Abstract
Thymic stromal lymphopoietin (TSLP) has recently emerged as a key cytokine in the development of type 2 immune responses. Although traditionally associated with allergic inflammation, type 2 responses are also recognised to contribute to the pathogenesis of tissue fibrosis. However, the role of TSLP in the development of non-allergen driven diseases, characterised by pro-fibrotic type 2 immune phenotypes and excessive fibroblast activation, remains underexplored. Fibroblasts represent the key effector cells responsible for extracellular matrix production, but additionally play important immunoregulatory roles, including choreographing immune cell recruitment through chemokine regulation. The aim of this study was to examine whether TSLP may be involved in the pathogenesis of a proto-typical fibrotic disease, idiopathic pulmonary fibrosis (IPF). We combined the immunohistochemical analysis of human IPF biopsy material with signalling studies employing cultured primary human lung fibroblasts (pHLFs) and report for the first time that TSLP and its receptor (TSLPR) are highly upregulated in IPF. We further show that lung fibroblasts represent both a novel cellular source and target of TSLP and that TSLP induces fibroblast CCL2 release (via STAT3) and subsequent monocyte chemotaxis. These studies extend our understanding of TSLP as a master regulator of type 2 immune responses beyond that of allergic inflammatory conditions and suggest a novel role for TSLP in the context of chronic fibrotic lung disease.
Introduction
Type 2 cytokine-induced inflammatory responses are critical components of the mucosal immune response required for host defence against helminth infection but are also involved in the pathogenesis of a number of common and highly debilitating diseases, including asthma/allergy (1) and ulcerative colitis (2). Type 2 immune responses have also been implicated in progressive and fatal fibrotic conditions, including idiopathic pulmonary fibrosis (IPF) (3), systemic sclerosis (4) and liver fibrosis resulting from persistent infections and non-alcoholic steatohepatitis (5). The mechanisms driving inappropriate type 2 cytokine responses remain poorly understood but recent studies have highlighted a key role for the IL-7 like cytokine, thymic stromal lymphopoietin (TSLP) in the context of allergic inflammatory conditions such as allergic asthma and atopic dermatitis (6, 7).
TSLP was originally identified as a growth factor capable of supporting the long term growth of pre B-cells in vitro (8,9). TSLP was subsequently found to be highly expressed by lung epithelial cells and epidermal keratinocytes (10, 11) and is now recognised to be a critical mediator involved in linking responses at the interface between mucosal barriers and the environment to type 2 immune responses. TSLP has been implicated in driving type 2 responses in the airways (6) skin (7) and gut (12) and mediates its effects by driving the activation of immature dendritic cells (DCs) into a type 2 polarising phenotype (10, 13, 14), as well as via direct effects on naïve and differentiated T-cells (15, 16).
The biological effects of TSLP are mediated by binding to a functional heterodimeric receptor complex composed of the TSLP receptor (TSLPR) and the IL-7Rα-chain (17, 18), signaling through STAT 3 (19) and STAT5 pathways (8). Recent evidence suggests that in addition to promoting type 2 cytokine responses, TSLP plays broader homeostatic roles, including controlling regulatory T Cell (Treg) differentiation in the thymus (20) and contributing to intestinal homeostasis (12). The key stimuli involved in regulating TSLP expression are also beginning to be identified, with current evidence suggesting a major role for environmental stimuli (including allergen exposure (21), viral and bacterial infections (22, 23), helminth infections (24), diesel exhaust (25), cigarette smoke (26)), pro-inflammatory cytokines such as TNF-α (27) and IL-1β (28), type 2 cytokines (22) and IgE (29). TSLP is also now known to be expressed by non-epithelial cell types including mesenchymal cells (30) and has recently been implicated in promoting tumour cell growth in breast (31) and pancreatic cancer (32), joint destruction in arthritis (33, 34) and fibrocyte function in atopic dermatitis (35). Current evidence suggests that the TSLP receptor complex is also more broadly expressed although cells of haematopoietic lineage, notably DCs, are still considered to be key cellular targets of TSLP (17,36).
The aim of this study was to begin to evaluate the potential importance of TSLP in non-atopic pathobiology characterized by type 2 cytokine responses, focusing on the proto-typical fibrotic lung disease, IPF. IPF is the most fatal of all fibrotic lung conditions and is characterized by a progressive decline in lung function leading to premature death as a result of respiratory failure. The pathomechanisms involved remain poorly understood but current hypotheses propose that this condition arises as a result of chronic or repetitive lung injury (of unknown origin) followed by a highly aberrant wound healing response (reviewed in (37)). The classical histopathological pattern of IPF is characterized by evidence of patchy epithelial damage, type II pneumocyte hyperplasia, together with abnormal proliferation of mesenchymal cells, varying degrees of fibrosis and overproduction and disorganized deposition of collagen and ECM. Fibrotic foci are commonly observed underlying injured and reparative epithelium – these fibrotic foci comprise accumulations of fibroblasts and myofibroblasts within extensive ECM and are felt to represent the leading edge of the fibrotic lesion. Although the contribution of inflammation to disease initiation and progression in IPF remains unclear (38), current evidence supports the notion that type 2 cytokines, in particular IL-13, may contribute to fibrotic responses by amplifying the dysregulated epithelial-mesenchymal crosstalk which is central to this condition (39, 40). The infiltration of activated DCs in the IPF lung provides further strong support for the notion that a maladaptive immune response may be responsible for driving fibrosis in this condition (41). The nature of the mediators responsible for DC migration and activation in this context remain poorly defined. However, DC trafficking to remodelling lung has recently been shown to be dependent on fibroblast-derived CCL2 (42), a chemokine implicated in facilitating type 2 immune responses (43-45), and which has also been implicated in the pathogenesis of several chronic diseases associated with tissue remodelling, including IPF. Furthermore, it is now increasingly recognised that DCs may be activated by non-antigenic endogenous danger signals, reflecting cellular injury or stress (46, 47) - supporting the notion that epithelial injury, sterile or otherwise, can initiate aberrant immune responses.
In this study, we combined the immunohistochemical analysis of human IPF biopsy material with signalling studies employing cultured primary human cells and report for the first time that TSLP and its receptor are highly upregulated in IPF. We further show that lung fibroblasts represent both a novel cellular source and target of TSLP and that TSLP induces fibroblast CCL2 release and subsequent monocyte chemotaxis. These studies extend our understanding of TSLP as a master regulator of type 2 immune responses beyond that of allergic inflammatory conditions and suggest a novel role for TSLP in the context of chronic fibrotic lung disease.
Materials and Methods
Reagents
Recombinant human (rh)TSLP and CCL2 were purchased from R & D Systems (Abingdon, UK); rhTNF-α was from PeproTech (NJM, USA). For Western blotting: Rabbit anti-human phospho-HSP27 (S78), rabbit anti-human phospho-p42/44 (T202/Y204), rabbit anti-human IκBα, rabbit anti-human phospho-c-Jun (S73), rabbit anti-human phospho-STAT3 (Y705), rabbit anti-human phospho-STAT5 (Y694), murine anti-human HSP27 mAB, rabbit anti-human p42/44, rabbit anti-human c-Jun, rabbit anti-human STAT3 and rabbit anti-human STAT5 were purchased from Cell Signalling Technology (Hitchin, UK). Goat anti-human ERK2 was acquired from Santa Cruz (CA, USA). For immunocytofluorescence: Rabbit anti-human TSLPR and goat anti-human IL-7Rα antibodies were purchased from Pro-Sci (CA, USA) and Santa Cruz (CA, USA) respectively. Donkey anti-rabbit AF488 and donkey anti-goat AF555 were from Invitrogen (Paisley, UK). Polyclonal rabbit IgG and polyclonal goat and sheep IgG isotype controls were purchased from Santa Cruz (CA, USA) and Vector Laboratories (UK). For immunohistochemistry: Mouse anti-human αSMA was purchased from Dako (Cambridgeshire, UK). Sheep anti-human TSLP was from R&D Systems (Abingdon, UK; Cat no. AF1398), rabbit anti-human TSLPR was purchased from ProSci (CA, USA; Cat no. 4207). For inhibition studies: Polyclonal anti-human CCL2 antibodies were purchased from R&D Systems (Abingdon, UK). The NFκB inhibitor, SC-514; p38 inhibitor, SB203580; MEK 1/2 inhibitor, U0126; JNK inhibitors (SP600125 and TI-JIP); STAT3 inhibitor (S3I-201) were all from Calbiochem (Nottingham, UK). Plasmids used for transfection were acquired from Addgene (Cambridge, USA); pFUGW containing GFP only (Addgene plasmid 14883) was a kind gift from David Baltimore (48), while pSTAT3C containing a constitutively-active mutant of STAT3 (Addgene plasmid 24983) was a kind gift from Linzhao Cheng (49). JetPRIME transfection reagent was purchased from Polyplus-transfection SA, France. All cell culture medium (DMEM), FBS and antibiotics (penicillin / streptomycin) were purchased from Invitrogen (Paisley, UK), aside from RPMI 1640 (PAA, Somerset, UK). Sterile tissue culture equipment was purchased from Nunc (Roskilde, Denmark). All other chemical reagents were from Sigma-Aldrich.
