Abstract
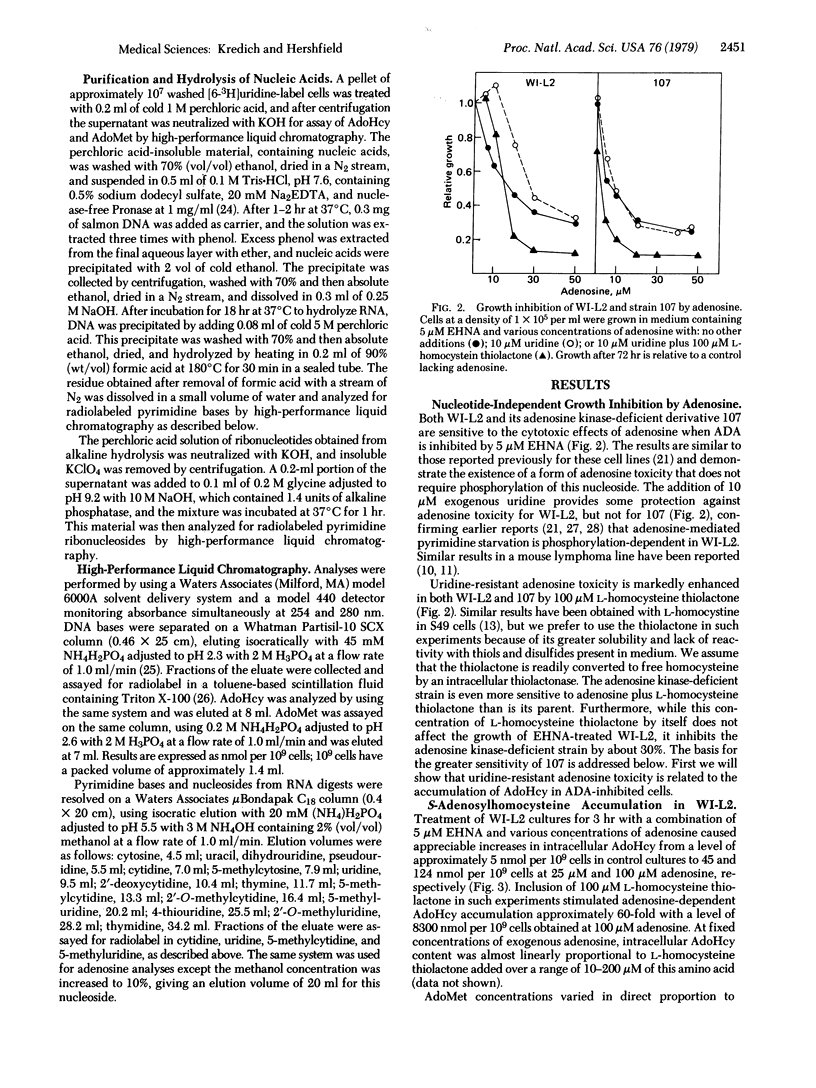
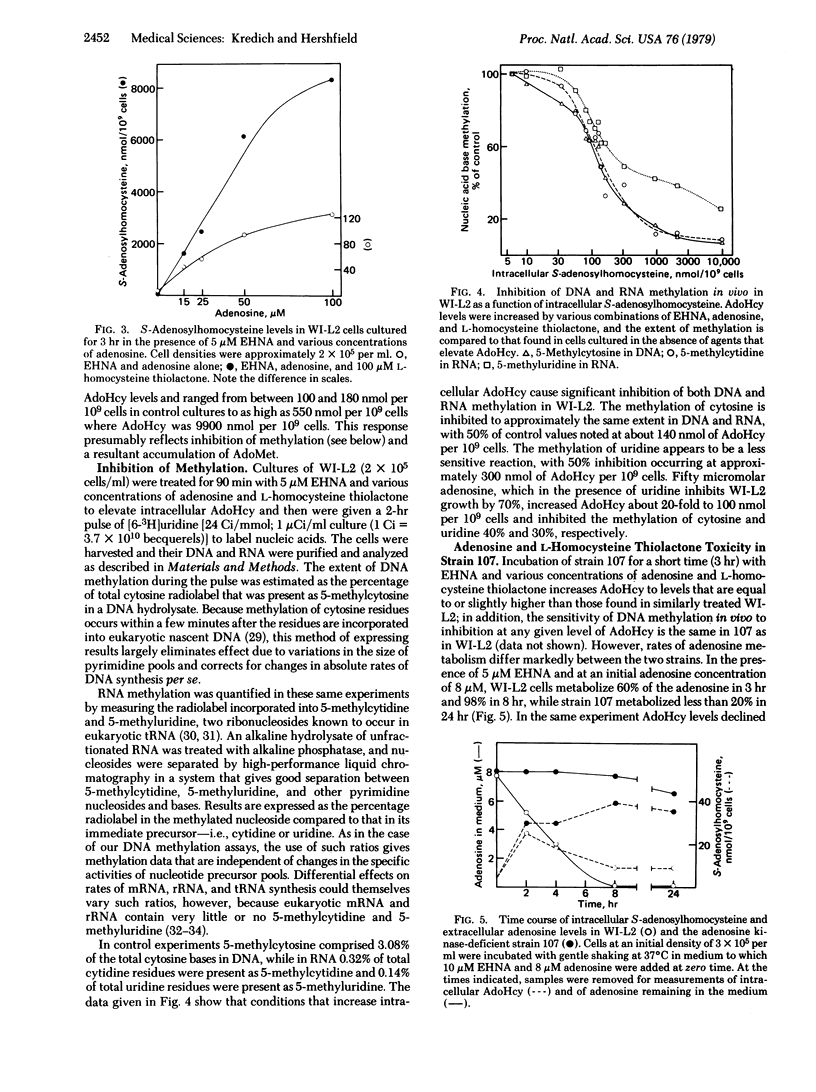
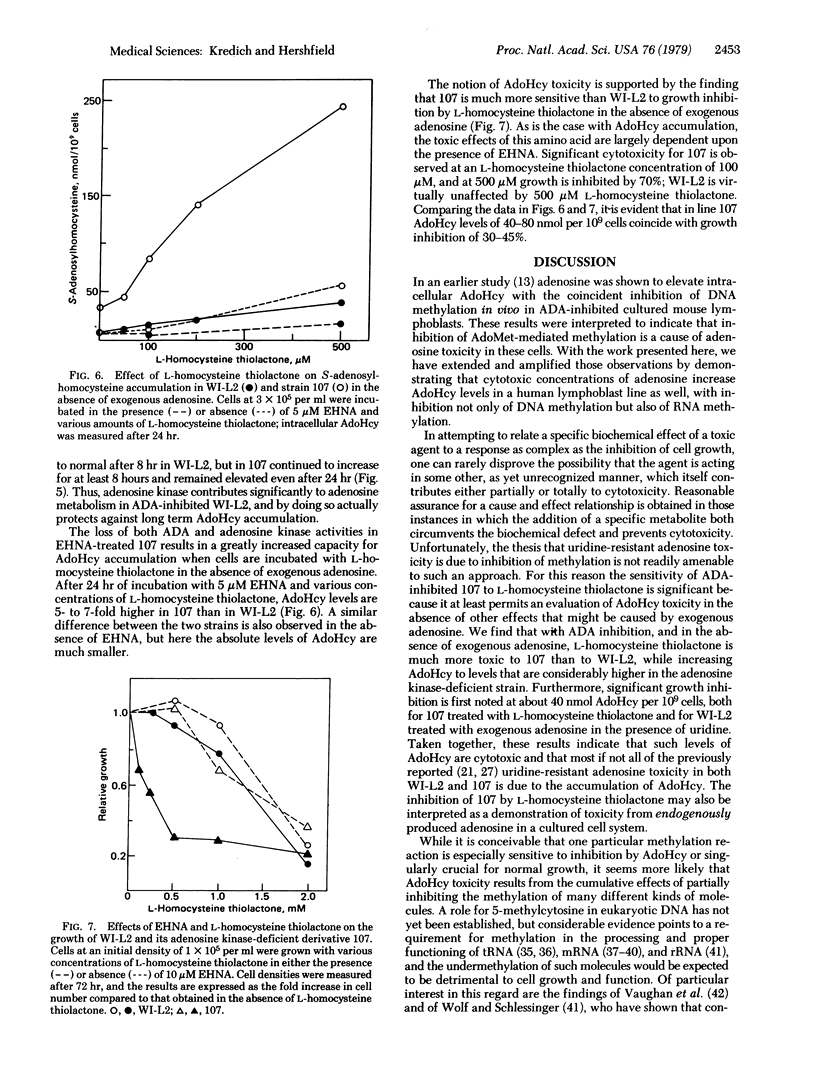
The human lymphoblast line WI-L2 is subject to growth inhibition by a combination of the adenosine deaminase (ADA; adenosine aminohydrolase, EC 3.5.4.4.) inhibitor erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) and adenosine. Although adenosine-induced pyrimidine starvation appears to contribute to this effect, uridine only partially reverses adenosine toxicity in WI-L2 and not at all in strain 107, an adenosine kinase-(ATP:adenosine 5′-phosphotransferase, EC 2.7.1.20) deficient derivative of WI-L2. Treatment of both cell lines with EHNA and adenosine leads to striking elevations in intracellular S-adenosyl-L-homocysteine (AdoHcy), a potent inhibitor of S-adenosyl-L-methionine (AdoMet)-dependent methylation reactions. The methylation in vivo of both DNA and RNA is inhibited by concentrations of EHNA and adenosine that elevate intracellular AdoHcy. Addition of 100 μM L-homocysteine thiolactone to cells treated with EHNA and adenosine enhances adenosine toxicity and further elevates AdoHcy to levels approximately 60-fold higher than those obtained in the absence of this amino acid, presumably by combining with adenosine to form AdoHcy in a reaction catalyzed by S-adenosylhomocysteine hydrolase (EC 3.3.1.1). In the adenosine kinase-deficient strain 107, a