/or modulation of EROs. Theta and delta EROs depend on the level of acetylcholine. Muscarinic acetylcholine (M2) receptors inhibit presynaptic release of acetylcholine, leading to inhibition of irrelevant networks (cf. [181]). M2 are especially concentrated in the forebrain and possibly serve to maintain the effective balance of relevant/irrelevant networks, hence, having a direct influence on P3 generation [182]. Our results with the CHRM2 gene and brain oscillations support the role of acetylcholine in the generation of N2 (theta oscillations) and in the P3 component (delta and theta oscillations). A role of acetylcholine has been reported with regard to stimulus significance [183], selective attention [184], and P3 generation [185]. Administration of cholinergic agonists and antagonists have resulted in modified memory performance, as well as modified P3 amplitudes in humans [186-188]. In vitro administration of moderate amounts of the muscarinic agonist carbachol in the rat hippocampus induces synchronized delta oscillations, whereas higher concentrations produced short episodes of theta oscillations, and the carbachol-induced delta rhythms were not observed concurrent with carbachol-theta [189,190].
Recent evidence indicates that the CHRM2 gene is not only associated with brain oscillations and cognition, but also clinical diagnosis. Significant linkage and association were reported for the CHRM2 gene and a diagnosis of alcohol dependence and depression [191], comorbid alcohol and drug dependence [192], as well as a spectrum of externalizing disorders [193] in the COGA study. Other groups have also replicated these findings, reporting that the CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders [194], and major depression in women [195]. Thus, genes important for the expression of the endophenotypes help in identification of genes that increase the susceptibility for risk of alcohol dependence and related disorders [196,197].
Under the same theta ERO linkage peak on chromosome 7 is a glutamate receptor (GRM8) gene that is another very likely candidate gene for modulating electrophysiological networks, particularly as the glutamatergic system is also involved in theta oscillations and P3 [182]. Family-based association analyses of theta EROs revealed significant associations with several SNPs in the GRM8 gene and theta EROs to target stimuli at frontal, central, and parietal regions [198]. An interesting finding is that several of the same GRM8 SNPs were also significant for diagnosis of alcohol dependence using ICD-10 diagnostic criteria.
Using 3-T proton magnetic resonance spectroscopy (1H-MRS), Gallinat et al. [199] have suggested the involvement of glutamatergic neurotransmission in integrative frontal-hippocampal processing [199] and the sensation seeking personality dimension [200]. The study demonstrated a strong relationship between glutamate levels in the hippocampus and frontal theta activity during auditory stimulus processing [199]. Glutamatergic neurotransmission and its neuroadaptive changes have been proposed as important molecular determinants of craving and relapse [201,202]. In particular, it is suggested that a hyperglutamatergic state mediates, at least in part, alcohol relapse behavior and maintenance of alcoholism [203]. Several studies have suggested the involvement of glutamate receptors, NMDA and metabotropic, in alcohol relapse [204-206]. Acamprosate, a drug used to prevent relapse in alcoholic patients [207], has been suggested to act through a suppression of a hyperglutamatergic state created by alcohol addiction [208,209]. Although there have been as yet no ERO/ERPs studies done in alcoholics during/after the administration of anti-craving/anti-dependence medications, such as acamprosate, naltrexone, etc., a few resting EEG studies in animals [210-212] and in humans [213-215] found EEG alterations after administration of these drugs. While animal studies indicated a slowing or reduction of frequency, human studies were equivocal. Future electrophysiological research (especially ERP and ERO studies) should further investigate this important aspect.
In a first genome-wide case-control association study (GWAS) of an ERO endophenotype (frontal theta ERO from the target response in visual oddball task), Zlojutro et al. [216] reported some important genetic associations. This was a two-stage GWAS study that first identified four markers, ARID5A, GNAS1, ANXA13, and HTR7 with nominally significant association with frontal theta ERO, with the most significance in ARID protein 5A gene (ARID5A) on chromosome 2q11. The most intriguing association was with a serotonin receptor gene (HTR7) on chromosome 10q23, implicating the serotonergic system in the neurophysiological underpinnings of theta EROs. They also found a significant association of alcohol dependence diagnosis (DSM-IV) with several HTR7 SNPs among GWAS case-controls. Significant recessive genetic effects were also detected for alcohol dependence in both the COGA case-control and family-based samples, with the HTR7 risk allele corresponding to theta ERO reductions among homozygotes. These results suggested a role of the serotonergic system in the biological basis of alcohol dependence, and therefore demonstrated the utility of brain oscillations as a powerful approach to understanding complex genetic psychiatric disorders such as alcoholism.
In summary, it can be said that the genetic underpinnings of evoked oscillations are likely to stem from regulatory genes which control the neurochemical processes of the brain, thereby influencing neural function. The major neurochemical substrates contributing to theta and delta rhythms and P3 has been suggested to be strongly glutamatergic, with strong modulatory influences from both cholinergic and GABAergic sources that elicit excitatory and inhibitory post-synaptic potentials, and indirect system interactions and contributions from the serotonergic, dopaminergic and noradrenergic systems [182,216].
