Abstract
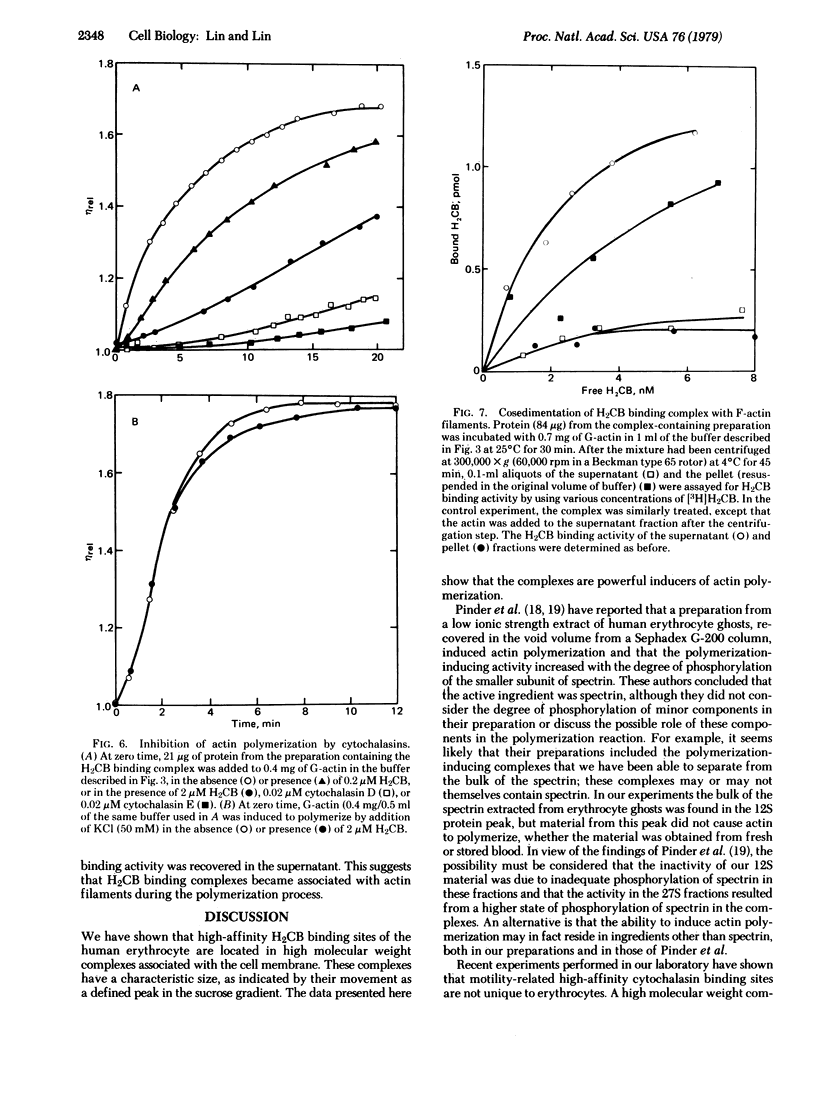
A high molecular weight complex (sedimentation coefficient ≈27 S) containing high-affinity binding site(s) for [3H]dihydrocytochalasin B has been isolated from a low ionic strength extract of human erythrocyte membranes by sucrose density gradient centrifugation. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis showed that actin, spectrin, and other minor components, including two polypeptides with the electrophoretic mobility of band 4.1, were present in the complex-containing fraction. Addition of this complex to a solution of muscle monomeric actin (G-actin) in a low ionic strength medium resulted in a rapid increase in viscosity to a level comparable to that of a solution of filamentous actin (F-actin). Electron microscopy showed that the viscosity increase reflected actin filament formation. The rate of induced actin polymerization was dependent on the amount of complex added to the G-actin; in less than 1 hr, less than 1 μg of protein from the complex-containing fraction induced the conversion of 0.4 mg of G-actin to the “F” from. Binding studies indicated that, upon polymerization of the actin, the cytochalasin binding complex became associated with the actin filaments. Low concentrations of cytochalasins D and E and dihydrocytochalasin B inhibited actin polymerization induced by the complex; the relative potencies of the drugs in inhibiting this process corresponded to their relative affinities for the complex, as well as their relative potencies in affecting cell motility. These results suggest that the cytochalasin binding complex functions as a regulatory site for cell motility by controlling formation and membrane attachment of actin-containing microfilaments in the cell.