Cell culture
Primary human lung fibroblasts (pHLFs) were isolated and maintained as previously described (50, 51) and were used at no more than passage 7. Type II AECs were isolated from lung tissue obtained at lung transplantation using the technique modified from Thorley and colleagues (52). Primary cells were obtained from macroscopically healthy segments of lung from patients undergoing lung cancer resection. Approval for the use of material was obtained from the Royal Brompton, Harefield, NHLI and the UCL/UCLH ethics committee and informed consent was obtained from patients. Human THP-1 monocytes were purchased from the American Type Culture Collection and grown in RPMI 1640 at 37°C (5% CO2), supplemented with penicillin (200 U/ml), streptomycin (200 U/ml), glutamine (4 mM) and 10% FBS (v/v), unless otherwise stated. Cells were routinely tested and found negative for mycoplasma infection.
ELISA analysis of cytokine/chemokine release by pHLFs in conditioned media
pHLFs were grown to 80% confluence and serum-starved for 24 hours prior to use in experiments. Conditioned media were collected at designated time points after exposure to varying concentrations of TNF-α or TSLP or media alone. Samples were centrifuged at 300g for 5 minutes at 4°C to remove cell debris and stored at −80°C until analysis by ELISA. For inhibitor studies, cells were incubated with designated concentrations of inhibitors for 30 minutes at 37°C prior to exposure to TNF-α or TSLP. Cell viability in all inhibitor studies was >95% as assessed by trypan blue exclusion. TSLP in conditioned media was quantified using ELISA with matched antibodies according the manufacturer’s instructions (R&D Systems, Abingdon, UK). The sensitivity limit of the TSLP ELISA is 7.8 pg/ml. Each data point represents the mean ± SEM from readings performed in triplicate from three independent assays. CCL2 in conditioned media was quantified by ELISA as previously described (53). Paired antibodies MAB679 and BAF279 for the human CCL2 ELISA were obtained from R&D Systems and human recombinant CCL2 protein standard was from Peprotech (NJM, USA).
RNA isolation / RT-PCR analysis / qRT-PCR analysis
Total RNA from cell cultures was isolated with TRIzol reagent as per the manufacturer’s protocol. RNA was DNase-treated using a DNAfree kit (Ambion, Huntingdon, United Kingdom). Reverse transcription was performed using 1 μg total RNA in a first-strand cDNA synthesis with qScript cDNA SuperMix kit (Quanta Biosciences, USA) in a reaction volume of 20μl as per manufacturer’s protocol. TSLPR and IL-7Rα mRNA expression in pHLFs was analysed by qualitative RT-PCR. Cycling conditions were as follows: activation step of 95°C for 10 minutes; 35 cycles of 95°C (10 seconds), 62°C (45 seconds). PCR products were run on 1% w/v agarose gel electrophoresis and visualized by GelRed (Biotium, CA, USA) staining. Size of products was estimated using a co-migrated DNA size marker (Roche Diagnostics).
Real-time RT-PCR was conducted using the Platinum SYBR Green qPCR SuperMix UDG (Invitrogen) on a Mastercycler EP Realplex (Eppendorf, Germany). Cycling conditions were as follows: activation step of 95°C for 10 minutes; 40 cycles of 95°C (10 seconds), 62°C (45 seconds). The specificity of the PCR product was confirmed by melting curve analysis. Fold change in expression was calculated using the 2−ΔΔCp formula, as previously described (51). Primers are shown in Table I.
Table I. Primer sequences used for RT-PCR and qPCR.
| Gene | Forward | Reverse |
|---|---|---|
| Human Tslp | 5′-TATGAGTGGGACCAAAAGTACCG-3 | 5′-GGGATTGAAGGTTAGGCTCTGG-3′ |
| Human Tslp (long splice) |
5′-GATTACATATATGAGTGGGAC-3′ | 5′-TTCATTGCCTGAGTAGCAT-3′ |
| Human Tslp (short splice) |
5′-CGTAAACTTTGCCGCCTATGA-3′ | 5′-TTCTTCATTGCCTGAGTAGCATTTAT-3′ |
| Human Tslpr | 5′-GCAAGTCGCTGGATGGTTA-3′ | 5′-GTCAGAACACGTCACCGTCA-3′ |
| Human Il7ra | 5′-GGAGCCAATGACTTTGTGGT-3′ | 5′-AGTGTCAGCTTTGTGCTGGA-3′ |
| Human Ccl2 | 5′-AGCAAGTGTCCCAAAGAAGC-3′ | 5′-CATGGAATCCTGAACCCTCT-3′ |
| Human Hprt | 5′-TCATTATGCCGAGGATTTGG-3′ | 5′-ACAGAGGGCCACAATGTGATGTTG-3′ |
Western blot analysis of HSP27, p42/44, IκBα, c-Jun, STAT3, STAT5, ERK2
(Phospho-) HSP27, (Phospho-) p42/44, IκBα, (Phospho-) c-Jun, (Phospho-) STAT3, (Phospho-) STAT5 and ERK2 expression were analysed by Western blotting. pHLFs were grown to 80% confluence before being quiesced for 24 hours in serum-free media. Cells were then stimulated in fresh serum-free media with TNF-α (10 ng/ml) or TSLP (1 ng/ml) for designated times. Inhibitor and antibody studies were performed as described above. After incubation, cells were washed with ice-cold PBS, then lysed on ice in 100 μl Phosphosafe buffer (VWR, Lutterworth, UK) supplemented with Complete Mini protease inhibitor cocktail (Roche, West Sussex, UK). Equal amounts of protein were loaded onto 8-16% LongLife pre-cast gels (Nusep, Wasserburg Germany), electrophoresed, transferred to nitrocellulose membranes (Hybond-ECL; Amersham Biosciences), and incubated with blocking buffer containing 5% non-fat dry milk in TBS/0/1% Tween-20 (TBST) for 1 hour. Blots were then incubated with antibodies specific for phosphorylated HSP27, p42/44, c-Jun, STAT3 and STAT5, or for IκBα, overnight at 4°C. All blots were then washed with TBST and incubated for 1 hour at room temperature with HRP-conjugated secondary antibody. After further washing in TBST, immunoreactive bands were visualized by standard chemiluminescence (ECL reagent; Amersham Biosciences) according to the manufacturer’s instructions. For examination of total HSP27, p42/44, c-Jun, STAT3, STAT5 and ERK2 (as loading controls), the blots were stripped before immunoblotting with antibodies described above, using the same protocol. Blots were scanned on an Epson Perfection 4870 photo scanner and densitometric analysis was performed using Image J software (NIH, USA) calibrated against Kodak photographic Step Tablet no.3.
Transfection of pHLFs with siRNA
pHLFs were grown to 80% confluence in antibiotic-free medium to maximise transfection efficiency. After being serum-starved for 24 hours, cells were transfected with siRNA directed against either c-Jun, STAT3 or control scrambled siRNA at final concentrations of 100 nM. Further control cells were mock-transfected at the same time. siRNA (Dharmacon, USA) was transfected into cells using a Gemini transfection reagent (a kind gift from GSK) in Optimem medium. Cells were then allowed to quiesce for 24 hrs before subsequent stimulation. After each specific treatment, cell extracts were prepared as described above, and Western blotting was used to evaluate expression of proteins of interest. In addition, time-point matched conditioned media were collected and analysed for TSLP and CCL2 protein release by ELISA, as described above. siRNA sequences for c-Jun and STAT3 are listed below in Table II.
Table II. Sequences of siRNA used for transfection of pHLFs.
| Target | Sequence |
|---|---|
| c-Jun | GAGCGGACCUUAUGGCUAC |
| STAT3 | CAACAGAUUGCCUGCAUUG |
| Scrambled controls | Sequences not provided by Dharmacon |
Plasmid Transfection of pHLFs
pHLFs were grown to 60% confluence (1.25 × 104 cells/well in 48-well plates) in DMEM/10% FBS. Cells were then transfected with 0.25 μg plasmid DNA (pFUGW as control, or pSTAT3C) per well, using JetPRIME® reagent (1:2 ratio of DNA to JetPRIME®, w/v) according to the manufacturer’s instructions. Four hours after addition of transfection complexes, pHLFs were incubated in fresh DMEM/10% FBS overnight. Cells were subsequently incubated in serum-free DMEM for 18 hours to assess the effect of constitutive STAT3C activity on CCL2 production (assessed by ELISA, as described above).
Monocyte chemotaxis
THP-1 human monocyte chemotaxis was assayed in a 48-well Boyden chamber using a 5 μm polyvinylpyrrolidone (PVP)-free polycarbonate filter as previously described (54). Graded concentrations of hrCCL2 (0 – 100 ng/ml) were prepared in DMEM/1% BSA, and the optimum [CCL2] for inducing monocyte chemotaxis in these studies was determined to be 3 ng/ml. To determine the ND50 of the anti-CCL2 antibody used for inhibition studies, hrCCL2 (3 ng/ml) was incubated with graded concentrations of antibody (0 – 30 μg/ml) for 1 hour at room temperature before being added to wells. The ND50 for this antibody in inhibiting CCL2 (3 ng/ml)-induced chemotaxis was determined to be 5.06 μg/ml, with complete abrogation of chemotaxis observed at 30 μg/ml. For experiments, conditioned media from pHLFs treated with graded concentrations of TSLP as designated were added to the lower wells. For inhibition studies, conditioned media was incubated with anti-CCL2 antibody as described as above (30 μg/ml), before being added to wells. Cells were suspended in DMEM/1% BSA (1 × 106 cells/ml) and added to the upper wells of the chamber and allowed to migrate for 2 hours at 37°C in a 5% CO2 atmosphere. After this, the filter was removed from the chamber, fixed in methanol and stained with a Diff-Quick stain kit. Cells that migrated through the membrane were counted under light microscopy (×100 objective) on 5 random high power fields (HPF). The results are expressed as mean number of cells per 5 HPF from experiments performed in triplicate on three independent occasions. Monocyte migration towards hrCCL2 (3 ng/ml) or DMEM/1% BSA were used as positive and negative controls respectively.
