Abstract
Deregulation of apoptosis is a hallmark of human cancer and contributes to therapeutic resistance. Recent advances in cancer genomics reveal a myriad of alterations in key pathways that directly or indirectly increase tumor cell survival. This review will outline the pathways of apoptosis in mammalian cells, and highlight the common alterations of apoptosis regulators found in colon cancer, the role of apoptosis and underlying mechanisms in colon cancer treatment and prevention, including recent advances on investigational agents, such as kinase inhibitors, proteasome inhibitors, HSP90 inhibitors, BH3 mimetics, TRAIL, and IAP antagonists. Topics will also include novel concepts, as well as opportunities and challenges for drug discovery and combination therapy by exploring cancer-specific genetic defects, and therefore selective induction of apoptosis in cancer cells. Although the emphasis is on colon cancer, the main theme and many of the aspects are applicable to other solid tumors.
Keywords: apoptosis, colon cancer, Bcl-2 family, BH3-only protein, mitochondria, death receptor, TRAIL, EGFR, K-RAS, b-Raf, c-Myc, PI3K, IAPs, targeted therapies, sorafenib, regorafinib, vemurafenib, protesome inhibitors, Hsp90 inhibitors, autophagy, necrosis, BH3 mimetics, SMAC mimetics, NSAIDs, synthetic lethality
Introduction
Apoptosis, also known as programmed cell death, is an evolutionally conserved cell suicidal process essential for managing stress and maintaining tissue homeostasis in multi-cellular organisms. It consists of a series of well-ordered biochemical events which are regulated by a network of proteins containing distinctive functional domains. Apoptosis serves as a safeguard mechanism against tumorigenesis. During oncogenic transformation, neoplastic cells become resistant to apoptosis as a result of genetic and epigenetic alterations (1). Defective apoptosis regulation drives other tumorigenic events, such as extended lifespan, accumulation of further genetic mutations, growth under stress conditions, and tumor angiogenesis and metastasis, and contributes to therapeutic resistance (1). Colorectal cancer (CRC) is one of the most common cancers, with estimated 140,000 new cases and over 50,830 deaths in 2013 in the US, and over 1.2 million new cases and 600,000 deaths worldwide (2). Research in colon cancer has provided fundamental insights in cancer biology, treatment and prevention. This review provides an overview of the current understanding of the apoptotic pathways, and their deregulation in colon cancer. Discussions will cover advances in selective induction of apoptosis in neoplastic cells, and explore novel concepts such as crosstalk among cell death and survival pathways and synthetic lethality for cancer drug development and combination.
1. Apoptotic pathways
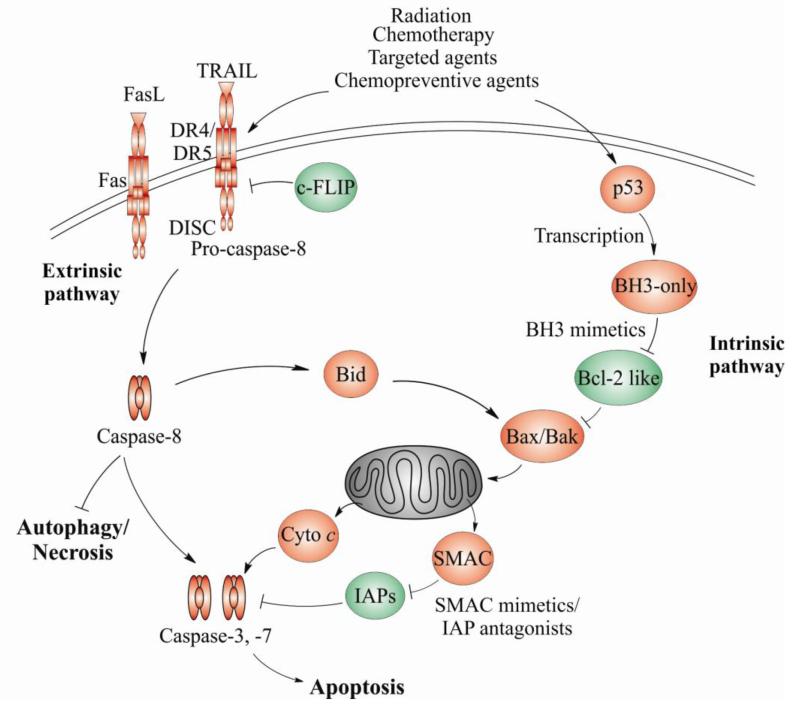
Apoptosis is initiated and executed by a series of well-ordered biochemical events and regulated by complex signaling networks. There are two major apoptotic pathways, termed as the extrinsic and intrinsic pathways (Figure 1), which are responsible for processing stress signals and executing cell demise in mammalian cells. Although distinctively regulated, these two pathways frequently cross-talk with each other.
Figure 1. Coaxing cancer cells into apoptosis.