Keywords: cytochalasin B, [3H]dihydrocytochalasin B, microfilament formation and membrane attachment, control of cell motility, viscosity changes
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas S. J., Lin S. Dihydrocytochalasin B. Biological effects and binding to 3T3 cells. J Cell Biol. 1978 Feb;76(2):360–370. doi: 10.1083/jcb.76.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Lin S. High affinity binding of [3H]dihydrocytochalasin B to peripheral membrane proteins related to the control of cell shape in the human red cell. J Biol Chem. 1978 Mar 10;253(5):1415–1419. [PubMed] [Google Scholar]
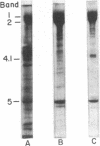
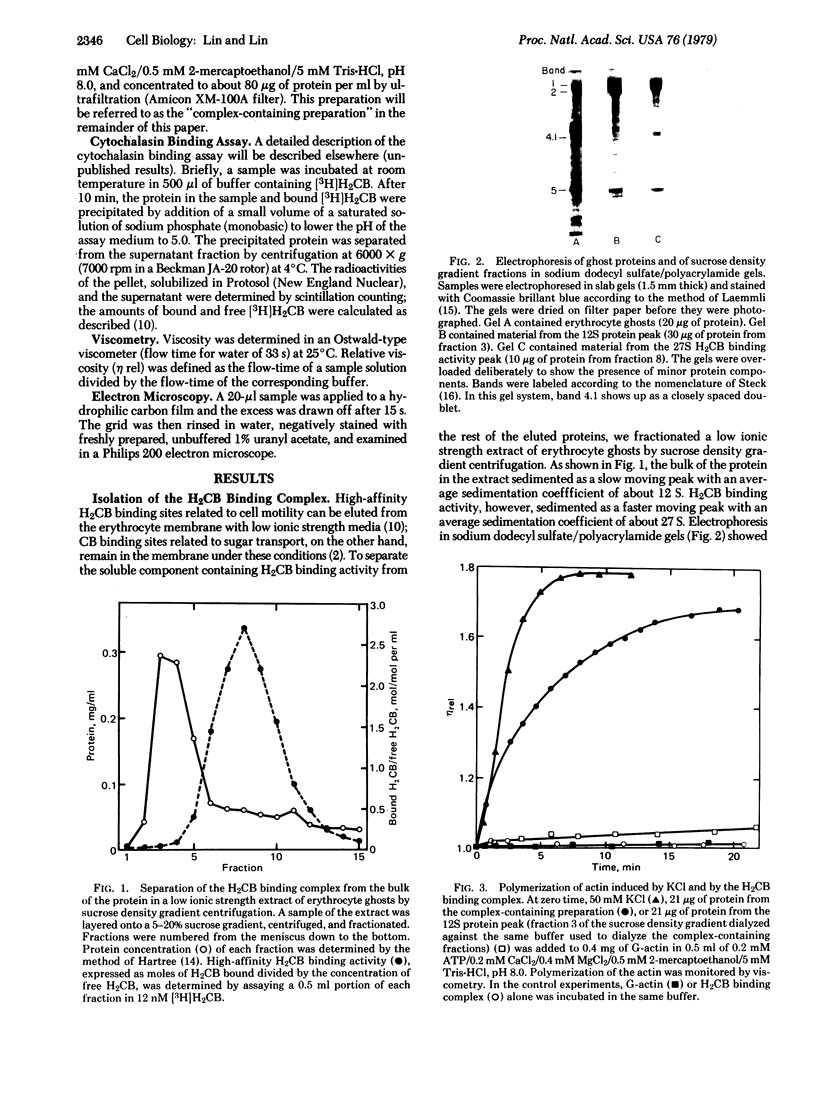
- Lin S., Lin D. C., Flanagan M. D. Specificity of the effects of cytochalasin B on transport and motile processes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):329–333. doi: 10.1073/pnas.75.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
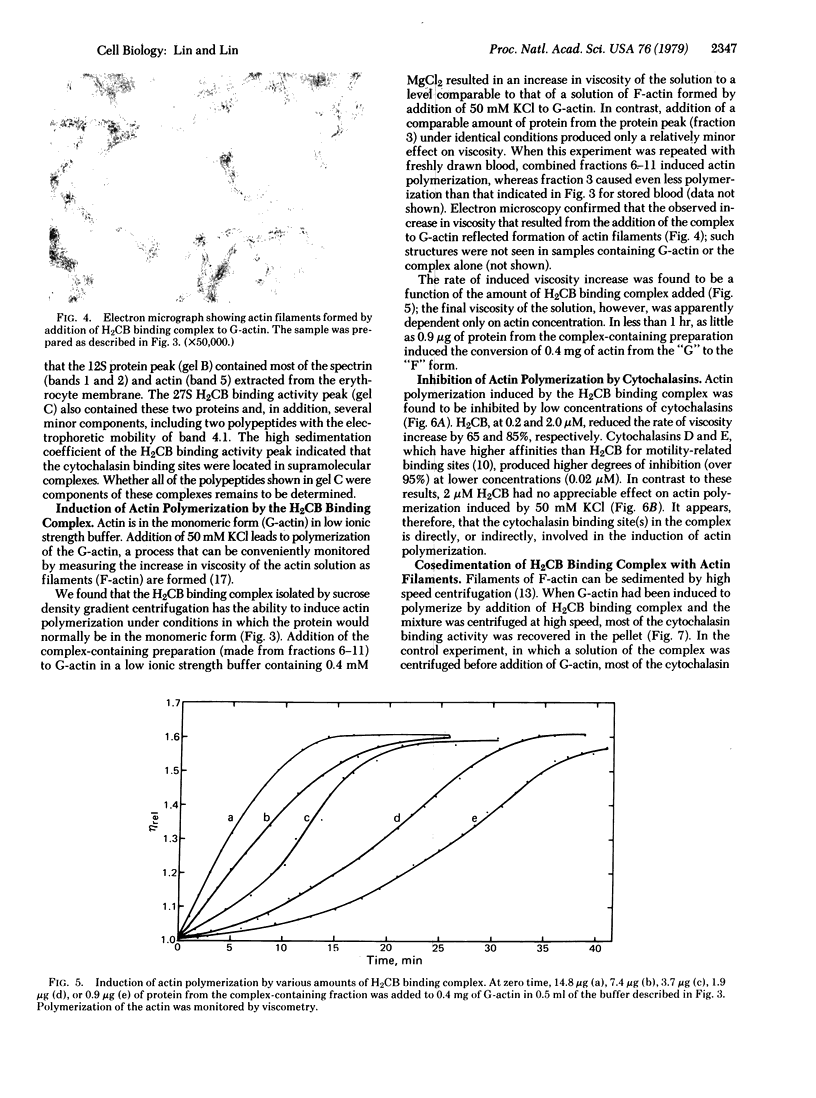
- Lin S., Santi D. V., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Preparation of (3H)cytochalasin B and studies on its binding of cells. J Biol Chem. 1974 Apr 10;249(7):2268–2274. [PubMed] [Google Scholar]
- Lin S., Snyder C. E., Jr High affinity cytochalasin B binding to red cell membrane proteins which are unrelated to sugar transport. J Biol Chem. 1977 Aug 10;252(15):5464–5471. [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Binding of cytochalasin B to a red cell membrane protein. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1471–1476. doi: 10.1016/s0006-291x(74)80449-3. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Cytochalasin B binding to red cell membrane in relation to glucose transport. J Biol Chem. 1974 Sep 25;249(18):5778–5783. [PubMed] [Google Scholar]
- Lin S., Spudich J. A. On the molecular basis of action of cytochalasin B. J Supramol Struct. 1974;2(5-6):728–736. doi: 10.1002/jss.400020516. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Actin polymerisation induced by spectrin. Nature. 1975 Dec 25;258(5537):765–766. doi: 10.1038/258765a0. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Bray D., Gratzer W. B. Control of interaction of spectrin and actin by phosphorylation. Nature. 1977 Dec 22;270(5639):752–754. doi: 10.1038/270752a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Sogin D. C., Hinkle P. C. Characterization of the glucose transporter from human erythrocytes. J Supramol Struct. 1978;8(4):447–453. doi: 10.1002/jss.400080407. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Lin S. Cytochalasin B, its interaction with actin and actomyosin from muscle (cell movement-microfilaments-rabbit striated muscle). Proc Natl Acad Sci U S A. 1972 Feb;69(2):442–446. doi: 10.1073/pnas.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Zoccoli M. A., Baldwin S. A., Lienhard G. E. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978 Oct 10;253(19):6923–6930. [PubMed] [Google Scholar]