Summary and Conclusions
EROs are measures of cognitive processing in response to a stimulus or event, and are considered to be building blocks of various neurocognitive functions; each frequency activity of EROs represents several specific cognitive functions [79,217]. EROs facilitate neural communication across brain regions through synchrony, with low frquency oscillations (such as delta, theta and alpha) for long-range communication and high frequency oscillations (such as beta and gamma) for short-range communication [108].
Studies with alcoholics and their offspring have consistently reported ERO deficits in delta, theta, and gamma bands in visual oddball tasks. Furthermore, deficits in theta ERO power have been reported across several task paradigms (e.g., oddball, Go/NoGo, and gambling) in these groups. Since theta ERO contributions to the P3 as well as N2 components of the ERP are mainly from frontocentral regions of the brain, it can be hypothesized that the deficits in theta ERO power in these groups may be associated with the dysfunctional frontal processing. Further, these frontal theta ERO deficits in the offspring of alcoholics indicate that they antecede the development of alcoholism. Importantly, the frontal theta EROs have successfully served as endophenotypes in genetic studies of alcoholism, and have been found to be significantly associated with SNPs in CHRM2 [179,180], GRM8 [198], and HTR7 [216] genes, SNPs that have also been found to be significantly associated with alcohol dependence. This suggests the involvement of glutamatergic, GABAergic and serotonergic systems in the modulation of theta oscillations especially in the context of alcoholism.
The use of quantitative brain oscillations as endophenotypes provides the power to more easily localize and characterize disease susceptibility genes than diagnostic categories. The recent identification of genetic loci associated with human brain oscillations indicates that they are under genetic control and are modulated by genes controlling neurotransmitters in the brain. Several receptor genes have emerged from the genetic studies of event-related oscillations as endophenotypes, that are involved in variation in brain oscillations and hence their cognitive correlates; specifically, theta EROs have been associated with the cholinergic muscarinic receptor (CHRM2), metabotropic glutamate receptor (GRM8), and serotonin receptor (HTR7) genes. Although no functional variant affecting the electrophysiological characteristics has yet been identified at the molecular level, a large body of pharmacological evidence attests to the relevance of these receptors to aspects of cognitive function. The advent of genomics and proteomics and a fuller understanding of gene regulation will open new horizons on the critical electrical events so essential for human brain function. This approach has the unprecedented potential to unravel the complex interplay of various neural subsystems relevant to the generation of brain oscillations elicited under different cognitive conditions and in the disease states.
It is interesting to note that in the genetic studies using brain oscillations as endophenotypes significantly associated SNPs lie in the same genes that are also associated with alcohol dependence and related disorders. Thus, genes underlying the variations in endophenotypes are also associated with the disease state. This highlights the utility and importance of using this endophenotype approach with quantitative brain oscillations in the identification and understanding of genes involved in alcoholism and related disorders. As alcohol dependence results from a complex interaction of changing genetic and environmental liabilities across development, it is hoped that by combining these approaches we can eventually understand how much of the capacity to modulate these oscillations, in the context of a disease state, lies in the control of genes and how environment influences this control; prospective studies of young individuals with “risk genotypes” can lead to an improved understanding of how neural and cognitive changes contribute to susceptibility across development, which in turn can lead to the design of well-targeted prevention initiatives. Understanding genetic control of brain electrical activity may provide clues about cerebral function, and may shed light on pathogenic mechanisms involved in neurological and psychiatric disorders, where impairment in brain electrical activity is apparent. Once genes are identified and understood, risk genotypes can be used in the development of both targeted treatment and prevention initiatives.
Acknowledgements
This review paper is dedicated to the memory of Henri Begleiter (deceased April 6, 2006), Distinguished Professor of Psychiatry and Neuroscience, and founder and director of the Neurodynamics Laboratory, now renamed in his honor. His scientific vision brought together the fields of alcoholism, brain oscillations, and genetics. Our laboratory continues to follow the path that he envisioned, inspired by his innovative approaches to research.
Our studies reviewed in this paper were supported by NIH Grants AA008401, AA05524, AA002686 from the National Institute of Alcohol Abuse and Alcoholism.
We are grateful for the valuable assistance of our team members David B. Chorlian, Niklas Manz, Sun J. Kang, Arthur T. Stimus, Carlene Haynes, Joyce Alonzia, Chamion Thomas, Aleksandr Razran, Christopher Horrax, Susan S. Goldstein, Kelly Makris, Lyubov Gorbach, Amanda Frudakis, and David Friedlander.
References
- 1.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 2.Begleiter H, Platz A. The effects of alcohol on the central nervous system in humans. In: Kissin B, Begleiter H, editors. The Biology of Alcoholism. Volume 2, Physiology and Behavior. Plenum; New York: 1972. pp. 293–343. [Google Scholar]
- 3.Begleiter H, Porjesz B, Tenner M. Neuroradiological and neurophysiological evidence of brain deficits in chronic alcoholics. Acta Psychiatrica Scandinavica. Acta Psychiatr Scand Suppl. 1980;286:3–13. doi: 10.1111/j.1600-0447.1980.tb08050.x. [DOI] [PubMed] [Google Scholar]
- 4.Porjesz B, Begleiter H. Evoked brain potentials and alcoholism. In: Parsons OA, Butter N, Nathan P, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford Press; New York: 1987. pp. 45–63. [Google Scholar]
- 5.Zhang XL, Begleiter H, Porjesz B, Litke A. Electrophysiological evidence of memory impairment in alcoholic patients. Biol Psychiatry. 1997;42:1157–1171. doi: 10.1016/s0006-3223(96)00552-5. [DOI] [PubMed] [Google Scholar]
- 6.Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, et al. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biol Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- 7.Ardekani BA, Choi SJ, Hossein-Zadeh GA, Porjesz B, Tanabe JL, et al. Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res Cogn Brain Res. 2002;14:347–356. doi: 10.1016/s0926-6410(02)00137-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen ACH, Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, et al. Impulsivity and visual P3 in alcoholism. Annual Research Day meeting of the SUNY Downstate Medical Center. 2005.