Apoptosis in mammalian cells is mediated through the intrinsic and extrinsic pathways. The intrinsic pathway is predominantly regulated by the Bcl-2 family and mitochondria. The extrinsic pathway is engaged upon binding of proapoptotic ligands, such as TRAILor Fas to their respective death receptors on the cell surface. Apoptosis initiation is regulated by transcription as well as the cross-talk of two apoptotic pathways via Bid. Apoptosis is activated by anticancer agents, such as radiation, chemotherapy, kinase inhibitors, BH3 or Smac mimetics, and chemopreventive agents acting on both pathways and various players, to trigger a caspase activation cascade. Inhibition of apoptosis or certain caspases such as caspase-8 can lead to increased autophagy or necrosis, providing additional targets for drug development. Antiapoptotic proteins are colored in green while proapoptotic proteins are colored in red. Cyto c, cytochrome c.
The extrinsic apoptotic pathway is engaged upon binding of pro-apoptotic ligands to cell surface death receptors of the tumor necrosis factor receptor (TNFR) family. All death receptors contain a cysteine-rich extracellular domain for ligand binding, and an intracellular death domain for transmitting signals through the recruitment of effectors. Pro-apoptotic ligands belong to the extended cytokine TNF superfamily, and are either present on the cell surface or secreted into the extracellular space (3). Upon binding of proapoptotic ligands to their respective receptors, each receptor can independently form a death-inducing signaling complex (DISC) by recruiting the adapter protein FAS-associated death domain protein (FADD), along with pro-caspase-8 and pro-caspase-10 (Figure 1) (3). FADD recruitment and DISC formation lead to processing and activation of caspase-8 and caspase-10, and their release into the cytoplasm triggers subsequent activation of effector caspases to execute apoptosis (Figure 1) (3). FLICE-like inhibitory protein (FLIP) is a caspase-like protein lacking the catalytic function, which suppresses caspase-8 activation (4).
The intrinsic apoptotic pathway is triggered by stresses such as DNA damage, deregulated oncogenes or growth factor deprivation, and is largely regulated by the Bcl-2 family of proteins and mitochondria (5) (Figure 1). For example, DNA damage activates the tumor suppressor p53, which transmits death signals by inducing the expression of BH3-only proteins to allow for the activation of Bax and Bak. This in turn leads to mitochondrial outer membrane permeabilization (MOMP) (6), and release of several mitochondrial apoptogenic proteins, including cytochrome c, SMAC/Diablo, Omi/HtrA2, AIF (Apoptosis-inducing factor), and EndoG (Endonuclease G). Cytochrome c promotes the assembly of apoptosome and subsequent activation of caspase 9, which is facilitated by SMAC/Diablo and HtrA2/Omi, which antagonize the inhibitors of apoptosis (IAP) family of proteins. AIF and Endo G promote DNA degradation (7-8). In cells with low levels of DISC, caspase-8-dependent cleavage of the BH3-only protein Bid generates truncated Bid (tBid) to amplify apoptotic signaling via Bax/Bak and the mitochondria (Figure 1) (5).
2. Deregulation of apoptosis in cancer
Maintenance of tumor phenotypes is highly dependent on apoptosis suppression by certain pro-survival proteins, to evade the fate of cell killing imposed by “oncogenic stress” during neoplastic transformation. Many key pathways controlling apoptosis and cell survival are therefore commonly altered in cancer (1, 9**). Genetic alterations are generally found in the more proximal components rather than the core apoptotic machinery or downstream effectors, suggesting a strong selective pressure during carcinogenesis to disable nodal points critical for apoptosis initiation, such as p53. Data from genetically manipulated mice also support apoptosis as an important mechanism for tumor suppression in the intestinal tract.
Alterations in the proximal regulators
Transcription factors
Apoptosis initiation is heavily regulated at the level of gene expression. The best studied and highly relevant to cancer are p53 and NF-κB transcription factors. p53 is essential for preventing inappropriate cell proliferation and for maintaining genome integrity following genotoxic stress, and is mutated or inactivated in over half of human cancers (6, 9). Tumor-derived p53 mutants are defective in transcription and apoptosis induction (6, 10). NF-κB plays a key role in cancer and inflammation, and its overexpression or activation is linked to chemoresistance (11). Upon stimulation by pro-inflammatory cytokines, cytoplasmic NF-κB is released from inhibitor of κB (IκB), and translocates into the nucleus to regulate gene expression. Well-known antiapoptotic targets of NF-κB include Bcl-2-like proteins, IAPs and c-FLIP (11).
Protein kinases
Kinases are key regulators of normal cell physiology and play critical roles in the development and progression of human cancer. Aberrant activation of receptor tyrosine kinases (RTKs) and non-RTKs ultimately contributes to tumor initiation and maintenance of tumor phenotypes such as cell survival, proliferation, migration, and tumor angiogenesis (1, 9), and confers resistance to cancer therapy (12**). Aberrant activation of RTKs, such as epidermal growth factor receptor (EGFR, erbB1), hepatocyte growth factor (HGF) receptor MET, platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR), engages intracellular kinase cascades such as Ras/b-Raf/ERK and phophotidylinositol-3-kinase (PI3K)/AKT/mTOR. Activation of these non-RTKs is also caused by gene mutations and amplifications in colon cancer (9).
Alterations in the Bcl-2 family of proteins
Antiapoptotic Bcl-2 family members are frequently overexpressed in solid tumors, while proapoptotic Bcl-2 family members are downregulated or mutated (5). In rare cases, homozygous deletions or inactivating mutations of BAX are found, particularly in DNA mismatch repair deficient colorectal tumors (13). The activities or levels of proapoptotic proteins are often suppressed by oncogenic kinases. For example, PI3K/AKT or ERK inactivates BAD by phosphorylation (14), and suppresses PUMA induction (15-17). PUMA ablation in mice leads to decreased apoptosis and increased tumor burden and invasiveness in the GI tract (18**).