Immunocytofluorescence
Serum-fed pHLFs were grown on 8-well chamber slides (Millipore, UK) and grown to 80% confluence. Slides were washed in PBS and fixed for 10 min with 4% formaldehyde in PBS. Cells were blocked with 10% FBS (v/v) / 0.2% fish skin gelatine (v/v) in PBS for 1 h at room temperature. After washing twice in PBS, slides were incubated with primary rabbit anti-TSLPR and goat anti-IL-7Rα antibodies or isotype control antibodies for 2 hours at room temperature. Slides were washed twice in PBS and incubated for 1 hour at room temperature with donkey anti-rabbit IgG AF488 and donkey anti-goat IgG AF555. The cell layer was then washed twice in PBS before being mounted with Prolong Gold anti-fade reagent with DAPI (Invitrogen). Sections were visualized using a Zeiss Axioskop 2 microscope (Carl Zeiss Ltd.), and images were captured using a Qicam 12-bit colour fast camera using Q capture software, version 2.81 (both from QImaging Corp.).
Human subject details
Lung biopsy specimens were obtained from 12 patients with IPF (obtained at diagnostic surgical lung biopsy) and 3 control patients (obtained from uninvolved tissue during cancer resection surgery). All biopsies used in this study were classified using the diagnostic criteria of the American Thoracic Society/European Respiratory Society Consensus (American Thoracic Society/European Respiratory Society International multidisciplinary consensus classification of the idiopathic interstitial pneumonias, 2002) demonstrating a pattern of usual interstitial pneumonia (UIP). Approval for the use of the material was obtained from the Royal Brompton, Harefield, NHLI and the UCL/UCLH ethic committee. Informed consent was obtained from each patient.
Histologic analysis
Fresh human lung biopsy material was processed for immunohistochemical analysis as previously described (53). Briefly, after fixation in 4% formaldehyde, specimens were placed in processing cassettes, dehydrated through a serial alcohol gradient a xylene using automated Leica Tissue Processor, and embedded in paraffin wax blocks. Before immunostaining, 3 μm-thick lung tissue sections were dewaxed in xylene, rehydrated through decreasing concentrations of ethanol to deionised water.
Immunohistochemistry
To examine the immunolocalisation of TSLP and TSLPR in human lung tissue, antigens were unmasked by microwaving sections in 10 mM citrate buffer, pH 6.0 (twice for 5 min, followed by 15 min cooling step). Immunolocalisation of α-smooth muscle actin (α-SMA) to allow identification of lung (myo)fibroblasts was performed as previously described (51).
Two 30 min blocking steps with 3% H2O2 in deionised water and 3% sera corresponding to secondary antibodies species made in 1% BSA/ PBS were performed before incubation with primary antibodies.
Immunostaining was undertaken by the avidin–biotinylated HRP enzyme complex method (Vector Laboratories, Burlingame, CA) with antibodies against human TSLP (0.5 μg/ml), human TSLPR (1 μg/ml), or equivalent concentrations of polyclonal non-immune IgG controls incubated for 16h at 4°C. After incubation with an appropriate biotin-conjugated secondary antibody for 30 mins at RT, and subsequently with Vector ABC complex PK-6100 (as per manufacturer’s protocol for 30 mins), 5 mins colour development was performed with 3,3′-diaminobenzidine (BioGenex) as a chromogen. Sections were counterstained with Gill-2 haematoxylin (Thermo-Shandon, Pittsburgh, PA), dehydrated, and coverslipped permanently. Comparative immunohistochemical analysis for TSLP, TSLPR and αSMA was performed on serial sections. Sections were digitally scanned with a Hamamatsu NanoZoomer (40× objective) and representative images are presented.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism software (GraphPad Software, San Diego, CA). All data are presented as mean ± standard errors of the mean (SEM), unless otherwise indicated, from experiments performed in triplicate on 3 independent occasions. All differences in mRNA levels compared differences in ΔCp values. All Western blot data are representative of three independent experiments performed, unless otherwise specified. Statistical comparison was performed between two treatment groups by student’s t-test, and between multiple treatment groups by ANOVA (one-way or two-way, as appropriate) with Tukey post hoc testing. A p value of less than 0.05 was considered significant.
RESULTS
TSLP and TSLPR expression in idiopathic pulmonary fibrosis (IPF)
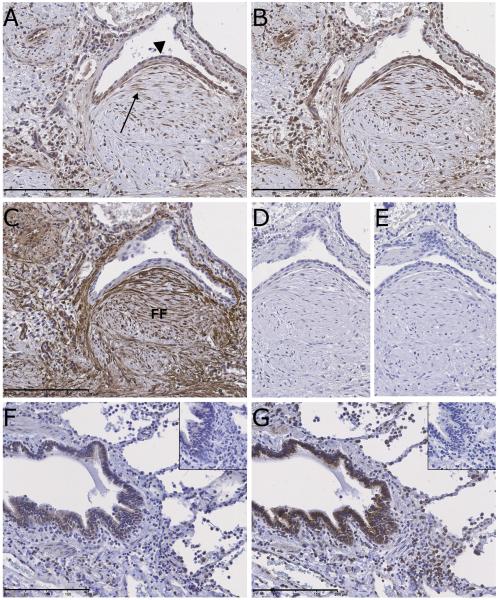
We first examined whether TSLP is expressed in IPF lung tissue by assessing TSLP immunoreactivity in IPF (n = 12) and control lung biopsy (n = 3) material. We found that tissue sections from IPF lung demonstrated strong TSLP immunoreactivity which was predominantly associated with alveolar epithelial cells (AEC), and fibroblasts within fibrotic foci - the histological hallmark of IPF (Fig 1A). Positive immunoreactivity was also detectable on airway smooth muscle cells (ASMC) and (alveolar) macrophages. In contrast, TSLP immunoreactivity in control lung was weak and limited to occasional macrophages and bronchial epithelium (Fig 1F). We next sought to identify potential cellular targets of TSLP in IPF. Serial sections of IPF lung demonstrated intense TSLPR immunostaining in AEC, ASMC and surprisingly also for fibroblasts within fibrotic foci (Fig 1B). Activated fibroblasts within fibrotic foci were identified by their characteristic spindle-shaped morphology, demonstrating strong αSMA immunoreactivity on serial sections (Fig 1C). As with TSLP, immunostaining for TSLPR in control lung was limited to macrophages and bronchial epithelium (Fig 1G). No immunoreactivity was observed on serial sections stained with isotype control antibodies for TSLP (Fig 1D), TSLPR (Fig 1E) or αSMA (data not shown). To confirm these results, additional immunohistochemical studies were undertaken using a different panel of antibodies, with highly concordant results (Supplementary Figures S1 and S2). Taken together, these data raise the intriguing possibility that lung fibroblasts may represent a novel cellular source and target for TSLP in IPF.
Figure 1. TSLP, TSLPR and α-smooth muscle actin (αSMA) immunostaining in idiopathic pulmonary fibrosis (IPF) identifies epithelial cells and fibroblasts as major cell types displaying strong immunoreactivity.
Shown is immunostaining for TSLP (A), TSLPR (B) and αSMA (C) in serial sections of IPF lung. (A) In IPF lung, strong immunoreactivity for TSLP is observed in alveolar epithelial cells (arrowhead) and spindle-shaped fibroblasts (arrows) located within fibrotic foci (FF). Immunoreactivity is also associated with macrophages and airway smooth muscle cells. (B) Strong TSLPR staining is also observed in IPF lung, again localising to AEC, fibroblasts, ASMC and macrophages. Spindle-shaped cells within fibrotic foci that stained positive for TSLP and TSLPR were also strongly immunoreactive for αSMA (C). (D & E) IPF serial tissue sections stained with isotype-specific non-immune primary antibodies as controls for TSLP and TSLPR shows no discernible staining. (F & G) Immunostaining for TSLP and TSLPR, respectively, in control lung tissue demonstrated staining associated predominantly with bronchial epithelium and macrophages. Inset images show negative immunostaining with isotype control antibodies on serial sections. Scale bars: 200 μm.
We next considered the potential mechanisms responsible for TSLP upregulation in IPF and focused our attention on the master cytokine, TNF-α, which has been strongly implicated in the pathogenesis of lung fibrosis (55, 56) and is a known inducer of TSLP expression (27, 28).