- 9.Kamarajan C, Porjesz B, Jones KA, Chorlian DB, Padmanabhapillai A, et al. Spatial-anatomical mapping of NoGo-P3 in the offspring of alcoholics: evidence of cognitive and neural disinhibition as a risk for alcoholism. Clin Neurophysiol. 2005;116:10491061. doi: 10.1016/j.clinph.2004.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamarajan C, Rangaswamy M, Tang Y, Chorlian DB, Pandey AK, et al. Dysfunctional reward processing in male alcoholics: an ERP study during a gambling task. J Psychiatr Res. 2010;44:576–590. doi: 10.1016/j.jpsychires.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill SY, Shen S, Lowers L, Locke-Wellman J, Matthews AG, et al. Psychopathology in offspring from multiplex alcohol dependence families with and without parental alcohol dependence: a prospective study during childhood and adolescence. Psychiatry Research. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper C, Kril J. Brain atrophy in chronic alcoholic patients: a quantitative pathological study. Journal J Neurol Neurosurg Psychiatry. 1985;48:211–217. doi: 10.1136/jnnp.48.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 15.Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, et al. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, et al. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol Clin Exp Res. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, et al. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolas JM, Catafau AM, Estruch R, Lomena FJ, Salamero M, et al. Regional cerebral blood flow-SPECT in chronic alcoholism: relation to neuropsychological testing. J Nucl Med. 1993;34:1452–1459. [PubMed] [Google Scholar]
- 19.Sachs H, Russell JA, Christman DR, Cook B. Alteration of regional cerebral glucose metabolic rate in non-Korsakoff chronic alcoholism. Arch Neurol. 1987;44:1242–1251. doi: 10.1001/archneur.1987.00520240024007. [DOI] [PubMed] [Google Scholar]
- 20.Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, et al. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones B, Parsons OA. Specific vs generalized deficits of abstracting ability in chronic alcoholics. Arch Gen Psychiatry. 1972;26:380–384. doi: 10.1001/archpsyc.1972.01750220090017. [DOI] [PubMed] [Google Scholar]
- 22.Parsons OA. Neuropsychological deficits in alcoholics: facts and fancies. Alcohol Clin Exp Res. 1977;1:51–56. doi: 10.1111/j.1530-0277.1977.tb05767.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller L. Neuropsychological assessment of substance abusers: review and recommendations. J Subst Abuse Treat. 1985;2:5–17. doi: 10.1016/0740-5472(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 24.Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. J Stud Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- 25.Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt MJ, Warren K, editors. Review of NIAAA’s Neuroscience and Behavioral Research Portfolio. National Institute on Alcohol Abuse and Alcoholism (NIAAA); Bethesda, MD: 2000. pp. 437–471. Research Monograph No. 34. NIAAA. [Google Scholar]
- 26.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrett HL, Carey PD, Thomas KG, Tapert SF, Fein G. Neuropsychological performance of South African treatment-naive adolescents with alcohol dependence. Drug Alcohol Depend. 2010;110:8–14. doi: 10.1016/j.drugalcdep.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S, Fein G. Cognitive performance in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2010;34:2097–2105. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celesia GG, Brigell M. Event-related potentials. Curr Opin Neurol Neurosurg. 1992;5:733–739. [PubMed] [Google Scholar]
- 30.Krieger S, Timmer J, Lis S, Olbrich HM. Some considerations on estimating event-related brain signals. J Neural Transm Gen Sect. 1995;99:103–129. doi: 10.1007/BF01271473. [DOI] [PubMed] [Google Scholar]
- 31.Hummel F, Gerloff C. Larger interregional synchrony is associated with greater behavioral success in a complex sensory integration task in humans. Cereb Cortex. 2005;15:670–678. doi: 10.1093/cercor/bhh170. [DOI] [PubMed] [Google Scholar]
- 32.Hummel FC, Gerloff C. Interregional long-range and short-range synchrony: a basis for complex sensorimotor processing. Prog Brain Res. 2006;159:223–236. doi: 10.1016/S0079-6123(06)59015-6. [DOI] [PubMed] [Google Scholar]
- 33.Chen CC, Hsieh JC, Wu YZ, Lee PL, Chen SS, et al. Mutual-information-based approach for neural connectivity during self-paced finger lifting task. Hum Brain Mapp. 2008;29:265–280. doi: 10.1002/hbm.20386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma A, Weisbrod M, Kaiser S, Markela-Lerenc J, Bender S. Deficits in fronto-posterior interactions point to inefficient resource allocation in schizophrenia. Acta Psychiatr Scand. 2011;123:125–135. doi: 10.1111/j.1600-0447.2010.01603.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan RF, Glueck BC, Hesselbrock MN, Reed HB., Jr. Power and coherence analysis of the EEG in hospitalized alcoholics and nonalcoholic controls. J Stud Alcohol. 1985;46:122–127. doi: 10.15288/jsa.1985.46.122. [DOI] [PubMed] [Google Scholar]
- 37.Bryant KJ, Rounsaville BJ, Babor TF. Coherence of the dependence syndrome in cocaine users. Br J Addict. 1991;86:1299–1310. doi: 10.1111/j.1360-0443.1991.tb01705.x. [DOI] [PubMed] [Google Scholar]
- 38.Chorlian DB, Rangaswamy M, Porjesz B. EEG coherence: topography and frequency structure. Exp Brain Res. 2009;198:59–83. doi: 10.1007/s00221-009-1936-9. [DOI] [PubMed] [Google Scholar]
