Abstract
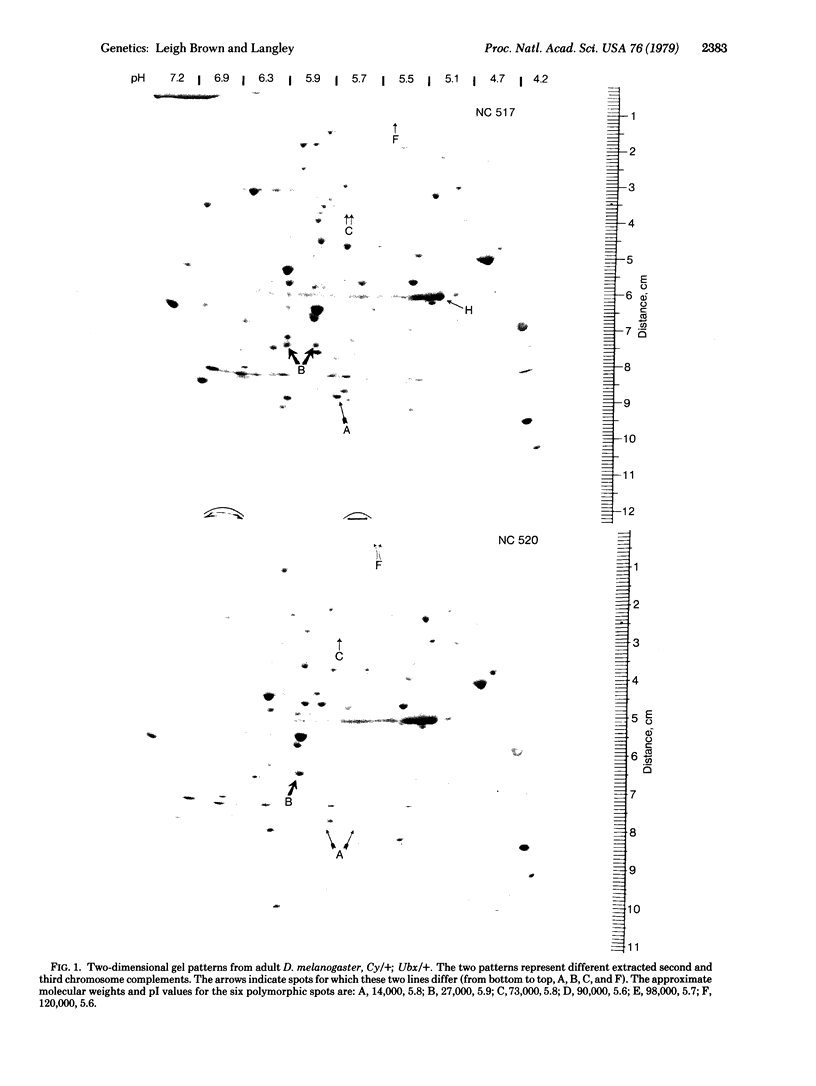
We have found the two-dimensional electrophoretic technique of O'Farrell to be highly efficient in the detection of charge-change substitutions in a large number of proteins. We have applied this method to determine the level of heterozygosity of the most abundant proteins in Drosophila melanogaster adults from a natural population. The estimate of per-locus heterozygosity obtained from approximately 54 loci screened was 4% with 6 loci polymorphic. This is much lower than overall estimates obtained by standard gel electrophoresis but is not different from estimates for "Group I" enzymes--i.e., those utilizing a narrow spectrum of substrates of intracellular origin. We consider these data to throw open the question of the level of genetic variability in nature.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala F. J., Tracey M. L., Barr L. G., McDonald J. F., Pérez-Salas S. Genetic variation in natural populations of five Drosophila species and the hypothesis of the selective neutrality of protein polymorphisms. Genetics. 1974 Jun;77(2):343–384. doi: 10.1093/genetics/77.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN L., JOHNSON F. M. VARIATIONS IN LARVAL ALKALINE PHOSPHATASE CONTROLLED BY APH ALLELES IN DROSOPHILA MELANOGASTER. Genetics. 1964 May;49:829–835. doi: 10.1093/genetics/49.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Felton A. A. Genic Heterogeneity at Two Alcohol Dehydrogenase Loci in DROSOPHILA PSEUDOOBSCURA and DROSOPHILA PERSIMILIS. Genetics. 1977 Oct;87(2):285–304. doi: 10.1093/genetics/87.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. H., Langley C. H. A general model to account for enzyme variation in natural populations. Genetics. 1974 Apr;76(4):837–848. doi: 10.1093/genetics/76.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. Enzyme polymorphisms in man. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):298–310. doi: 10.1098/rspb.1966.0032. [DOI] [PubMed] [Google Scholar]
- Harris H., Hopkinson D. A., Edwards Y. H. Polymorphism and the subunit structure of enzymes: a contribution to the neutralist-selectionist controversy. Proc Natl Acad Sci U S A. 1977 Feb;74(2):698–701. doi: 10.1073/pnas.74.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubby J. L., Lewontin R. C. A molecular approach to the study of genic heterozygosity in natural populations. I. The number of alleles at different loci in Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):577–594. doi: 10.1093/genetics/54.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON F. M., DENNISTON C. GENETIC VARIATION OF ALCOHOL DEHYDROGENASE IN DROSOPHILIA MELANOGASTER. Nature. 1964 Nov 28;204:906–907. doi: 10.1038/204906a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Alcohol dehydrogenase of Drosophila: interconversion of isoenzymes. Science. 1968 Jan 19;159(3812):324–325. doi: 10.1126/science.159.3812.324. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C., Hubby J. L. A molecular approach to the study of genic heterozygosity in natural populations. II. Amount of variation and degree of heterozygosity in natural populations of Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):595–609. doi: 10.1093/genetics/54.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978 Jul;14(3):545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Parker J., Pollard J. W., Friesen J. D., Stanners C. P. Stuttering: high-level mistranslation in animal and bacterial cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1091–1095. doi: 10.1073/pnas.75.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J. A., Eanes W. F. Study of the charge-state model for electrophoretic variation using isoelectric focusing of esterase-5 from Drosophila pseudoobscura. Nature. 1978 Sep 7;275(5675):68–70. doi: 10.1038/275068a0. [DOI] [PubMed] [Google Scholar]
- Rodgers M. E., Shearn A. Patterns of protein synthesis in imaginal discs of Drosophila melanogaster. Cell. 1977 Dec;12(4):915–921. doi: 10.1016/0092-8674(77)90155-6. [DOI] [PubMed] [Google Scholar]
- Selander R. K., Yang S. Y. Protein polymorphism and genic heterozygosity in a wild population of the house mouse (Mus musculus). Genetics. 1969 Nov;63(3):653–667. doi: 10.1093/genetics/63.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Hall M. E., Stone G. C., Rubin R. W. Some improvements in two-dimensional gel electrophoresis of proteins. Protein mapping of eukaryotic tissue extracts. Anal Biochem. 1977 Nov;83(1):33–44. doi: 10.1016/0003-2697(77)90506-1. [DOI] [PubMed] [Google Scholar]