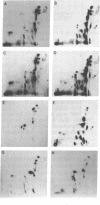
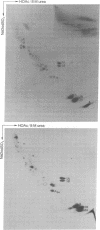
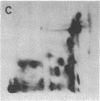
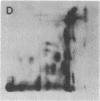
Abstract
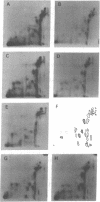
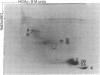
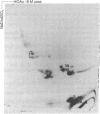
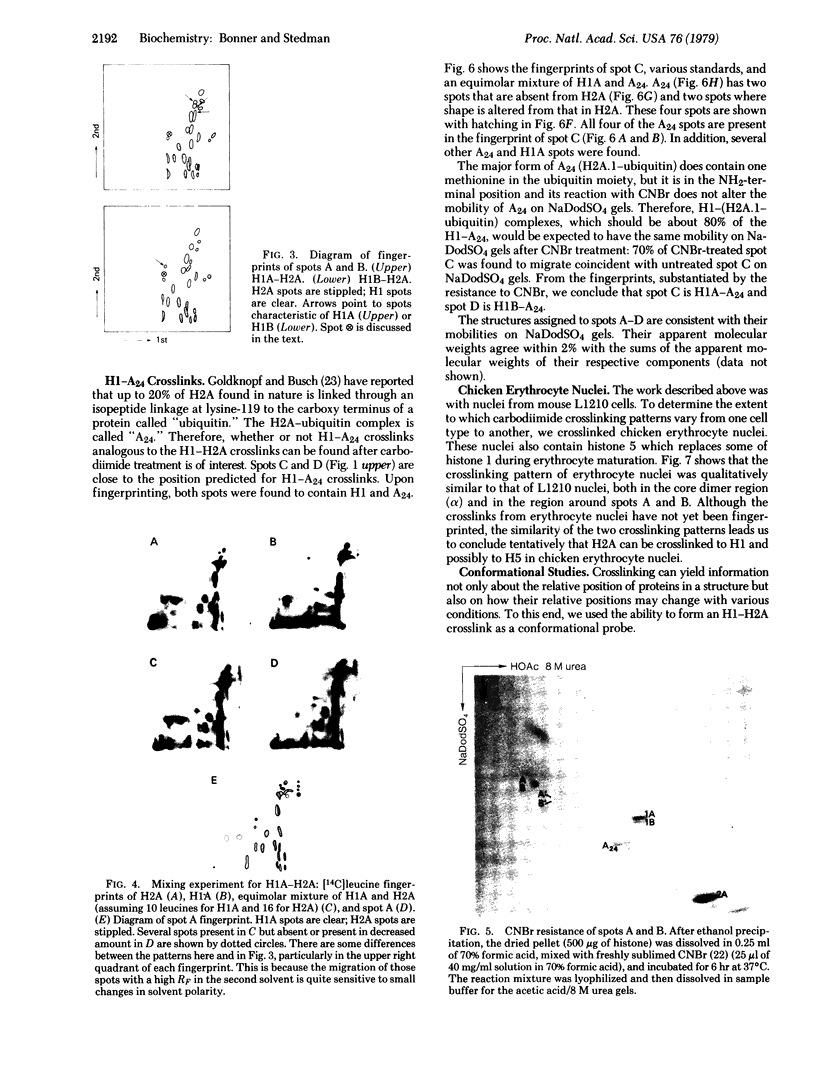
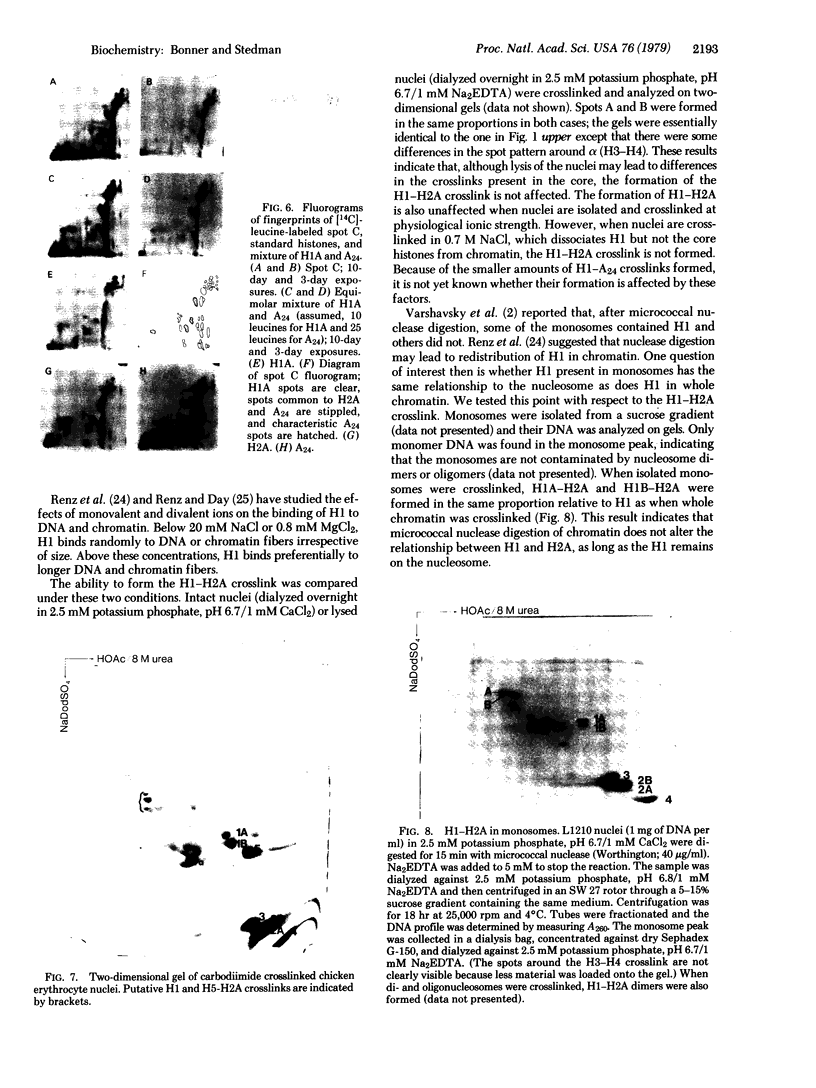
Water-soluble carbodiimide crosslinks histones 1A and 1B to histone 2A and to semi-histone A24 in chromatin from mouse cells. The identities of the histone species present in the crosslinked dimers were determined by fingerprinting. The molar ratio of H1--A24 to H2A is the same as the molar ratio of A24 to H2A in these cells. The H1-H2A crosslinks form equally well in whole nuclei, lysed nuclei, and H1-containing mononucleosomes isolated from a sucrose gradient. These results suggest that there exist major H1 interactions within the nucleosome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Berkowitz D. M., Kakefuda T., Sporn M. A simple and rapid method for the isolation of enzymatically active HeLa cell nuclei. J Cell Biol. 1969 Sep;42(3):851–854. doi: 10.1083/jcb.42.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Pollard H. B. The presence of F3-F2a1 dimers and F1 oligomers in chromatin. Biochem Biophys Res Commun. 1975 May 5;64(1):282–288. doi: 10.1016/0006-291x(75)90250-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Proximity and accessibility studies of histones in nuclei and free nucleosomes. Nucleic Acids Res. 1978 Jan;5(1):71–85. doi: 10.1093/nar/5.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A histone cross-complexing pattern. Biochemistry. 1974 Nov 19;13(24):4992–4997. doi: 10.1021/bi00721a019. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Franklin S. G., Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977 Mar 17;266(5599):273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R. C., Eichner M. E., Chalkley R. An approach to histone nearest neighbours in extended chromatin. Nucleic Acids Res. 1975 Oct;2(10):1751–1770. doi: 10.1093/nar/2.10.