- 39.Coutin-Churchman P, Moreno R. Intracranial current density (LORETA) differences in QEEG frequency bands between depressed and non-depressed alcoholic patients. Clin Neurophysiol. 2008;119:948–958. doi: 10.1016/j.clinph.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Pandey AK, Kamarajan C, Tang Y, Chorlian DB, Roopesh BN, et al. Neurocognitive deficits in male alcoholics: An ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol. 2012;89:170–182. doi: 10.1016/j.biopsycho.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 42.Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, et al. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 43.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 44.Haas LF. Hans Berger (1873-1941) Richard Caton (1842-1926), and electroencephalography. J Neurol Neurosurg Psychiatry. 2003;74:9. doi: 10.1136/jnnp.74.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedermeyer E, Lopes da Silva F. Electroencephalography: basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 46.Propping P, Kruger J, Mark N. Genetic disposition to alcoholism. An EEG study in alcoholics and their relatives. Hum Genet. 1981;59:51–59. doi: 10.1007/BF00278854. [DOI] [PubMed] [Google Scholar]
- 47.Costa L, Bauer L. Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug Alcohol Depend. 1997;46:87–93. doi: 10.1016/s0376-8716(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 48.Winterer G, Kloppel B, Heinz A, Ziller M, Dufeu P, et al. Quantitative EEG (QEEG) predicts relapse in patients with chronic alcoholism and points to a frontally pronounced cerebral disturbance. Psychiatry Res. 1998;78:101–113. doi: 10.1016/s0165-1781(97)00148-0. [DOI] [PubMed] [Google Scholar]
- 49.Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25:332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- 50.Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, et al. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- 51.Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- 52.Rugg MD, Coles MGH. Electrophysiology of mind event-related brain potentials and cognition. Oxford University Press; Oxford; New York: 1996. [Google Scholar]
- 53.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- 55.Almasy L, Porjesz B, Blangero J, Chorlian DB, O’Connor SJ, et al. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88:383–390. [PubMed] [Google Scholar]
- 56.Polich J, Bloom FE. P300, alcoholism heritability, and stimulus modality. Alcohol. 1999;17:149–156. doi: 10.1016/s0741-8329(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 57.van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- 58.van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- 59.van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- 60.Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 61.Carlson SR, McLarnon ME, Iacono WG. P300 amplitude, externalizing psychopathology, and earlier- versus later-onset substance-use disorder. J Abnorm Psychol. 2007;116:565–577. doi: 10.1037/0021-843X.116.3.565. [DOI] [PubMed] [Google Scholar]
- 62.Luijten M, van Meel CS, Franken IH. Diminished error processing in smokers during smoking cue exposure. Pharmacol Biochem Behav. 2011;97:514–520. doi: 10.1016/j.pbb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 63.Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Patrick G, Straumanis JJ, Struve FA, Nixon F, Jo Fitz-Gerald M, et al. Auditory and visual P300 event related potentials are not altered in medically and psychiatrically normal chronic marihuana users. Life Sci. 1995;56:2135–2140. doi: 10.1016/0024-3205(95)00199-g. [DOI] [PubMed] [Google Scholar]
- 65.Kempel P, Lampe K, Parnefjord R, Hennig J, Kunert HJ. Auditoryevoked potentials and selective attention: different ways of information processing in cannabis users and controls. Neuropsychobiology. 2003;48:95–101. doi: 10.1159/000072884. [DOI] [PubMed] [Google Scholar]
- 66.Gamma A, Brandeis D, Brandeis R, Vollenweider FX. The P3 in ‘ecstasy’ polydrug users during response inhibition and execution. J Psychopharmacol. 2005;19:504–512. doi: 10.1177/0269881105056535. [DOI] [PubMed] [Google Scholar]
- 67.Halgren E, Squires NK, Wilson CL, Rohrbaugh JW, Babb TL, et al. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- 68.Halgren E, Baudena P, Clarke JM, Heit G, Liegeois C, Chauvel P, Musolino A. Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol. 1995;94:191–220. doi: 10.1016/0013-4694(94)00259-n. [DOI] [PubMed] [Google Scholar]
- 69.Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- 70.Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A. Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport. 1997;8:3029–3037. doi: 10.1097/00001756-199709290-00007. [DOI] [PubMed] [Google Scholar]
- 71.Kiehl KA, Liddle PF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophr Res. 2001;48:159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- 72.Basar E, Basar-Eroglu C, Rosen B, Schutt A. A new approach to endogenous event-related potentials in man: relation between EEG and P300-wave. Int J Neurosci. 1984;24:1–21. doi: 10.3109/00207458409079530. [DOI] [PubMed] [Google Scholar]
- 73.Basar-Eroglu C, Basar E, Demiralp T, Schurmann M. P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- 74.Devrim M, Demiralp T, Ademoglu A, Kurt A. A model for P300 generation based on responses to near-threshold visual stimuli. Brain Research. Brain Res Cogn Brain Res. 1999;8:37–43. doi: 10.1016/s0926-6410(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 75.Basar-Eroglu C, Demiralp T, Schurmann M, Basar E. Topological distribution of oddball ‘P300’ responses. Int J Psychophysiol. 2001;39:213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 76.Demiralp T, Ademoglu A, Istefanopulos Y, Basar-Eroglu C, Basar E. Wavelet analysis of oddball P300. Int J Psychophysiol. 2001;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- 77.Rodionov V, Sohmer H. The contribution of the time locking of EEG waves to the generation of the auditory P300. J Basic Clin Physiol Pharmacol. 2004;15:71–105. doi: 10.1515/jbcpp.2004.15.1-2.71. [DOI] [PubMed] [Google Scholar]