Alterations in the extrinsic pathway and other apoptotic components
In contrast to the deregulation in the upstream regulators or Bcl-2 family, direct impairments of the extrinsic pathway and downstream apoptotic effectors by genetic alterations are generally rare in human cancer, while altered expression is common (3, 19-20). Overexpression of c-FLIP (4), particularly c-FLIP-L, functions as a survival factor in colon cancer (21). Elevated XIAP (22), cIAP2 (23) or reduced SMAC expression (24), are correlated with disease progression, metastasis or poor survival in colon cancer patients. SMAC levels were inversely correlated with cIAP2 levels and tumor grade, and its ablation accelerates cancer progression in mice (25). Lost expression or deletion of DR4 and DR5, overexpression of decoy receptors DcR3 or DcR1 (3), Caspase-8 (CASP8) polymorphisms or deletion have been reported in other cancers, however not in colon cancer.
3. Apoptosis induction in colon cancer treatment and resistance mechanisms
Induction of apoptosis is a major cytotoxic mechanism of anticancer therapies, including radiation, chemotherapy, and targeted therapies (Figure 1) (3, 5, 12, 26). Targeted therapies are expected to have improved specificity and reduced toxicity, and ultimately help move oncology practice toward personalized treatment (27). Cancer cells can develop various mechanisms to evade apoptosis. Deficiency in p53, Bax, PUMA, or caspase activation can cause resistance to radiation and chemotherapy, while overexpression of antiapoptotic proteins such as Bcl-XL, c-FLIP and IAPs are frequently associated with therapeutic resistance. Mutations in kinases and their cross-talk modulate therapeutic responses to targeted therapies in patients.
Radiation and chemotherapy
Radiation and the majority of conventional chemotherapeutic agents induce DNA damage. Following DNA damage, p53 undergoes extensive post-translational modifications, including phosphorylation and acetylation, which stabilize p53 and enhance its transcriptional activities (6). p53 mediates DNA damage-induced apoptosis primarily via the intrinsic pathway, through the BH3-only protein PUMA, and Noxa to a lesser extent, in various cancer cells and normal tissues (28-30). In addition, DR5 and PIDDosome/caspase 2 (31) are implicated in 5-FU-induced apoptosis in colon cancer cells, which is partially dependent on p53 (32). Under some conditions, cytoplasmic p53 inactivates Bcl-2 like proteins at the mitochondria (33), though the significance and mechanisms are not well understood. Moreover, genotoxic stress can activate p53 family proteins such as p73 and p63, which share many proapoptotic targets with p53 (34).
On the other hand, p53 plays a key role in the pathologic apoptosis of tissue stem cells and acute side effects induced by radiation and chemotherapy in the bone marrow and GI tract (29-30, 35). Given the prevalence of p53 alterations in cancer (6), it is tempting to speculate that enhancement of p53-independent induction of apoptosis genes in cancer cells and/or suppression of p53-dependent apoptosis in normal tissues can be exploited to widen the therapeutic index.
Targeted therapies
Anti-EGFR therapy
EGFR-targeting agents, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have been used to treat different tumor types. EGFR TKIs are particularly useful in treating a subset of lung cancers harboring oncogenic EGFR mutations. Cetuximab and panitumumab, two anti-EGFR monoclonal antibodies, are effective in combination with chemotherapy or as single agents for the treatment of metastatic colon cancer. However, mutations in K-RAS, b-Raf, and PIK3CA are associated with resistance to anti-EGFR therapies (27). Apoptosis is induced by EGFR TKIs in a variety of tumor cells through the intrinsic pathway by modulating the BH3-only proteins, such as Bim or PUMA (17, 36-37). Clinical response to EGFR TKIs is correlated with induction of apoptosis in tumor cells, and defective apoptosis regulation contributes to resistance of EGFR-targeted therapy (38).
Anti-VEGF therapy
Bevacizumab (Avstin), a recombinant humanized monoclonal antibody against VEGF, is used in combination with conventional chemotherapy for treating metastatic colorectal cancer. Several studies suggest induction of apoptosis in tumor cells contributes to the therapeutic effect of Bevacizumab. In xenograft models, antitumor effects of Bevacizumab are correlated with hypoxia-induced apoptosis (39). Combined use of Bevacizumab and irinotecan (CPT-11) strongly induced apoptosis and improved therapeutic efficacy against lung metastasis from colon cancer cells (40). In Bevacizumab-treated metastatic colon cancer patients, the serum level of proapoptotic death receptor ligand TRAIL (sTRAIL) was found to be elevated, which was correlated with patient survival (41-42).