TNF-α upregulates TSLP expression in pHLFs in a JNK/c-Jun dependent manner
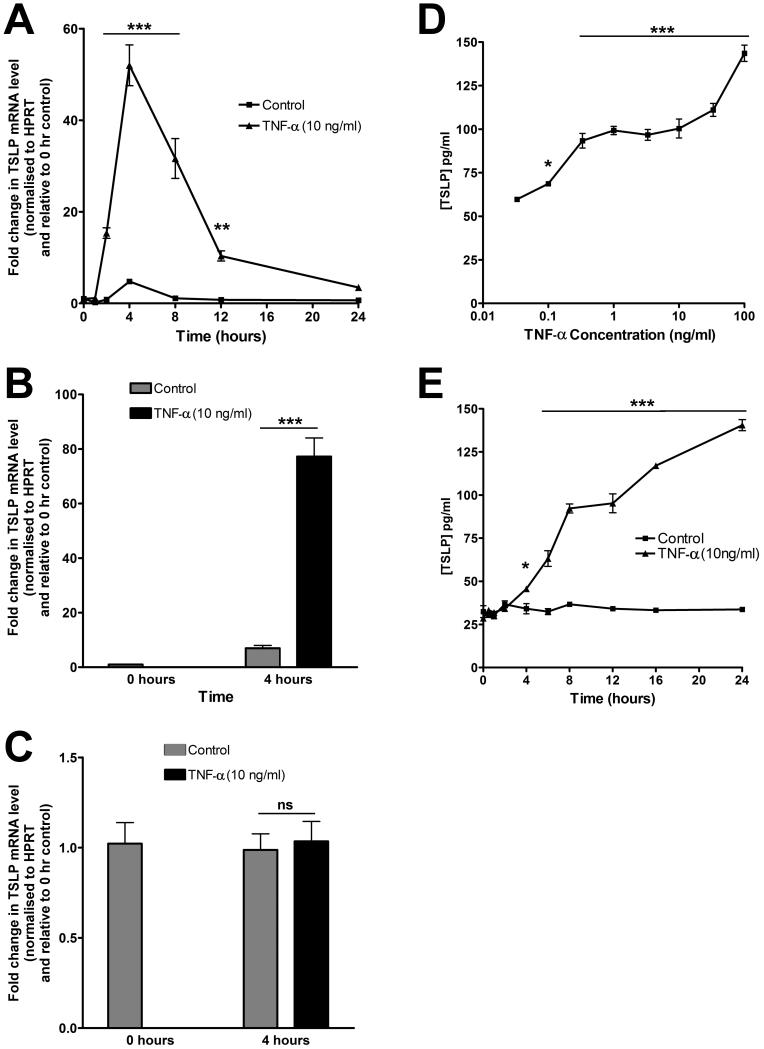
IPF IHC data suggested that the hyperplastic alveolar epithelium and fibroblasts might represent potential cellular sources of TSLP in the fibrotic lung. To address this possibility, we examined whether primary human alveolar epithelial cells (pAECs) and primary human lung fibroblasts (pHLFs) express TSLP in vitro. Although the alveolar epithelium displayed strong TSLP immunoreactivity in IPF lung, we were unable to demonstrate baseline or inducible TSLP protein release by pAEC following exposure to TNF-α (data not shown). In contrast, pHLFs were found to constitutively express TSLP mRNA at baseline and this was significantly increased in response to TNF-α over time (Fig 2A).
Figure 2. TNF-α stimulates pHLF TSLP gene expression and protein production.
Serum-starved pHLFs were exposed to control medium (DMEM) or graded concentrations of TNF-α for varying durations as designated. (A) Time-course data for the effect of TNF-α (10 ng/ml) on pHLF TSLP mRNA levels. TSLP mRNA levels, at each designated time point, were assessed by qPCR. Data are expressed as fold change for each time point relative to time zero, normalized to HPRT mRNA levels (mean ± SEM of triplicates from three independent experiments). (B and C) TSLP mRNA levels of the long (B) and short (C) splice variant following exposure to TNF- α (10 ng/ml) or control medium for 4 hours were assessed as in (A) and data expressed as in (A); ** p<0.01, *** p<0.001, comparison with time-matched media controls. (D and E) Concentration-response and time-course data for the effect of TNF-α on pHLF TSLP protein levels. pHLFs were exposed to graded concentrations of TNF-α or control medium for 6 hours (D) or TNF-α (10 ng/ml) or control medium for varying durations (E). TSLP protein release into conditioned media was measured by ELISA and the amount of secreted TSLP is expressed as pg/ml (mean ± SEM of triplicates from three independent experiments). * p<0.05; *** p<0.001 compared with unstimulated control.
We next examined which of the two known TSLP splice-variants (57) are expressed by pHLFs. We found that pHLFs express both transcript variants constitutively but that TNF-α only increases the expression of the long splice-variant (Fig 2B & C). Confirmation of TSLP induction at the protein level (ELISA) revealed that TNF-α induces TSLP protein release in both a concentration- and time-dependent manner (Fig 2D & E).
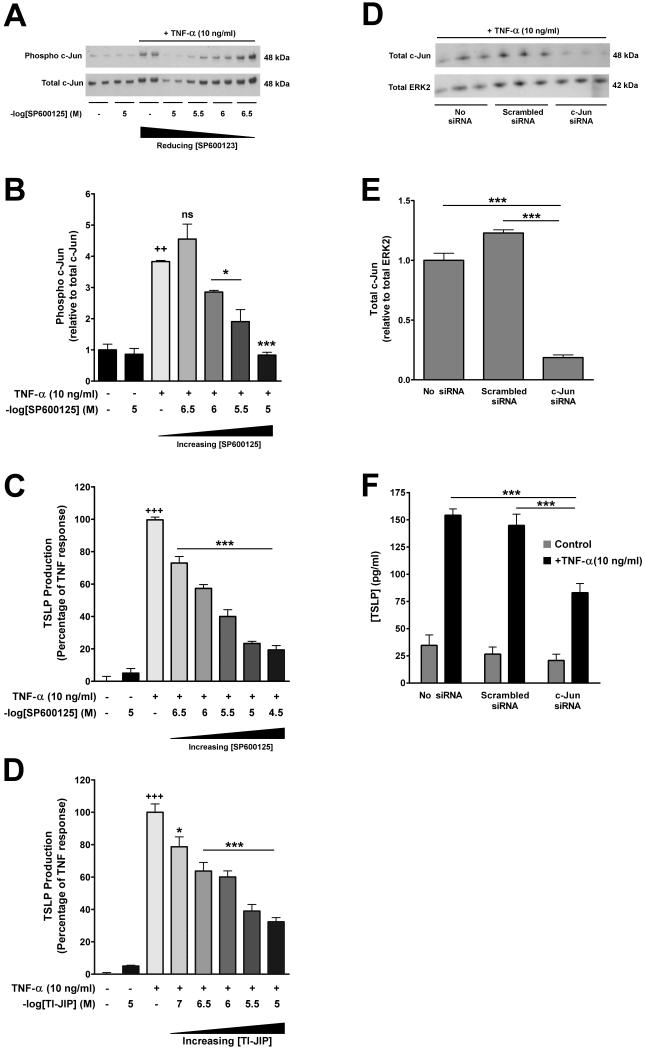
To delineate the signalling pathways responsible for mediating TNF-α-induced TSLP expression by pHLFs, we employed a combined pharmacological and genetic approach. Pre-incubation of pHLFs with SC-514 (IKK-2 inhibitor), U0126 (MEK 1/2 inhibitor) and SB203580 (p38 inhibitor) had no effect on TNF-α-induced TSLP protein release into conditioned media despite engagement of their respective targets, as assessed by Western blot analysis of cell lysates (data not shown). Taken together these data ruled out a significant role for NFκB, ERK1/2 and p38. In contrast, pharmacological inhibition of JNK activity with SP600125, significantly inhibited TNF-α-induced c-Jun phosphorylation and TSLP protein release in a concentration-dependent manner from around 10−6 M onwards (Fig 3A, B & C). These findings were corroborated using a second structurally unrelated inhibitor of JNK activity, TI-JIP (Fig 3D), which unlike SP600125, inhibits JNK activity in an ATP non-competitive manner (58). To confirm the importance of the JNK/AP-1 signalling pathway in mediating TNF-α-induced TSLP protein release, we also knocked down c-Jun by siRNA transfection which resulted in a significant attenuation in TNF-α-induced TSLP protein release (Fig 3E & F). To the best of our knowledge, this is the first report that TNF-α induces TSLP expression in a JNK/c-Jun dependent manner.
Figure 3. TNF-α-induced TSLP expression in pHLFs requires c-Jun phosphorylation.