- 78.Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, et al. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117:2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 79.Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- 80.Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- 81.Basar E, Schurmann M, Demiralp T, Basar-Eroglu C, Ademoglu A. Event-related oscillations are ‘real brain responses’--wavelet analysis and new strategies. Int J Psychophysiol. 2001;39:91–127. doi: 10.1016/s0167-8760(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 82.Demiralp T, Ademoglu A, Istefanopulos Y, Gulcur HO. Analysis of event-related potentials (ERP) by damped sinusoids. Biol Cybern. 1998;78:487–493. doi: 10.1007/s004220050452. [DOI] [PubMed] [Google Scholar]
- 83.Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- 84.Basar E. The theory of the whole-brain-work. Int J Psychophysiol. 2006;60:133–138. doi: 10.1016/j.ijpsycho.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Klimesch W, Doppelmayr M, Schwaiger J, Winkler T, Gruber W. Theta oscillations and the ERP old/new effect: independent phenomena? Clin Neurophysiol. 2000;111:781–793. doi: 10.1016/s1388-2457(00)00254-6. [DOI] [PubMed] [Google Scholar]
- 86.Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, et al. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Res Cogn Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb Cortex. 2005;15:371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- 88.Hanslmayr S, Klimesch W, Sauseng P, Gruber W, Doppelmayr M, et al. Alpha phase reset contributes to the generation of ERPs. Cereb Cortex. 2007;17:1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- 89.Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, et al. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 90.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 91.Basar E. Brain Function and Oscillations. Vol. I: Principles and Approaches. Springer Verlag; Berlin: 1999. [Google Scholar]
- 92.Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 93.Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:1793–1796. doi: 10.1097/00001756-199705060-00045. [DOI] [PubMed] [Google Scholar]
- 94.Doppelmayr M, Klimesch W, Schwaiger J, Auinger P, Winkler T. Theta synchronization in the human EEG and episodic retrieval. Neurosci Lett. 1998;257:41–44. doi: 10.1016/s0304-3940(98)00805-2. [DOI] [PubMed] [Google Scholar]
- 95.Basar-Eroglu C, Demiralp T. Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int J Psychophysiol. 2001;39:167–195. doi: 10.1016/s0167-8760(00)00140-9. [DOI] [PubMed] [Google Scholar]
- 96.Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 97.Schurmann M, Basar-Eroglu C, Kolev V, Basar E. Delta responses and cognitive processing: single-trial evaluations of human visual P300. Int Jo Psychophysiol. 2001;39:229–239. doi: 10.1016/s0167-8760(00)00144-6. [DOI] [PubMed] [Google Scholar]
- 98.Klimesch W, Doppelmayr M, Yonelinas A, Kroll NE, Lazzara M, et al. Theta synchronization during episodic retrieval: neural correlates of conscious awareness. Brain Research. Cognitive Brain Research. 2001;12:33–38. doi: 10.1016/s0926-6410(01)00024-6. [DOI] [PubMed] [Google Scholar]
- 99.Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, et al. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- 101.Klimesch W, Doppelmayr M, Pachinger T, Russegger H. Event-related desynchronization in the alpha band and the processing of semantic information. Brain Res Cogn Brain Res. 1997;6:83–94. doi: 10.1016/s0926-6410(97)00018-9. [DOI] [PubMed] [Google Scholar]
- 102.Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 103.Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 104.Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- 105.Basar-Eroglu C, Struber D, Kruse P, Basar E, Stadler M. Frontal gamma-band enhancement during multistable visual perception. Int J Psychophysiol. 1996;24:113–125. doi: 10.1016/s0167-8760(96)00055-4. [DOI] [PubMed] [Google Scholar]
- 106.Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol. 1996;24:101–12. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- 107.Karakas S, Basar-Eroglu C, Ozesmi C, Kafadar H, Erzengin OU. Gamma response of the brain: a multifunctional oscillation that represents bottom-up with top-down processing. Int J Psychophysiol. 2001;39:137–150. doi: 10.1016/s0167-8760(00)00137-9. [DOI] [PubMed] [Google Scholar]
- 108.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 109.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- 111.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 112.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 113.Roehm D, Schlesewsky M, Bornkessel I, Frisch S, Haider H. Fractionating language comprehension via frequency characteristics of the human EEG. Neuroreport. 2004;15:409–412. doi: 10.1097/00001756-200403010-00005. [DOI] [PubMed] [Google Scholar]
- 114.von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A. 2000;97:14748–1453. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cereb Cortex. 1999;9:137–150. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- 116.Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, et al. Analysis and visualization of single-trial event-related potentials. Hum Brain Mapp. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front Psychol. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, et al. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mineva A, Popivanov D. Method for single-trial readiness potential identification, based on singular spectrum analysis. J Neurosci Methods. 1996;68:91–99. doi: 10.1016/0165-0270(96)00012-x. [DOI] [PubMed] [Google Scholar]