Sorafenib/Regorafinib
Sorafenib (Nexavar) is the first FDA-approved oral multi-kinase inhibitor drug that targets the RAS/Raf/MEK/ERK pathway. Sorafenib inhibits c-Raf, b-Raf, PDGFR, and VEGFRs, and has been used for the treatment of advanced kidney and liver tumors (43-44). Regorafenib (BAY 73-4506, Stivarga), a Sorafenib analogue with an extra fluoro group, has been recently approved for the treatment of metastatic colorectal cancer and advanced gastrointestinal stromal tumors that are resistant to other therapies. The anticancer effects of Sorafenib/Regorafenib are thought to be mediated by apoptosis induction, in addition to its anti-proliferative and anti-angiogenic effects, and are enhanced when combined with cytotoxic agents (43-44). Sorafenib-induced apoptosis is associated with downregulation of Mcl-1 and inhibition of eIF4E (eukaryotic translation initiation factor 4E) phosphorylation (45-46), as well as the induction of PUMA through NF-κB in colon cancer cells (47*), and Bim in leukemia cells (48).
Other targeted therapies and investigational agents
Numerous investigational drugs are in the various stages of clinical development for colon cancer and found to stimulate apoptosis. Vemurafenib (PLX4032), a selective inhibitor of b-Raf V600E mutant, has shown significant activity in b-Raf mutant (V600E) melanomas, but only very limited efficacy in colon cancers with the same mutation. The intrinsic and acquired resistance to b-Raf inhibitors involves feedback activation of the PI3K/AKT, mTOR, or EGFR pathway, and can be overcome by the inhibitors of these pathways via apoptosis induction (49-51*). Non-selective kinase inhibitors such as UCN-01(15) or Sunitinib (52) induced PUMA-dependent, but p53-independent apoptosis in colon cancer cells. Met/ALK inhibitors such as crizotinib induced both p53-dependent and -independent apoptosis, and synergized with EGFR-TKI or Raf inhibitors to induce PUMA and apoptosis (53*). Additional inhibitors against Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways are in development (12). Therefore, systematic approaches are needed to determine optimal use of these agents in the correct patients (see synthetic lethality).
In addition to kinase inhibitors, several other classes of agents also induce apoptosis in cancer cells. The proteasome inhibitor Bortezomib (Velcade), approved for treating multiple myeloma, triggers apoptosis alone or in combination with chemotherapy by modulating the Bcl-2 family and NF-κB pathway (54-55). Heat shock protein 90 (Hsp90) is one of the most abundant molecular chaperones in eukaryotes and expressed at much higher levels in tumor cells. Currently, over 17 Hsp90 inhibitors have entered clinical trials. These agents are found to induce apoptosis and overcome resistance to bRaf or PI3K/ATK/mTOR inhibitors in preclinical models (56). Several proapoptotic mechanisms, likely to be cell-type specific, have been suggested, including down-regulation of Hsp90 client oncoproteins such as mutant p53, Raf-1, AKT, NF-κB, Her2 (56), or activation of wild-type p53 (57).
4. Apoptosis and cancer prevention
Cancer prevention is expected to have a great impact on cancer mortality. Chemoprevention is a promising anti-cancer strategy, referring to the use of pharmacologic agents or natural products to intervene early in the process of tumor development (58). Epidemiological studies, clinical trials and animal studies have shown that the widely used non-steroidal anti-inflammatory drugs (NSAIDs) prevent intestinal cancer in human and mice, particularly in high-risk individuals who carry germ-line APC mutations (9, 59). NSAIDs are also useful in preventing other types of cancer, and as an adjuvant therapy (60).
Several anticancer mechanisms of NSAIDs and other cancer preventive agents have been described, such as inhibition of cyclooxygeneses (COXs) enzymes (60). and induction of apoptosis (61). Mechanistic studies demonstrated that Bax and SMAC/Diablo mediate the anti-cancer effects of NSAIDs by promoting apoptotic cell death (62-65*), likely by crosstalk between the death receptor and the mitochondrial apoptotic pathways (Figure 1). Manipulating apoptosis or using a combination of different classes of chemopreventive agents can enhance the effectiveness of NSAIDs (64), and reduce toxicity by lowering the doses (66).
One of the earliest events in colorectal tumorigenesis is genetic alterations in the APC tumor suppressor pathway leading to aberrant Wnt signaling and subsequent nuclear translocation of β-catenin, and activation of oncogenes such as c-Myc and cyclin D1 (9), and likely targeting the intestinal stem cells (67). NSAID treatment selectively induced apoptosis to remove intestinal stem cells with aberrant Wnt signaling (65). Adenoma samples from patients taking NSAIDs were also found to have enhanced apoptosis induction in cells with expression of stem cell markers and aberrant Wnt signaling (65), suggesting that NSAIDs eliminate stem cells that have acquired oncogenic mutations to prevent intestinal tumorigenesis.
5. Apoptosis cross talk with other death and survival mechanisms
Although apoptosis is a major mode of cell death in response to anticancer agents, several alternative cell death pathways have recently been identified, in particular autophagy and programmed necrosis (Figure 1). These pathways are engaged in response to specific stimuli, upon a restricted condition, or within a time window during cell death. The interplay among different cell death pathways can sometimes play a critical role in determining therapeutic response and development of drug resistance, and is partially mediated by the competition of shared regulators or adaptors.