(A – D) Serum-starved pHLFs were pre-incubated with graded or designated concentrations of SP600125 or TI-JIP for 30 minutes prior to exposure to TNF-α (10 ng/ml) or control medium (DMEM) for 30 minutes (A, B) or 6 hours (C, D). Final concentrations of DMSO were kept constant for all experimental conditions (0.1% in DMEM). (A & B) The effect of SP600125 on TNF-α-induced c-Jun phosphorylation. Phosphorylated c-Jun was assessed by Western blot analysis of total cell lysates (A) using an anti–phospho c-Jun antibody (upper panel). The same blots were stripped, re-probed with an anti-total c-Jun antibody (lower panel) and used to verify protein loading. Densitometric analysis was performed on c-Jun phosphorylation (B) and data are presented as the mean ratio of phospho over total c-Jun, normalised to unstimulated control of triplicates from three independent experiments, ++ p<0.01, comparison with unstimulated control; * p<0.05, *** p<0.001, comparison to cells stimulated with TNF-α only. (C & D) The effect of SP600125 and TI-JIP on TNF-α-induced TSLP protein release. TSLP protein release into conditioned media was measured by ELISA at 6 hours. Data are presented as a percentage of the maximal response obtained with TNF-α and drug vehicle alone. The first bars represents control medium with drug vehicle alone; the second bars represents the highest concentration of SP600125 (C) or TI-JIP (D) examined, and show that these compounds have no effect on basal TSLP protein production. Negative log of the concentrations of SP600125 and TI-JIP are presented. Data represent the mean ± SEM of triplicates from three independent experiments. (E, F) The effect of siRNA knockdown of c-Jun expression on TNF-α-induced TSLP protein release. pHLFs were transfected with siRNA targeting c-Jun, scrambled siRNA (final siRNA concentration 100nM) or mock-transfected. Cells were then exposed to TNF-α (10 ng/ml) or control medium for 6 hours before total cell lysates were prepared for Western blot analysis; matched conditioned media was also removed for TSLP protein measurements by ELISA. Knockdown of c-Jun expression was assessed by Western blot analysis of total cell lysates from cells exposed to TNF-α (10 ng/ml) using an anti-total c-Jun antibody (E; upper panel). The same blots were stripped, re-probed with an anti-total ERK1/2 antibody (E; lower panel) and used to verify protein loading. Densitometric analysis was performed on total-Jun expression (F) and data are presented as the mean ratio of total c-Jun over total ERK2, normalised to mock-transfected control of triplicates from three independent experiments. (G) TSLP protein release into conditioned media was measured by ELISA and data are presented as pg/ml (mean ± SEM of triplicates from three independent experiments). *** p < 0.001, comparison with mock-transfected cells.
pHLFs express a functional TSLP receptor complex
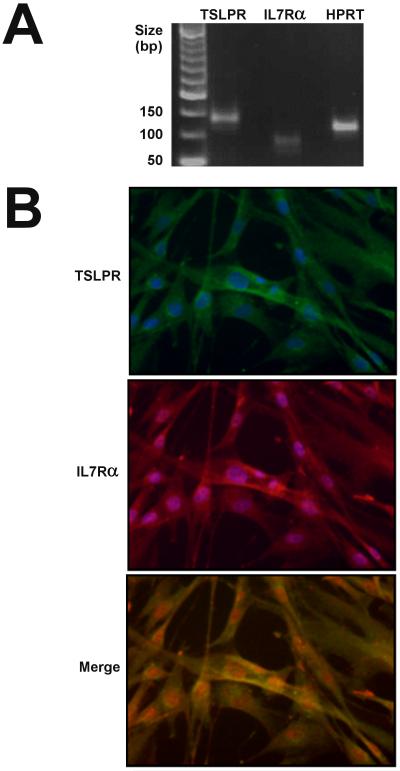
To determine whether lung fibroblasts express a functional TSLP receptor complex, we first examined baseline expression of the Tslpr and IL7ra genes in pHLFs by RT-PCR. pHLFs were found to express mRNA transcripts for both constituent chains of the TSLP receptor complex (Fig 4A). Examination of the expression of these chains by dual immunocytofluorescence (Fig 4B) revealed co-localization of TSLPR and IL7Rα in pHLFs.
Figure 4. pHLFs express the TSLP receptor complex.
pHLFs were grown to 80% confluence and TSLPR and IL-7Rα expression was analysed by (A) RT-PCR and (B) double immunofluorescence as described in Materials and Methods. (A) pHLFs express mRNA transcripts for both components of the TSLP receptor. Following RNA extraction, PCR products were run on 1% w/v agarose gel electrophoresis; HPRT cDNA served as an internal control and non-template samples served as negative controls. (B) TSLPR (green) and IL7Rα (red) immunoreactivity is detectable in pHLFs and shown to co-localise in a merged image (yellow signal). pHLFs were counterstained with DAPI to enable nuclear localisation. Original magnifications ×20.
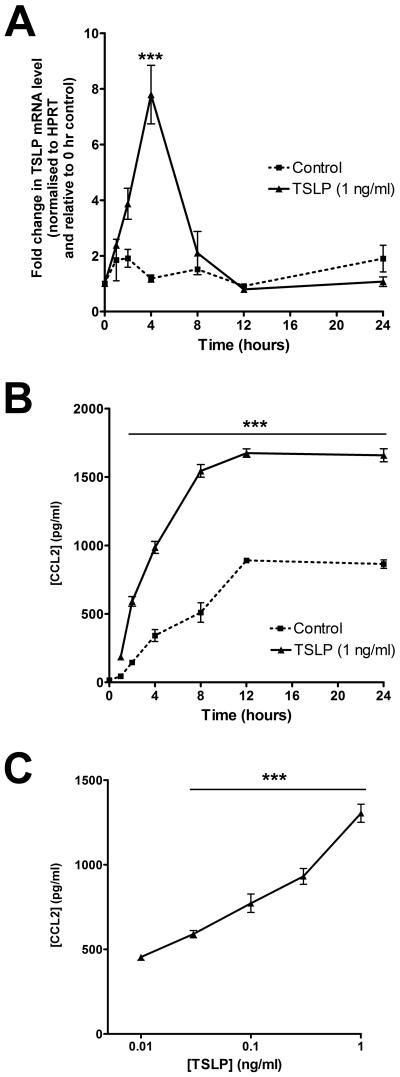
Recent evidence suggests that human mesenchymal cells, such as airway smooth muscle cells, are capable of releasing chemokines, including CCL2, in response to stimulation by TSLP in vitro (59). Moreover, there is strong evidence that this chemokine, in particular, plays a pathogenic role in diseases characterised by tissue remodelling and a T-2 immune phenotype, including IPF (53). Having demonstrated increased immunoreactivity for TSLP in IPF lung, we contemplated the presence of a biologically relevant TSLP-CCL2 axis in this disease. We therefore examined whether lung fibroblasts express a functional TSLP receptor and were capable of upregulating expression of CCL2 following exposure to this type 2 polarising cytokine. (Fig 5). These studies revealed that TSLP increased CCL2 mRNA levels within 4 hours post-stimulation; this response was transient, with levels returned back to baseline levels by 8 hours (Fig 5A). This upregulation was accompanied by the concentration- and time-dependent release of CCL2 protein into conditioned media (Fig 5B & C respectively). Taken together, these data demonstrate that pHLFs express a functional TSLP receptor complex and upregulate CCL2 expression and release in response to TSLP stimulation.
Figure 5. TSLP induces expression of CCL2 in pHLFs.
pHLFs were exposed to control medium (DMEM) or graded concentrations of TSLP for varying durations as designated. Time-course data (A) for the effect of TSLP (1 ng/ml) on pHLF CCL2 mRNA levels. CCL2 mRNA levels, at each designated time point, were assessed by qRT-PCR. Data are expressed as fold change for each time point relative to time zero, normalized to HPRT mRNA levels (mean ± SEM of triplicates from three independent experiments). (B and C) Time-course and concentration-response data for the effect of TSLP on pHLF CCL2 protein levels. pHLFs were exposed to TSLP (1 ng/ml) or control medium for varying durations (B) or graded concentrations of TSLP or control medium for 6 hours (C). CCL2 protein release into conditioned media was measured by ELISA and the amount of secreted CCL2 is expressed as pg/ml (mean ± SEM of triplicates from three independent experiments, *** p<0.001, comparison with time-point matched media control.
TSLP-induced upregulation of CCL2 expression by pHLFs is STAT3-dependent
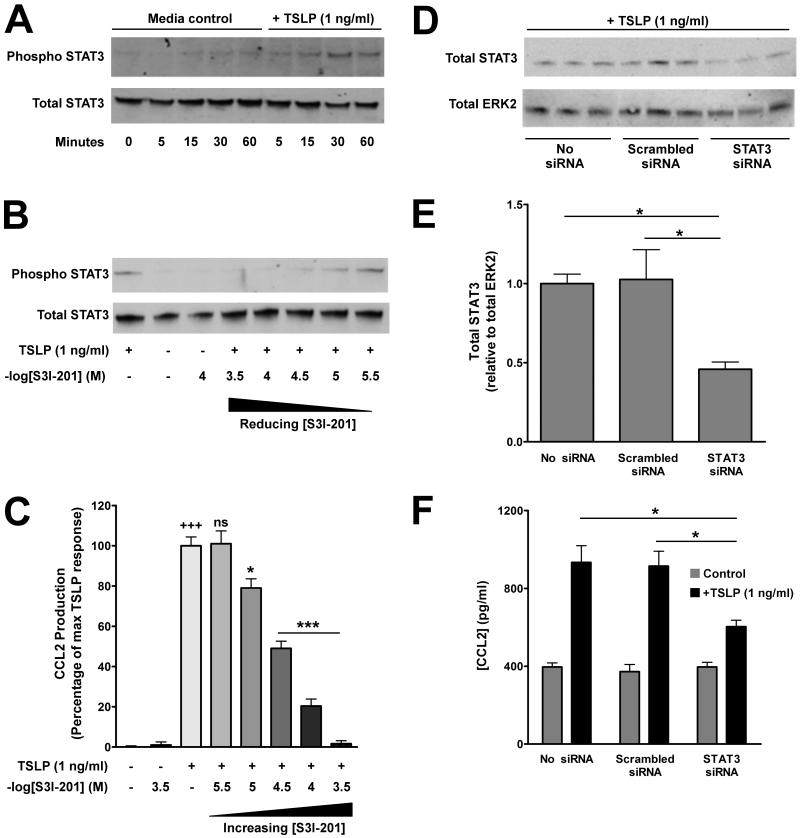
Signal transduction downstream of the heterodimeric TSLP receptor comprises functional activation of STAT3 and STAT5 (36). To investigate the potential involvement of STAT3 and STAT5 in TSLP-induced CCL2 expression in pHLFs, we performed Western blot analysis on cell lysates prepared from pHLFs exposed to TSLP using specific antibodies directed against phosphorylated regulatory sites on these transcription factors. No STAT5 phosphorylation was observed in pHLFs exposed to TSLP over a period of 1 hour (Supplementary Figure S3). In contrast, TSLP induced STAT3 phosphorylation in a time-dependent manner from 15 minutes onwards which was maintained for 1 hour (Fig 6A).