- 120.Chorlian DB, Porjesz B, Begleiter H. Amplitude modulation of gamma band oscillations at alpha frequency produced by photic driving. Int J Psychophysiol. 2006;61:262–278. doi: 10.1016/j.ijpsycho.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Rangaswamy M, Porjesz B. From event-related potential to oscillations: genetic diathesis in brain (dys)function and alcohol dependence. Alcohol Research & Health. 2008;31:238–242. [PMC free article] [PubMed] [Google Scholar]
- 122.van Leeuwen TH, Steinhausen HC, Overtoom CC, Pascual-Marqui RD, van’t Klooster B, et al. The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav Brain Res. 1998;94:97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- 123.Ashley-Koch A, Bonner ER, Gaskell PC, West SG, Tim R, et al. Fine mapping and genetic heterogeneity in the pure form of autosomal dominant familial spastic paraplegia. Neurogenetics. 2001;3:91–97. doi: 10.1007/s100480000098. [DOI] [PubMed] [Google Scholar]
- 124.Baron M, Knowles JA. Chromosome 18p11 and linkage to bipolar disorder revisited. Psychiatr Genet. 1999;9:201–203. 205–206. doi: 10.1097/00041444-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 125.Baron M, Knowles JA. Bipolar disorder and chromosome 18p11: uncertainties redux. Psychiatr Genet. 2000;10:55–58. doi: 10.1097/00041444-200010010-00010. [DOI] [PubMed] [Google Scholar]
- 126.Bellisari A, Newman TK, Greenberg C, Rogers J, Towne B. Individual variation in the growth of captive infant gorillas. Am J Phys Anthropol. 2001;115:110–132. doi: 10.1002/ajpa.1062. [DOI] [PubMed] [Google Scholar]
- 127.Rebai M, Bernard C, Lannou J. The Stroop’s test evokes a negative brain potential, the N400. Int J Neurosci. 1997;91:85–94. doi: 10.3109/00207459708986367. [DOI] [PubMed] [Google Scholar]
- 128.Miller R. Cortico-hippocampal interplay and the representation of contexts in the brain. Springer-Verlag; Berlin: 1991. [Google Scholar]
- 129.Klimesch W, Doppelmayr M, Schimke H, Ripper B. Theta synchronization and alpha desynchronization in a memory task. Psychophysiology. 1997;34:169–176. doi: 10.1111/j.1469-8986.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 130.Demiralp T, Basar E. Theta rhythmicities following expected visual and auditory targets. Int J Psychophysiol. 1992;13:147–160. doi: 10.1016/0167-8760(92)90054-f. [DOI] [PubMed] [Google Scholar]
- 131.Karakas S. A descriptive framework for information processing: an integrative approach. Int J Psychophysiol. 1997;26:353–368. doi: 10.1016/s0167-8760(97)00775-7. [DOI] [PubMed] [Google Scholar]
- 132.Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- 133.Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- 134.Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 135.Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 136.Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Max Planck Institute of Cognitive Neuroscience; Leipzig: 2004. [Google Scholar]
- 137.Cohen MX, Elger CE, Ranganath C. Reward expectation modulates feedback-related negativity and EEG spectra. Neuroimage. 2007;35:968–978. doi: 10.1016/j.neuroimage.2006.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marco-Pallares J, Cucurell D, Cunillera T, Garcia R, Andres-Pueyo A, et al. Human oscillatory activity associated to reward processing in a gambling task. Neuropsychologia. 2008;46:241–248. doi: 10.1016/j.neuropsychologia.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 139.Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, et al. Theta oscillations during the processing of monetary loss and gain: a perspective on gender and impulsivity. Brain Res. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duncan CC, Barry RJ, Connolly JF, Fischer C, Michie PT, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120:1883–1908. doi: 10.1016/j.clinph.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 141.Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, et al. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63:3–15. doi: 10.1016/j.ijpsycho.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34:669–680. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andrew C, Fein G. Induced theta oscillations as biomarkers for alcoholism. Clin Neurophysiol. 2010;121:350–358. doi: 10.1016/j.clinph.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behav Genet. 2010;40:186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr Clin Neurophysiol. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- 147.Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, et al. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biological Psychiatry. 2006;59:625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 149.Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kamarajan C, Rangaswamy M, Manz N, Chorlian DB, Pandey AK, et al. Topography, power, and current source density of theta oscillations during reward processing as markers for alcohol dependence. Hum Brain Mapp. 2012 doi: 10.1002/hbm.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bernat EM, Nelson LD, Steele VR, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain-loss feedback in a simulated gambling task: dissociable components of brain response revealed by time-frequency analysis. J Abnorm Psychol. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- 154.Fell J, Fernandez G, Klaver P, Elger CE, Fries P. Is synchronized neuronal gamma activity relevant for selective attention? Brain Research. Brain Res Brain Res Rev. 2003;42:265–272. doi: 10.1016/s0165-0173(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 155.Yordanova J, Banaschewski T, Kolev V, Woerner W, Rothenberger A. Abnormal early stages of task stimulus processing in children with attention-deficit hyperactivity disorder--evidence from event-related gamma oscillations. Clin Neurophysiol. 2001;112:1096–108. doi: 10.1016/s1388-2457(01)00524-7. [DOI] [PubMed] [Google Scholar]