Autophagy
Autophagy is a self-eating process of cells that has recently emerged as an important stress response and cell death mechanism, and regulated by a group of evolutionarily conserved autophagy-related genes (ATGs) (68). It is characterized by the formation double membrane autophagosomes, which fuse with lysosomes to promote degradation of damaged organelles or cytosolic materials (69). Autophagy can either promote or inhibit cell death, and is often cytoprotective in cancer cells (70). Inhibition of autophagy sensitizes cancer cells to chemotherapy, and autophagy inhibitors such as hydroxychloroquine are being tested in a number of clinical trials (71). Autophagy interconnects with apoptosis in a number of ways (72). Bcl-2-interacting protein-1 (Beclin 1), a key regulator of autophagy, contains a BH3 domain that can interact with Bcl-2 and Bcl-XL (73), which inhibit autophagy (74). Several ATG proteins, including Beclin 1, ATG5 and ATG4D, can be cleaved by caspases and other proteases during apoptosis (75-76). Specific ablation of caspase-8-mediated Beclin 1 cleavage restored autophagy in apoptotic cells, and enhanced cancer cell survival and therapeutic resistance (77*).
Necrosis
Necrosis is characterized by ATP depletion, swelling of organelles and spilling of cellular contents, and some forms of necrosis are found to be regulated. In the absence of cIAPs, TNFα stimulates the formation of a secondary cytoplasmic complex similar to DISC, leading to rapid activation of caspase 8 and subsequently apoptosis (64, 78-79), which is largely blocked by caspase inhibitors in most cell types. In some cells, caspase inhibitors or genetic ablation of caspase-8 can switch the apoptotic response to necrosis mediated by receptor-interacting protein kinase 1 (RIPK1 or RIP1) or RIPK3 (80-81*). Necrosis often leads to inflammation, whose significance in cancer biology and therapy and underlying mechanisms remain to be elucidated.
6. Apoptosis targeted agents in development for cancer therapy
Several strategies have been devised to induce cell killing by manipulating apoptotic regulators directly. Small-molecule or antisense inhibitors of pro-survival Bcl-2 family members or the IAP antagonists promote apoptosis by inhibiting antiapoptotic signaling within the cells. The protein-based agents trigger apoptosis from cell surface by stimulating death receptors. Most of these agents have limited toxicity to cancer cells as single agents, while potently synergize with radiation or conventional chemotherapy in preclinical studies.
Bcl-2 antagonists
Compared to peptidyl and small-molecule BH3 mimetics, antisense oligonucleotides against various antiapoptotic Bcl-2 family members have limited success. Only the Bcl-2 antisense oligo oblimersen advanced into clinic trials. Due to more desirable pharmacological properties, extensive preclinical studies have been conducted on various BH3 mimetics, including HA14-1, Antimycin A and analogs, BH3Is, gossypol and analogs, GX015-070 (82). The most promising BH3 mimetic is ABT-737, which potently inhibits Bcl-2 and Bcl-XL, but not MCL-1, to induce Bax/Bak-dependent apoptosis (83-84). As a single agent, ABT-737 and most BH3 mimetics had limited efficacy in selective types of cancer cells (83), but they exhibited striking synergy with a variety of anticancer agents in colon cancers (15, 47, 52). ABT-263, an orally available derivative of ABT-737, is currently being evaluated in clinical trials (85).
Death receptor ligands or agonists
Recombinant human TRAIL (rhTRAIL) has shown promise in both preclinical and clinical studies, suggesting the importance of DR4 and DR5 in cancer cell killing (19). Deficiency in CASP8 (3, 19), BAX (62) or SMAC (63) , but not p53 (3) is associated with TRAIL resistance. Mapatumumab (HGS-ETR1) is the only human agonistic antibody for DR4 that has advanced into clinical trials, showing some efficacy in combination regimens (3). The clinical outlook of FasL or TNF- is limited due to severe side effects associated with inflammation (3).
IAP antagonists
SMAC/Diablo and Omi/HtrA2 are endogenous IAP inhibitors, also called IAP antagonists, and relieve the antiapoptotic activities of several IAPs (20). The synthesis of SMAC mimetics that resemble either monomeric or dimeric N-terminal IAP-binding AVPI peptide of SMAC is a great example of structure-based drug design (20, 86). Both monovalent and divalent SMAC mimetics are currently in Phase I and Phase II trials (20, 86). As single agents, SMAC mimetics exert little or no toxicity in non-malignant human cells, and only induce apoptosis in a limited number of cancer cell lines via TNFα production (79). SMAC mimetics or forced SMAC expression sensitizes cancer cells to apoptosis induced by various classes of anticancer agents, including chemotherapeutics, radiation, death receptor agonists and kinase inhibitors, by enhancing caspase activation (64), release of endogenous SMAC (87) and cIAP1/2 degradation (20).
7. Exploring synthetic lethality in cancer therapy and prevention
The concept of synthetic lethality borrowed from yeast genetics has recently been used to discover new anticancer agents or targets based on cancer-specific genetic changes (88). Using isogenic cells combined with siRNA or small-molecule library screens has identified several synthetic lethal interactions in colon cancer cells. For example, K-RAS mutation is synthetic lethal with the inhibition of the mitotic machinery, proteasome (89), kinase STK33 (90) and TAK1 (91), transcription factor GATA2, DNA replication factor CDC6 (92), and MEK/Bcl-XL (93*). c-Myc overexpression is synthetic lethal with Aurora B kinase inhibition (94). Interestingly, TRAIL is synthetic lethal with either RAS or c-Myc (95-96). Cell killing in these settings is likely mediated through apoptosis. Synthetic lethal interactions with DNA repair deficiencies are of therapeutic interest, such as the PARP inhibitors with BRCA1/2 deficiency in a subset of ovarian or breast cancer (97), and mismatch repair proteins MSH2 and DNA polymerase (polβ), and MLH1 with polγ in colon cancer cells (98-99).