Figure 6. TSLP-induced upregulation of CCL2 expression by pHLFs is STAT3-dependent.
TSLP induces STAT3 phosphorylation in pHLFs (A). Serum-starved pHLFs were exposed to TSLP (1 ng/ml) or control medium for the designated times and phosphorylation of STAT3 (p-STAT3) was assessed by Western blot analysis of total cell lysates using an anti-p-STAT3 antibody (A, upper panel). The same blot was stripped, re-probed with anti-total STAT3 antibody (A, lower panel), to verify protein loading. (B & C) The effect of S3I-201 on TSLP-induced STAT3 phosphorylation and CCL2 protein release. pHLFs were pre-incubated with graded concentrations of S3I-201 for 30 minutes prior to exposure to TSLP (1 ng/ml) or control medium for the designated times. Final concentrations of DMSO were kept constant for all experimental conditions (0.1% in DMEM). (B) p- and t-STAT3 following exposure to TSLP for 30 mins was assessed as above. (C) CCL2 protein release into conditioned media at 6 hours was measured by ELISA. Data are presented as a percentage of the maximal response obtained with TSLP and drug vehicle alone. The first bar represents the CCL2 response to TSLP and drug vehicle alone. The second bar represents the response to control medium alone. The third bar represents highest concentration of S3I-201 examined and shows that this compound has no effect on basal CCL2 release. Negative log of the concentrations of S3I-201 are presented. Data represent the mean ± SEM of triplicates from three independent experiments. +++ p<0.001, comparison with untreated cells; * p<0.05; *** p<0.001, comparison with TSLP alone. (D, E & F) The effect of siRNA knockdown of STAT3 expression on TSLP-induced CCL2 protein release by pHLFs. pHLFs were transfected with siRNA targeting STAT3, scrambled siRNA (final siRNA concentration 100nM) or mock-transfected. Cells were then exposed to TSLP (1 ng/ml) or control medium for 6 hours before total cell lysates were prepared for Western blot analysis (D & E); matched conditioned media was also removed for CCL2 protein measurements by ELISA (F). Knockdown of STAT3 expression was assessed by Western blot analysis of total cell lysates from cells exposed to TSLP (1 ng/ml) (D) using an anti-t-STAT3 antibody (upper panel). The same blots were stripped, re-probed with an anti-total ERK1/2 antibody (lower panel) and used to verify protein loading. Densitometric analysis was performed on total STAT3 expression (E) and data are presented as the mean ratio of total STAT3 over total ERK2, normalised to mock-transfected control, Data represent the mean ± SEM of triplicates from three independent experiments Data represent the mean ± SEM of triplicates from three independent experiments. (F) CCL2 protein release into conditioned media was measured by ELISA and data are presented as pg/ml (mean ± SEM of triplicates from three independent experiments). *** p < 0.001, comparison with mock-transfected cells.
We next determined whether STAT3 was required for TSLP-induced CCL2 protein release. Pre-incubation of pHLFs with the STAT3 inhibitor, S3I-201 resulted in a significant concentration-dependent inhibition of both TSLP-induced STAT3 phosphorylation and CCL2 protein release from 10−6M onwards (Fig 6B & C, respectively). The importance of STAT3 in this response was further confirmed by siRNA knockdown of STAT3 which also significantly attenuated CCL2 production (Fig 6D, E & F), while overexpression of constitutively-active STAT3 enhanced baseline expression of CCL2 in pHLFs (144 ± 10% versus control vector, p<0.05).
TSLP induces chemotaxis of human monocytes in a CCL2-dependent manner
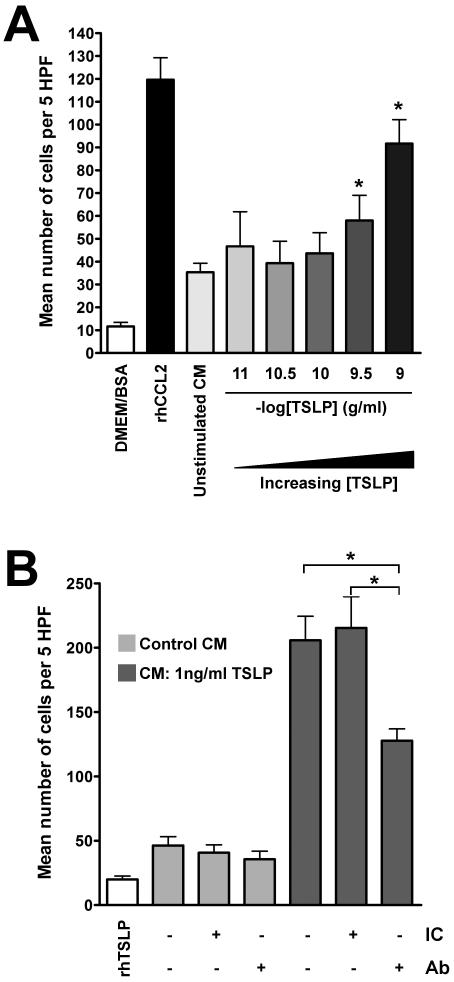
It is well established that fibroblasts represent an important cellular source of chemokines, including CCL2 (60), and the generation of a stromally-derived chemokine gradient is increasingly recognised as crucial to the accumulation, differentiation and survival of immune cells in non-lymphoid target tissue (61). We therefore examined whether TSLP was indirectly capable of generating a functional fibroblast-derived chemokine gradient in vitro. Chemotaxis assays were performed in a Boyden chamber using the human monocyte cell line, THP-1. First, we confirmed the chemotactic property of hrCCL2 for THP-1 cells (please see Supplementary Figure S4). In subsequent studies, conditioned media from fibroblasts exposed to TSLP (CM-TSLP) was found to promote a significant increase in monocyte migration compared with conditioned media from unstimulated fibroblasts (Figure 7A). To determine if this chemotactic response was mediated by CCL2, CM-TSLP was pre-incubated with a neutralising anti-hCCL2 antibody prior to exposure to monocytes. Monocyte chemotaxis in response to CM-TSLP was significantly attenuated (~40%) in the presence of an anti-CCL2 antibody suggesting that the chemotactic effect of CM-TSLP was, at least in part, mediated by CCL2 (Fig 7B). Finally, rhTSLP (1 ng/ml) exerted no chemotactic effect on THP-1 cells demonstrating that the observed chemotactic response was not due to direct stimulation by TSLP.
Figure 7. Conditioned media from TSLP-treated pHLFs induces chemotaxis of THP-1 monocytes via CCL2.
Conditioned media (CM) from pHLFs exposed to TSLP induces chemotaxis of human THP-1 monocytes (A). Human THP-1 monocytes were exposed to CM collected from pHLFs treated with graded concentrations of TSLP (0-1 ng/ml) for 6 hours; chemotaxis was assessed using a Boyden chamber as described in Materials and Methods. The first bar represents the chemotactic response of THP-1 cells to DMEM/BSA (1%, w/v) only. The second bar represents the chemotactic response to rhCCL2. Data show the mean number of cells per 5 high powered fields (HPF) ± SEM of triplicates from three independent experiments. * p<0.05, comparison with conditioned media from unstimulated cells. TSLP-induced CCL2 mediates monocyte chemotaxis (B). THP-1 cells were exposed to CM from pHLFs (treated with 1 ng/ml TSLP) which had been pre-incubated with an anti-CCL2 neutralizing antibody (Ab) or isotype control (IC) antibody (both at 30 μg/ml) and chemotaxis assessed as above. The first bar represents the chemotactic response of cells to recombinant human (rh) TSLP (1 ng/ml). Data are presented as above. * p<0.05, comparison with CM from pHLFs exposed to TSLP only.
Discussion
The data presented in this article examined the potential role and regulation of TSLP in the context of fibrotic lung disease. We report for the first time that TSLP and TSLPR are highly upregulated in idiopathic pulmonary fibrosis (IPF) and that fibroblasts represent both a novel cellular source and target of TSLP. We further show that TNF-α is a potent inducer of TSLP expression by human lung fibroblasts and that TSLP, signalling via STAT3, induces the release of biologically relevant concentrations of the potent monocyte chemoattractant, CCL2.
Fibroblasts express and release TSLP
Recent work has highlighted the importance of TSLP as a critical regulator of type 2 dominated inflammatory responses in the lung (6, 62). Although TSLP has been strongly implicated in the pathogenesis of atopic asthma (11) and the associated bronchial sub-epithelial fibrosis (6), the role of TSLP in non-atopic lung diseases associated with increased expression of type 2 cytokines, such as IPF (63), is unknown. Our report of consistently strong TSLP and TSLPR immunoreactivity within active fibrotic lesions in IPF lung supports the notion that TSLP may play a role in promoting type 2 immune responses in this condition. It is also in keeping with a recent article published during the course of our studies, in the context of skin fibrosis associated with the auto-immune disorder, systemic sclerosis (64, 65).