- 156.Gruber T, Muller MM. Oscillatory brain activity in the human EEG during indirect and direct memory tasks. Brain Res. 2006;1097:194–204. doi: 10.1016/j.brainres.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 157.Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci. 1999;16:449–459. doi: 10.1017/s0952523899163065. [DOI] [PubMed] [Google Scholar]
- 158.Padmanabhapillai A, Porjesz B, Ranganathan M, Jones KA, Chorlian DB, et al. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. 2006;60:15–26. doi: 10.1016/j.ijpsycho.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 159.Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, et al. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int J Psychophysiol. 2006;62:262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 160.Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- 161.Little HJ. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Ther. 1999;84:333–353. doi: 10.1016/s0163-7258(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 162.Yeung N, Cohen JD. The impact of cognitive deficits on conflict monitoring. Predictable dissociations between the error-related negativity and N2. Psychol Sci. 2006;17:164–171. doi: 10.1111/j.1467-9280.2006.01680.x. [DOI] [PubMed] [Google Scholar]
- 163.Krause CM, Aromaki A, Sillanmaki L, Astrom T, Alanko K, et al. Alcohol-induced alterations in ERD/ERS during an auditory memory task. Alcohol. 2002;26:145–153. doi: 10.1016/s0741-8329(01)00204-x. [DOI] [PubMed] [Google Scholar]
- 164.Jaaskelainen IP, Hirvonen J, Saher M, Pekkonen E, Sillanaukee P, et al. Dose-dependent suppression by ethanol of transient auditory 40-Hz response. Psychopharmacology (Berl) 2000;148:132–135. doi: 10.1007/s002130050034. [DOI] [PubMed] [Google Scholar]
- 165.Boha R, Molnar M, Gaal ZA, Czigler B, Rona K, et al. The acute effect of low-dose alcohol on working memory during mental arithmetic: I. Behavioral measures and EEG theta band spectral characteristics. Int J Psychophysiol. 2009;73:133–137. doi: 10.1016/j.ijpsycho.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 166.Molnar M, Boha R, Czigler B, Gaal ZA, Benyovszky M, et al. The acute effect of low-dose alcohol on working memory during mental arithmetic: II. Changes of nonlinear and linear EEG-complexity in the theta band, heart rate and electrodermal activity. Int J Psychophysiol. 2009;73:138–142. doi: 10.1016/j.ijpsycho.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 167.de Bruin EA, Bijl S, Stam CJ, Bocker KB, Kenemans JL, et al. Abnormal EEG synchronisation in heavily drinking students. Clin Neurophysiol. 2004;115:2048–2055. doi: 10.1016/j.clinph.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 168.Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 169.Ehlers CL, Somes C. Long latency event-related potentials in mice: effects of stimulus characteristics and strain. Brain Res. 2002;957:117–128. doi: 10.1016/s0006-8993(02)03612-0. [DOI] [PubMed] [Google Scholar]
- 170.Criado JR, Ehlers CL. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience. 2009;163:506–523. doi: 10.1016/j.neuroscience.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP) Alcohol. 2010;44:335–342. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ehlers CL, Criado JR. Event-related oscillations in mice: effects of stimulus characteristics. J Neurosci Methods. 2009;181:52–57. doi: 10.1016/j.jneumeth.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ehlers CL, Somes C, Li TK, Lumeng L, Kinkead B, et al. Neurontensin studies in alcohol naive, preferring and non-preferring rats. Neuroscience. 1999;93:227–236. doi: 10.1016/s0306-4522(99)00113-x. [DOI] [PubMed] [Google Scholar]
- 174.Tsuang MT, Faraone SV. The frustrating search for schizophrenia genes. Am J Med Genet. 2000;97:1–3. doi: 10.1002/(sici)1096-8628(200021)97:1<1::aid-ajmg1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 175.Gottesman II, Shields J, Meehl PE. Schizophrenia and genetics; a twin study vantage point. Academic Press; New York: 1972. [Google Scholar]
- 176.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 177.Almasy L. Quantitative risk factors as indices of alcoholism susceptibility. Ann Med. 2003;35:337–343. doi: 10.1080/07853890310004903. [DOI] [PubMed] [Google Scholar]
- 178.de Geus EJ. From genotype to EEG endophenotype: a route for post-genomic understanding of complex psychiatric disease? Genome Med. 2010;2:63. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, et al. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- 180.Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, et al. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 181.Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys) function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Res. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- 183.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 184.Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- 185.Callaway E. Presidential address, 1982. The pharmacology of human information processing. Psychophysiology. 1983;20:359–370. doi: 10.1111/j.1469-8986.1983.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 186.Dierks T, Frolich L, Ihl R, Maurer K. Event-related potentials and psychopharmacology. Cholinergic modulation of P300. Pharmacopsychiatry. 1994;27:72–74. doi: 10.1055/s-2007-1014282. [DOI] [PubMed] [Google Scholar]