Discovery of novel synthetic lethal interactions has important implications in cancer treatment and prevention. RAS mutant tumors account for 40-50% of colon cancer, while c-Myc overexpression is an early and nearly universal event in colon cancer resulting from the loss of tumor suppressor APC (9). The advantages of this approach include reduction of side effects by using lower doses of conventional therapies, and increase in therapeutic efficacy through rationale combinations (100*). It is likely that cancer-driver mutations play a key role in the understanding of gene-gene, gene-drug interactions and therapeutic resistance as discussed before.
Conclusion
Apoptosis in mammalian cells is regulated by two major pathways. Integration of apoptotic signals is achieved through the cross-talk among many upstream signals and downstream effectors, influencing the decision between life and death. Alterations in the apoptotic pathways are complex, with genetic alterations found mostly in the upstream regulatory proteins, providing many potential targets for drug development. One of the main goals of cancer research is to develop safer and more effective therapies, which might capitalize on a better understanding of cancer genome as well as cancer-specific synthetic lethal interactions. A great challenge ahead is to understand in biochemical detail the integration modules and nodes of apoptotic pathways to help drug development and delineation of therapeutic resistance mechanisms. Another big challenge is to identify biomarkers that can help stratify patients for treatments and follow-up. Such efforts are expected to ultimately lead towards personalized medicine.
Acknowledgements
Research in the authors’ laboratories is supported by National Institutes of Health (NIH) grants CA106348, CA121105, CA172136, the V Foundation for Cancer Research (L.Z.), and American Cancer Society grant RSG-10-124-10-CCE, and NIH grants CA129829 and U01DK085570 (J.Y.).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Lin Zhang has received compensation for U.S. patents 5,695,937; 5,866,330; 6,383,743; 6,746,845; and 6,333,152, and has received royalties from Johns Hopkins University for the preparation of genetically engineered cells.
Jian Yu has received compensation for U.S. patent WO 02/064790, and has received royalties from Johns Hopkins University for the preparation of genetically engineered cells.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interests, published recently, have been highlighted as
*Of importance
**Of major importance
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008 Dec;7(12):1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 4.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010 Feb;42(2):210–3. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007 Feb 26;26(9):1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009 May 1;137(3):413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Joza N, Susin SA, Daugas E, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001 Mar 29;410(6828):549–54. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science. 2002 Nov 22;298(5598):1587–92. doi: 10.1126/science.1076194. [DOI] [PubMed] [Google Scholar]
- 9**.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr., Kinzler KW. Cancer genome landscapes. Science. 2013 Mar 29;339(6127):1546–58. doi: 10.1126/science.1235122. This review summarizes key genetic alterations from recent genomics studies on human cancers including colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005 Jun 10;331(3):851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010 Mar 19;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.McCubrey JA, Steelman LS, Kempf CR, et al. Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol. 2011 Nov;226(11):2762–81. doi: 10.1002/jcp.22647. This review summarizes key findings on the therapeutic responses to various targeted agents and mutations in key survival pathways in human cancer, including colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 13.Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997 Feb 14;275(5302):967–9. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 14.Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007 Sep 15;6(18):2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- 15.Dudgeon C, Wang P, Sun X, et al. PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Mol Cancer Ther. 2010 Nov;9(11):2893–902. doi: 10.1158/1535-7163.MCT-10-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 Activate PUMA Following Serum Starvation. Carcinogenesis. 2008 Jun 25;2(9):9–1878. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Q, Ming L, Thomas SM, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009 May 4;18(28):2348–57. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Qiu W, Carson Walter EB, Kuan SF, Zhang L, Yu J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009 Jun 15;69(12):4999–5006. doi: 10.1158/0008-5472.CAN-09-0262. This study showed that blocked apoptosis increases intestinal cancer initiation and invasiveness in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008 Oct;8(10):782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 20.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012 Feb;11(2):109–24. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 21.Wilson TR, McLaughlin KM, McEwan M, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007 Jun 15;67(12):5754–62. doi: 10.1158/0008-5472.CAN-06-3585. [DOI] [PubMed] [Google Scholar]
- 22.Xiang G, Wen X, Wang H, Chen K, Liu H. Expression of X-linked inhibitor of apoptosis protein in human colorectal cancer and its correlation with prognosis. J Surg Oncol. 2009 Dec 15;100(8):708–12. doi: 10.1002/jso.21408. [DOI] [PubMed] [Google Scholar]
- 23.Krajewska M, Kim H, Kim C, et al. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res. 2005 Aug 1;11(15):5451–61. doi: 10.1158/1078-0432.CCR-05-0094. [DOI] [PubMed] [Google Scholar]
- 24.Endo K, Kohnoe S, Watanabe A, et al. Clinical significance of Smac/DIABLO expression in colorectal cancer. Oncol Rep. 2009 Feb;21(2):351–5. [PubMed] [Google Scholar]