Lung fibroblasts occupy a unique sentinel position in the interstitium which enables them to relay signals of epithelial injury and modulate the immune response accordingly, for instance, by participating in chemokine regulation and immune cell trafficking. We hypothesise that following epithelial injury, increased expression of the early wave alarm-type cytokine, TNF-α, induces fibroblast TSLP expression, a notion supported by our in vitro findings demonstrating that TNF-α is a potent inducer of TSLP expression and release by primary human lung fibroblasts via activation of JNK/c-Jun. Fibroblast-derived TSLP may then in turn promote a pro-fibrotic type 2 cytokine microenvironment via its well-documented actions on immature DCs.
A number of recent studies have highlighted the potential importance of DCs in the pathogenesis of lung fibrosis (41, 66). In terms of their origin, it has been postulated that activated DCs in IPF originate from a pool of recruited cells which mature locally (41, 67). This is consistent with the evidence that trafficking of DCs to lymph nodes is not a prerequisite for functional T-cell interactions (68), which can occur in situ (69). Although the mediators involved in local DC activation in lung fibrosis have not yet been identified, it is interesting that non-antigenic danger signals for DCs, such as uric acid and ATP (47, 70), have recently been reported to play key roles in the fibroproliferative response to tissue injury (71, 72). In light of these observations and our findings, it is now tempting to speculate that TSLP may promote the activation of DCs recruited to sites of injury. These TSLP-activated DCs (TSLP-DCs) would then be in a position to induce and maintain a local T-2 dominated microenvironment through their ability to induce proliferation of type 2 memory cells which retain their capacity for effector cytokine function. Furthermore, in light of recent evidence suggesting that naive T-cells circulate through non-lymphoid tissues (73), including lung (74), and can differentiate in situ (65), it is also conceivable that TSLP-DCs instruct programmes of type 2 differentiation of naïve T-cells cells locally, thus further promoting the development of a pro-fibrotic type 2 phenotype. Moreover, our observation that pHLFs are capable of upregulating TSLP release in response to a pro-inflammatory stimulus lends further support to the growing evidence that fibroblasts represent an important immunomodulatory cell type in several disease contexts. Indeed, global gene expression studies suggest that the transcriptional profile of fibroblasts may be modified towards a more immuno-centric phenotype following exposure to TNF-α (75). Our data therefore lend further support to the view that fibroblasts be regarded as important sentinel cells, receptive to local tissue injury and capable of choreographing immune cell behaviour (76).
Lung fibroblasts express a functional TSLP receptor complex and release CCL2
A second major novel finding reported in this article is the observation that fibroblasts constitutively express a functional TSLP receptor complex and release CCL2 downstream of TSLP signalling via STAT3. Recent studies have highlighted the importance of STAT3 (19) and STAT5 (8) in mediating functional downstream effects following TSLP receptor activation. We were unable to demonstrate TSLP-induced STAT5 phosphorylation in pHLFs, but found instead that exogenous TSLP promoted STAT3 phosphorylation within 15 minutes. This pattern of STAT activation is also observed in human airway smooth muscle cells (59) and may reflect mesenchymal cell-specific signalling events downstream of TSLP receptor ligation. Our finding that STAT3 mediates CCL2 expression is further consistent with previous observations that STAT3 plays an important role in mediating CCL2 expression in a wide variety of cell types (77-79). Taken together, these observations identify TSLP as a novel mediator of stromal-derived chemokine expression, and are consistent with the recent notion that fibroblasts are capable of generating a “stromal address code” regulating the recruitment of effector immune cells to sites of injury (80). The subsequent functional interaction between recruited immune cells, in the presence of fibroblast-derived TSLP, may serve to promote a local type 2 dominated pro-fibrotic cytokine milieu. During the course of this study, fibrocytes were also reported to express a functional TSLP receptor complex and respond to TSLP by promoting collagen deposition in a model of atopic dermatitis (35). There is some evidence that (myo)fibroblasts, the key effector cells in fibrosis, may be derived from circulating collagen I+/CD34+/CD45RO+ fibrocytes of haematopoietic lineage (81), though whether such cells represent an important source of pathogenic fibroblasts in IPF remains a matter of debate. Interestingly, the intradermal administration of TSLP in mice has been reported to lead to the development of sub-cuticular fibrosis associated with a significant inflammatory cell infiltrate. Taken together, these finding in the skin and our observations in the lung, support the notion that TSLP may contribute to the development of organ fibrosis in a type 2 cytokine-dominated milieu.
Chemokines are best known for their pivotal role in influencing chemotactic responses in a variety of cell types. However, they are also increasingly recognised to play additional important immunoregulatory roles, including regulating T-cell differentiation. Exposure of T cells to CCL2 in vitro promotes type 2 cytokine expression (43), an effect which is enhanced in recently-activated or memory T-cells. This finding is consistent with the observation that the major CCL2 receptor, CCR2, is not expressed by naïve T-cells, but rather by recently activated CD4+ cells (82, 83)While the pathogenic involvement of T cells in IPF remains an unresolved issue, the presence of T cells in fibrotic lung tissue is a consistent observation (84). Moreover, the majority of T cells organised within lymphoid aggregates in IPF display an activated phenotype (41) which would render them susceptible to further modulation by CCL2. Neutralisation of fibroblast-derived CCL2 has been shown to attenuate CD4+ T cell IL-4 production, with a concomitant increase in IFNγ expression (44), suggesting that CCL2 is capable of modulating CD4+ T cell behaviour directly. The importance of these in vitro findings have been confirmed in animal models; despite normal lymphocyte trafficking responses, CCL2 knock-out mice are unable to mount a type 2 immune response (45). It is therefore tempting to speculate that the induction of CCL2 production and release by TSLP serves to amplify the generation of a local Th2 effector cell population at sites of injury, initiated by the effects of TSLP itself on the trafficking of DCs and T-cells. In our monocyte chemotaxis assays, neutralization of CCL2 did not completely block the chemotactic potential of conditioned media from fibroblasts exposed to TSLP. These data suggest that TSLP may induce the release of alternative chemotactic mediators in addition to CCL2. Studies aimed at identifying these mediators are ongoing but are beyond the scope of the current work.
During the course of our studies, a pro-fibrotic role for TSLP was also suggested in the context of the auto-immune disease, systemic sclerosis (SSc) (85). Although both SSc and IPF are characterised by progressive fibrosis, the pathomechanisms underlying these conditions are distinct. Whereas SSc is primarily felt to be an immune-driven disease, current evidence suggests that the fibrotic response in IPF is driven by an aberrant wound healing programme following repetitive epithelial injury. Indeed, recent reports highlighting success in targeting SSc-associated lung pathology with anti-inflammatory strategies (86) are in stark contrast to the dismal failure of such treatment in modifying the natural history of IPF, and again highlights important differences in the underlying pathogenetic mechanisms of these two conditions.
In conclusion, this study extends our current understanding of TSLP (patho)biology beyond that of allergic/atopic inflammation, supporting the notion that this type 2 polarising cytokine may play a role in the pathogenesis of non-allergic diseases characterised by a type 2 phenotype and organ fibrosis. Furthermore, our data demonstrates a previously unrecognised functional TSLP-TSLPR signalling axis in fibroblasts which may contribute to dysregulated remodelling associated with both allergic and non-allergic inflammation. We envisage several potential mechanisms by which TSLP may contribute. First, TSLP might regulate the activation of a pro-fibrotic type 2 immune response following epithelial injury in a manner akin to danger signal/alarmin-induced activation of immature DCs. Second, TSLP may promote the recruitment of immune and inflammatory cells crucial to wound repair to sites of injury. Third, TSLP may further enhance a type 2 cytokine milieu through the generation of immunomodulatory chemokines. We propose that the therapeutic potential of anti-TSLP strategies may therefore ultimately reach beyond allergic inflammatory conditions to include fibroproliferative lung diseases, such as IPF, and possibly other fibrotic conditions including SSc.
Supplementary Material
Acknowledgements
The authors are grateful to Dr Robin McAnulty (UCL, Centre for Inflammation and Tissue Repair) for providing primary human lung fibroblasts.
The authors are grateful to the following grant agencies for funding: Wellcome Trust (A.D. Clinical Research Training Fellowship 084382/Z/07/Z); Medical Research Council UK (C.J.S. Career Development Award G0800340; R.C.C. CASE Award 2009-12); European Commission (R.C.C Framework 7 Programme HEALTH-F2-2007-2224; European IPF Network).