- 187.Hammond EJ, Meador KJ, Aung-Din R, Wilder BJ. Cholinergic modulation of human P3 event-related potentials. Neurology. 1987;37:346–350. doi: 10.1212/wnl.37.2.346. [DOI] [PubMed] [Google Scholar]
- 188.Potter DD, Pickles CD, Roberts RC, Rugg MD. Scopolamine impairs memory performance and reduces frontal but not parietal visual P3 amplitude. Biol Psychol. 2000;52:37–52. doi: 10.1016/s0301-0511(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 189.Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5-2 Hz), theta (5-12 Hz), and gamma (35-70 Hz) bands. Hippocampus. 2000;10:187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 190.Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus. 2001;11:251–274. doi: 10.1002/hipo.1041. [DOI] [PubMed] [Google Scholar]
- 191.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 192.Dick DM, Agrawal A, Wang JC, Hinrichs A, Bertelsen S, et al. Alcohol dependence with comorbid drug dependence: genetic and phenotypic associations suggest a more severe form of the disorder with stronger genetic contribution to risk. Addiction. 2007;102:1131–1139. doi: 10.1111/j.1360-0443.2007.01871.x. [DOI] [PubMed] [Google Scholar]
- 193.Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, et al. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- 194.Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, et al. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case-control structured association study. Hum Molecul Genet. 2005;14:2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- 195.Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, et al. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. Am J Med Genet. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 196.Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 197.Dick DM, Jones K, Saccone N, Hinrichs A, Wang JC, et al. Endophenotypes successfully lead to gene identification: results from collaborative study on the genetics of alcoholism. Behav Genet. 2006;36:112–126. doi: 10.1007/s10519-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 198.Chen AC, Tang Y, Rangaswamy M, Wang JC, Almasy L, et al. Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:359–368. doi: 10.1002/ajmg.b.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gallinat J, Kunz D, Senkowski D, Kienast T, Seifert F, et al. Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology (Berl) 2006;187:103–111. doi: 10.1007/s00213-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 200.Gallinat J, Kunz D, Lang UE, Neu P, Kassim N, et al. Association between cerebral glutamate and human behaviour: the sensation seeking personality trait. Neuroimage. 2007;34:671–678. doi: 10.1016/j.neuroimage.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 201.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- 203.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 204.Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- 205.Holter SM, Linthorst AC, Reul JM, Spanagel R. Withdrawal symptoms in a long-term model of voluntary alcohol drinking in Wistar rats. Pharmacol Biochem Behav. 2000;66:143–151. doi: 10.1016/s0091-3057(00)00196-9. [DOI] [PubMed] [Google Scholar]
- 206.Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 207.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 208.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 209.Spanagel R, Heilig M. Addiction and its brain science. Addiction. 2005;100:1813–1822. doi: 10.1111/j.1360-0443.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 210.Freye E, Hartung E, Kaliebe S. Prevention of late fentanyl-induced respiratory depression after the injection of opiate antagonists naltrexone and S-20682: comparison with naloxone. Br J Anaesth. 1983;55:71–77. doi: 10.1093/bja/55.1.71. [DOI] [PubMed] [Google Scholar]
- 211.Freye E, Hartung E, Schenk GK. Effects of three narcotic antagonists (naltrexone, diprenorphine, and S-20682) on blood pressure, heart rate and electrical cortical activity. Pharmacology. 1983;26:110–116. doi: 10.1159/000137792. [DOI] [PubMed] [Google Scholar]
- 212.Pietrzak B, Czarnecka E. Effect of the combined administration of ethanol and acamprosate on rabbit EEG. Pharmacol Rep. 2005;57:61–69. [PubMed] [Google Scholar]
- 213.Volavka J, James B, Reker D, Mallya A, Cho D, et al. EEG and other effects of naltrexone and heroin in man. Pharmakopsychiatr Neuropsychopharmakol. 1979;12:79–85. doi: 10.1055/s-0028-1094597. [DOI] [PubMed] [Google Scholar]
- 214.Knott VJ, Fisher DJ. Naltrexone alteration of the nicotine-induced EEG and mood activation response in tobacco-deprived cigarette smokers. Exp Clin Psychopharmacol. 2007;15:368–381. doi: 10.1037/1064-1297.15.4.368. [DOI] [PubMed] [Google Scholar]
- 215.Campanella S, Petit G, Verbanck P, Kornreich C, Noel X. How cognitive assessment through clinical neurophysiology may help optimize chronic alcoholism treatment. Neurophysiol Clin. 2011;41:115–123. doi: 10.1016/j.neucli.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 216.Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, et al. Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:44–58. doi: 10.1002/ajmg.b.31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Oscillatory brain theory: a new trend in neuroscience. IEEE Eng Med Biol Mag. 1999;18:56–66. doi: 10.1109/51.765190. [DOI] [PubMed] [Google Scholar]