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R. C., Zeitler D. P., Murphy J. M., Chalkley R. Histone neighbors in nuclei and extended chromatin. Cell. 1977 Oct;12(2):417–427. doi: 10.1016/0092-8674(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Johns E. W. The electrophoresis of histones in polyacrylamide gel and their quantitative determination. Biochem J. 1967 Jul;104(1):78–82. doi: 10.1042/bj1040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Wright E. B. Glutaraldehyde fixation of isolated eucaryotic nuclei. Evidence for histone-histone proximity. J Cell Biol. 1973 Nov;59(2 Pt 1):304–317. doi: 10.1083/jcb.59.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Chalkley R. An electrophoretic analysis of Drosophila histones. I. Isolation and identification. Exp Cell Res. 1972 Aug;73(2):295–302. doi: 10.1016/0014-4827(72)90051-1. [DOI] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Renz M., Day L. A. Transition from noncooperative to cooperative and selective binding of histone H1 to DNA. Biochemistry. 1976 Jul 27;15(15):3220–3228. doi: 10.1021/bi00660a010. [DOI] [PubMed] [Google Scholar]
- Renz M., Nehls P., Hozier J. Involvement of histone H1 in the organization of the chromosome fiber. Proc Natl Acad Sci U S A. 1977 May;74(5):1879–1883. doi: 10.1073/pnas.74.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Simpson R. T. Removal of histone H1 exposes a fifty base pair DNA segment between nucleosomes. Biochemistry. 1976 Jul 27;15(15):3307–3314. doi: 10.1021/bi00660a022. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Vincendon G., Mandel P., Gombos G. The utilisation of I-dimethylaminonaphthalene-5-sulphonyl chloride for quantitative determination of free amino acids and partial analysis of primary structure of proteins. J Chromatogr. 1970 Sep 23;51(3):441–458. doi: 10.1016/s0021-9673(01)96893-1. [DOI] [PubMed] [Google Scholar]