References
- 1.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 2.Heller F, Fromm A, Gitter AH, Mankertz J, Schulzke JD. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol. 2008;1(Suppl 1):S58–61. doi: 10.1038/mi.2008.46. [DOI] [PubMed] [Google Scholar]
- 3.Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff HL, Hogaboam CM, Das AM. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGFbeta1, IL-13 and CCL2. Int J Biochem Cell Biol. 2008;40:2174–2182. doi: 10.1016/j.biocel.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Fuschiotti P. Role of IL-13 in systemic sclerosis. Cytokine. 56:544–549. doi: 10.1016/j.cyto.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Weng HL, Liu Y, Chen JL, Huang T, Xu LJ, Godoy P, Hu JH, Zhou C, Stickel F, Marx A, Bohle RM, Zimmer V, Lammert F, Mueller S, Gigou M, Samuel D, Mertens PR, Singer MV, Seitz HK, Dooley S. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–243. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
- 6.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 7.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, Comeau MR, Campbell DJ, Ziegler SF. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 9.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 10.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 11.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, Corrigan C. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 12.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type2 cells. Eur J Immunol. 2011 doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 19.Wohlmann A, Sebastian K, Borowski A, Krause S, Friedrich K. Signal transduction by the atopy-associated human thymic stromal lymphopoietin (TSLP) receptor depends on Janus kinase function. Biol Chem. 2010;391:181–186. doi: 10.1515/bc.2010.029. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 21.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato A, Favoreto S, Jr., Avila PC, Schleimer RP. TLR3- and Th2 Cytokine-Dependent Production of Thymic Stromal Lymphopoietin in Human Airway Epithelial Cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, Kamijo S, Hiramatsu K, Ikeda S, Ogawa H, Okumura K, Takai T. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol. 126:985–993. 993 e981–983. doi: 10.1016/j.jaci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 25.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol. 2008;28:147–156. doi: 10.1007/s10875-007-9149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H, Nakao A. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L375–382. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 29.Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, Fueki M, Sugiyama K, Takeda K, Fukuda T, Saito H, Ra C. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34:425–435. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 30.Redhu NS, Saleh A, Halayko AJ, Ali AS, Gounni AS. Essential role of NF-kappaB and AP-1 transcription factors in TNF-alpha-induced TSLP expression in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L479–485. doi: 10.1152/ajplung.00301.2009. [DOI] [PubMed] [Google Scholar]
- 31.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 186:5656–5662. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartgring SA, Willis CR, Dean CE, Jr., Broere F, van Eden W, Bijlsma JW, Lafeber FP, van Roon JA. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheum. 63:1878–1887. doi: 10.1002/art.30336. [DOI] [PubMed] [Google Scholar]
- 34.Koyama K, Ozawa T, Hatsushika K, Ando T, Takano S, Wako M, Suenaga F, Ohnuma Y, Ohba T, Katoh R, Sugiyama H, Hamada Y, Ogawa H, Okumura K, Nakao A. A possible role for TSLP in inflammatory arthritis. Biochem Biophys Res Commun. 2007;357:99–104. doi: 10.1016/j.bbrc.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 35.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, Zhu Z, Zheng T. IL-13 Induces Skin Fibrosis in Atopic Dermatitis by Thymic Stromal Lymphopoietin. J Immunol. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 37.Datta A, Scotton CJ, Chambers RC. Novel therapeutic approaches for pulmonary fibrosis. Br J Pharmacol. 2011;163:141–172. doi: 10.1111/j.1476-5381.2011.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc. 2008;5:305–310. doi: 10.1513/pats.200710-160DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the Pathogenesis of Bleomycin-Induced Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2002;27:419–427. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 40.Borowski A, Kuepper M, Horn U, Knupfer U, Zissel G, Hohne K, Luttmann W, Krause S, Virchow JC, Jr., Friedrich K. Interleukin-13 acts as an apoptotic effector on lung epithelial cells and induces pro-fibrotic gene expression in lung fibroblasts. Clin Exp Allergy. 2008;38:619–628. doi: 10.1111/j.1365-2222.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 41.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 42.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, Wolters P, Hill A, Marks JD, Lou J, Pittet JF, Gauldie J, Baron JL, Nishimura SL. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J Clin Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 44.Hogaboam CM, Lukacs NW, Chensue SW, Strieter RM, Kunkel SL. Monocyte chemoattractant protein-1 synthesis by murine lung fibroblasts modulates CD4+ T cell activation. J Immunol. 1998;160:4606–4614. [PubMed] [Google Scholar]
- 45.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 46.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 47.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 48.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 49.Hillion J, Dhara S, Sumter TF, Mukherjee M, Di Cello F, Belton A, Turkson J, Jaganathan S, Cheng L, Ye Z, Jove R, Aplan P, Lin YW, Wertzler K, Reeves R, Elbahlouh O, Kowalski J, Bhattacharya R, Resar LM. The high-mobility group A1a/signal transducer and activator of transcription-3 axis: an achilles heel for hematopoietic malignancies? Cancer Res. 2008;68:10121–10127. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keerthisingam CB, Jenkins RG, Harrison NK, Hernandez-Rodriguez NA, Booth H, Laurent GJ, Hart SL, Foster ML, McAnulty RJ. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, Moffatt JD, Laurent GJ, Derian CK, Eickelberg O, Chambers RC. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorley AJ, Goldstraw P, Young A, Tetley TD. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3alpha)-induced dendritic cell migration. Am J Respir Cell Mol Biol. 2005;32:262–267. doi: 10.1165/rcmb.2004-0196OC. [DOI] [PubMed] [Google Scholar]
- 53.Mercer PF, Johns RH, Scotton CJ, Krupiczojc MA, Konigshoff M, Howell DC, McAnulty RJ, Das A, Thorley AJ, Tetley TD, Eickelberg O, Chambers RC. Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:414–425. doi: 10.1164/rccm.200712-1827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falk W, Goodwin RH, Jr., Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 55.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, Kollias G, Aidinis V. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piguet PF, Ribaux C, Karpuz V, Grau GE, Kapanci Y. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am J Pathol. 1993;143:651–655. [PMC free article] [PubMed] [Google Scholar]
- 57.Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, Miyatake A, Enomoto T, Noguchi E, Yoshihara S, Ebisawa M, Saito H, Matsumoto K, Nakamura Y, Ziegler SF, Tamari M. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 58.Barr RK, Boehm I, Attwood PV, Watt PM, Bogoyevitch MA. The critical features and the mechanism of inhibition of a kinase interaction motif-based peptide inhibitor of JNK. J Biol Chem. 2004;279:36327–36338. doi: 10.1074/jbc.M402181200. [DOI] [PubMed] [Google Scholar]
- 59.Shan L, Redhu NS, Saleh A, Halayko AJ, Chakir J, Gounni AS. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol. 2010;184:7134–7143. doi: 10.4049/jimmunol.0902515. [DOI] [PubMed] [Google Scholar]
- 60.Deng X, Mercer PF, Scotton CJ, Gilchrist A, Chambers RC. Thrombin induces fibroblast CCL2/JE production and release via coupling of PAR1 to Galphaq and cooperation between ERK1/2 and Rho kinase signaling pathways. Mol Biol Cell. 2008;19:2520–2533. doi: 10.1091/mbc.E07-07-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 62.Li YL, Li HJ, Ji F, Zhang X, Wang R, Hao JQ, Bi WX, Dong L. Thymic stromal lymphopoietin promotes lung inflammation through activation of dendritic cells. J Asthma. 2010;47:117–123. doi: 10.3109/02770900903483816. [DOI] [PubMed] [Google Scholar]
- 63.Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA) Clin Exp Immunol. 1995;101:436–441. doi: 10.1111/j.1365-2249.1995.tb03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usategui A, Criado G, Izquierdo E, Del Rey MJ, Carreira PE, Ortiz P, Leonard WJ, Pablos JL. A profibrotic role for thymic stromal lymphopoietin in systemic sclerosis. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2012-202279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christmann RB, Mathes A, Affandi AJ, Padilla C, Nazari B, Bujor AM, Stifano G, Lafyatis R. Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor beta. Arthritis Rheum. 2013;65:1335–1346. doi: 10.1002/art.37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P. A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am J Respir Crit Care Med. 2010;182:385–395. doi: 10.1164/rccm.200907-1164OC. [DOI] [PubMed] [Google Scholar]
- 67.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Kambouchner M, Valeyre D, Crestani B, Soler P. Dendritic cells accumulate in human fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2007;176:1007–1014. doi: 10.1164/rccm.200609-1347OC. [DOI] [PubMed] [Google Scholar]
- 68.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med. 2011;208:1707–1719. doi: 10.1084/jem.20101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 71.Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, Couillin I. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 72.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 73.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 74.Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 75.Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, Martin S, Salmon M, Buckley CD. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb Haemost. 2003;90:688–697. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- 76.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 77.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee HY, Lee SY, Kim SD, Shim JW, Kim HJ, Jung YS, Kwon JY, Baek SH, Chung J, Bae YS. Sphingosylphosphorylcholine stimulates CCL2 production from human umbilical vein endothelial cells. J Immunol. 2011;186:4347–4353. doi: 10.4049/jimmunol.1002068. [DOI] [PubMed] [Google Scholar]
- 79.Schroer N, Pahne J, Walch B, Wickenhauser C, Smola S. Molecular pathobiology of human cervical high-grade lesions: paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Cancer Res. 2011;71:87–97. doi: 10.1158/0008-5472.CAN-10-2193. [DOI] [PubMed] [Google Scholar]
- 80.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Qin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Buthcher EC, Mackay CR. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 83.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luzina IG, Todd NW, Iacono AT, Atamas SP. Roles of T lymphocytes in pulmonary fibrosis. J Leukoc Biol. 2008;83:237–244. doi: 10.1189/jlb.0707504. [DOI] [PubMed] [Google Scholar]
- 85.Denton CP, Ong VH. Targeted therapies for systemic sclerosis. Nature reviews. Rheumatology. 2013 doi: 10.1038/nrrheum.2013.46. [DOI] [PubMed] [Google Scholar]
- 86.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Renzoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Respir J. 2012;40:641–648. doi: 10.1183/09031936.00163911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.