- 25.Qiu W, Liu H, Sebastini A, et al. An apoptosis-independent role of SMAC in tumor suppression. Oncogene. 2012 Jul;:2. doi: 10.1038/onc.2012.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Zhang L. Apoptosis in human cancer cells. Curr Opin Oncol. 2004 Jan;16(1):19–24. doi: 10.1097/00001622-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Martini M, Vecchione L, Siena S, Tejpar S, Bardelli A. Targeted therapies: how personal should we go? Nat Rev Clin Oncol. 2012 Feb;9(2):87–97. doi: 10.1038/nrclinonc.2011.164. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008 Dec;27(Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu W, Carson Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008 Jun;2(6):576–83. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010 Apr 29;115(17):3472–80. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson M, Vakifahmetoglu H, Abruzzo PM, Hogstrand K, Grandien A, Zhivotovsky B. DISC-mediated activation of caspase-2 in DNA damage-induced apoptosis. Oncogene. 2009 May 7;28(18):1949–59. doi: 10.1038/onc.2009.36. [DOI] [PubMed] [Google Scholar]
- 32.Bunz F, Hwang PM, Torrance C, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999 Aug;104(3):263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent proapoptotic functions of p53. Curr Opin Cell Biol. 2005 Dec;17(6):631–6. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Dotsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol. 2010 Sep;2(9):a004887. doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007 Oct 9;4(10):e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa DB, Halmos B, Kumar A, et al. 2007 Oct;4(10):1669–79. doi: 10.1371/journal.pmed.0040315. discussion 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004 Aug 20;305(5687):1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 39.Selvakumaran M, Yao KS, Feldman MD, O’Dwyer PJ. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem Pharmacol. 2008 Feb 1;75(3):627–38. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizobe T, Ogata Y, Murakami H, et al. Efficacy of the combined use of bevacizumab and irinotecan as a postoperative adjuvant chemotherapy in colon carcinoma. Oncol Rep. 2008 Sep;20(3):517–23. [PubMed] [Google Scholar]
- 41.Kargi A, Yalcin AD, Erin N, Savas B, Polat HH, Gorczynski RM. IL8 and serum soluble TRAIL levels following anti-VEGF monoclonal antibody treatment in patients with metastatic colon cancer. Clinical laboratory. 2012;58(5-6):501–5. [PubMed] [Google Scholar]
- 42.Bisgin A, Kargi A, Yalcin AD, et al. Increased serum sTRAIL levels were correlated with survival in bevacizumab-treated metastatic colon cancer. BMC cancer. 2012:12–58. doi: 10.1186/1471-2407-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 44.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 45.Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005 Oct 20;24(46):6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006 Dec 15;66(24):11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 47*.Dudgeon C, Peng R, Wang P, Sebastiani A, Yu J, Zhang L. 2012 Jan;30 doi: 10.1038/onc.2011.644. 2012 Jan. [Epub ahead of print]. This study showed that sorafenib induces apoptosis in colon cancer cells by inducing the BH3-only protein PUMA through the NF-κB pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008 Apr;22(4):808–18. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 49.Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013 Feb 1;19(3):657–67. doi: 10.1158/1078-0432.CCR-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012 Apr;11(4):909–20. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 51*.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012 Mar 1;483(7387):100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Sun Q, Brown MF, et al. The Multi-Targeted Kinase Inhibitor Sunitinib Induces Apoptosis in Colon Cancer Cells via PUMA. PLoS One. 2012;7(8):e43158. doi: 10.1371/journal.pone.0043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Zheng X, He K, Zhang L, Yu J. Crizotinib induces PUMA-dependent apoptosis in colon cancer cells. Mol Cancer Ther. 2013 May;12(5):777–86. doi: 10.1158/1535-7163.MCT-12-1146. This study showed that ALK/Met inhibitor Crizotinib promotes PUMA-dependent apoptosis through both p53-dependent and independent mechanisms in colon cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J, Tiwari S, Steiner P, Zhang L. Differential Apoptotic Response to the Proteasome Inhibitor Bortezomib [VELCADE(TM), PS-341] in Bax-Deficient and p21-Deficient Colon Cancer Cells. Cancer Biol Ther. 2003 Nov-Dec;2(6):694–9. [PubMed] [Google Scholar]
- 55.Milano A, Iaffaioli RV, Caponigro F. The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer. 2007 May;43(7):1125–33. doi: 10.1016/j.ejca.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 56.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clinical Cancer Research. 2012 Jan 1;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaseva AV, Yallowitz AR, Marchenko ND, Xu S, Moll UM. Blockade of Hsp90 by 17AAG antagonizes MDMX and synergizes with Nutlin to induce p53-mediated apoptosis in solid tumors. Cell death & disease. 2011;2:e156. doi: 10.1038/cddis.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lippman SM. The future of molecular-targeted cancer chemoprevention. Gastroenterology. 2008 Dec;135(6):1834–41. doi: 10.1053/j.gastro.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 59.Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Drug Targets. 2004 Feb;4(1):29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]
- 60.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001 Oct;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 61.Sun SY, Hail N, Jr., Lotan R. Apoptosis as a novel target for cancer chemoprevention. J Natl Cancer Inst. 2004 May 5;96(9):662–72. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000 Nov 3;290(5493):989–92. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 63.Kohli M, Yu J, Seaman C, et al. SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci U S A. 2004 Nov 30;101(48):16897–902. doi: 10.1073/pnas.0403405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bank A, Wang P, Du C, Yu J, Zhang L. SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced apoptosis by promoting caspase-3-mediated cytochrome c release. Cancer Res. 2008 Jan 1;68(1):276–84. doi: 10.1158/0008-5472.CAN-07-5242. [DOI] [PubMed] [Google Scholar]
- 65**.Qiu W, Wang X, Leibowitz B, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010 Nov 1;107(46):20027–32. doi: 10.1073/pnas.1010430107. This study used a mouse tumor model to show that intestinal stem cells containing gatekeeper mutations are the key target by chemoprevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyskens FL, Jr., McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008 Jun;1(1):32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2008 Dec 17;457(7229):608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 68.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008 Jan 11;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010 Apr;22(2):124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006 Jul;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009 Sep 1;15(17):5308–16. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007 Sep;8(9):741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 73.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007 Apr 27;282(17):13123–32. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 74.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005 Sep 23;122(6):927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006 Oct;8(10):1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 76.Djavaheri Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010 Mar 25;29(12):1717–9. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 77*.Li H, Wang P, Sun Q, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011 May 15;71(10):3625–34. doi: 10.1158/0008-5472.CAN-10-4475. This study used a knock-in approach to demonstrate a cross-talk mechanism between chemotherapy-induced apoptosis and autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008 May 16;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 79.Petersen SL, Wang L, Yalcin Chin A, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007 Nov;12(5):445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaiser WJ, Upton JW, Long AB, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011 Mar 17;471(7338):368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81**.Oberst A, Dillon CP, Weinlich R, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011 Mar 17;471(7338):363–7. doi: 10.1038/nature09852. This study provided mechanistic insight on how apoptosis and programmed necrosis are co-regulated sharing components of the extrinsic apoptotic pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007 Dec;10(6):207–17. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 84.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006 Nov;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008 May 1;68(9):3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 86.Wang S. Design of small-molecule Smac mimetics as IAP antagonists. Curr Top Microbiol Immunol. 2011;3(48):48–89. doi: 10.1007/82_2010_111. [DOI] [PubMed] [Google Scholar]
- 87.Sun Q, Zheng X, Zhang L, Yu J. Smac Modulates Chemosensitivity in Head and Neck Cancer Cells through the Mitochondrial Apoptotic Pathway. Clin Cancer Res. 2011 Jan;:17. doi: 10.1158/1078-0432.CCR-10-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278(5340):1064–8. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 89.Luo J, Emanuele MJ, Li D, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009 May 29;137(5):835–48. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scholl C, Frohling S, Dunn IF, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009 May 29;137(5):821–34. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 91.Singh A, Sweeney MF, Yu M, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 2012 Feb 17;148(4):639–50. doi: 10.1016/j.cell.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steckel M, Molina Arcas M, Weigelt B, et al. Determination of synthetic lethal interactions in KRAS oncogene-dependent cancer cells reveals novel therapeutic targeting strategies. Cell Res. 2012 Aug;22(8):1227–45. doi: 10.1038/cr.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93*.Corcoran RB, Cheng KA, Hata AN, et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013 Jan 14;23(1):121–8. doi: 10.1016/j.ccr.2012.11.007. This study showed that co-targeting Bcl-XL and MEK triggered a synthetic lethal interaction in KRAS mutant colorectal tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang D, Liu H, Goga A, Kim S, Yuneva M, Bishop JM. Therapeutic potential of a synthetic lethal interaction between the MYC proto-oncogene and inhibition of aurora-B kinase. Proc Natl Acad Sci U S A. 2010 Aug 3;107(31):13836–41. doi: 10.1073/pnas.1008366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Quon KC, Knee DA, Nesterov A, Kraft AS. 2005 Feb 15;65(4):1615–6. doi: 10.1158/0008-5472.CAN-04-2757. author reply 6-7. [DOI] [PubMed] [Google Scholar]
- 96.Nieminen AI, Partanen JI, Hau A, Klefstrom J. c-Myc primed mitochondria determine cellular sensitivity to TRAIL-induced apoptosis. Embo J. 2007 Feb 21;26(4):1055–67. doi: 10.1038/sj.emboj.7601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008 Aug 1;26(22):3785–90. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 98.Martin SA, McCabe N, Mullarkey M, et al. DNA polymerases as potential therapeutic targets for cancers deficient in the DNA mismatch repair proteins MSH2 or MLH1. Cancer Cell. 2010 Mar 16;17(3):235–48. doi: 10.1016/j.ccr.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin SA, Hewish M, Sims D, Lord CJ, Ashworth A. Parallel high-throughput RNA interference screens identify PINK1 as a potential therapeutic target for the treatment of DNA mismatch repair-deficient cancers. Cancer Res. 2011 Mar 1;71(5):1836–48. doi: 10.1158/0008-5472.CAN-10-2836. [DOI] [PubMed] [Google Scholar]
- 100*.Chan DA, Giaccia AJ. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov. 2011 May;10(5):351–64. doi: 10.1038/nrd3374. This review discusses potential use of synthetic lethal interactions for discovering new anticancer drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]