combination of ADA inhibition and L-homocysteine thiolactone markedly increases intracellular AdoHcy and inhibits growth even in the absence of exogenous adenosine. These results demonstrate a form of toxicity from endogenously produced adenosine and support the view that AdoHcy, by inhibiting methylation, is a mediator of uridine-resistant adenosine toxicity in these human lymphoblast lines. Furthermore, they suggest that AdoHcy may play a role in the pathogenesis of the severe combined immunodeficiency disease found in most children with heritable ADA deficiency.
Keywords: S-adenosylmethionine, methylation, adenosine deaminase, combined immunodeficiency disease
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borum P. R., Broquist H. P. Purification of S-adenosylmethionine: epsilon-N-L-lysine methyltransferase. The first enzyme in carnitine biosynthesis. J Biol Chem. 1977 Aug 25;252(16):5651–5655. [PubMed] [Google Scholar]
- Bynum J. W., Volkin E. Wasting of 18 S ribosomal RNA by human myeloma cells cultured in adenosine. J Cell Physiol. 1976 Jun;88(2):197–206. doi: 10.1002/jcp.1040880209. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- DUNN D. B. The isolation of 5-methylcytidine from RNA. Biochim Biophys Acta. 1960 Feb 12;38:176–178. doi: 10.1016/0006-3002(60)91219-1. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto D. J., Jr, Veiveros O. H., Axelrod J. Subcellualr distribution of protein carboxymethylase and its endogenous substrates in the adrenal medulla: possible role in excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4050–4054. doi: 10.1073/pnas.73.11.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Axelrod J. Regional and subcellular distribution of protein carboxymethylase in brain and other tissues. J Neurochem. 1976 Jun;26(6):1159–1165. doi: 10.1111/j.1471-4159.1976.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Dubin D. T., Stollar V. Methylation of Sindbis virus "26S" messenger RNA. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1373–1379. doi: 10.1016/0006-291x(75)90511-2. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Glick J. M., Ross S., Leboy P. S. S-adenosylhomocysteine inhibition of three purified tRNA methyltransferases from rat liver. Nucleic Acids Res. 1975 Oct;2(10):1639–1651. doi: 10.1093/nar/2.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Cohen A., Ullman B., Martin D. W., Jr Analysis of adenosine-mediated pyrimidine starvation using cultured wild-type and mutant mouse T-lymphoma cells. Somatic Cell Genet. 1978 Mar;4(2):201–219. doi: 10.1007/BF01538985. [DOI] [PubMed] [Google Scholar]
- Gunthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ J., GOLD M., ANDERS M. THE ENZYMATIC METHYLATION OF RIBONUCLEIC ACID AND DEOXYRIBONUCLEIC ACID. IV. THE PROPERTIES OF THE SOLUBLE RIBONUCLEIC ACID-METHYLATING ENZYMES. J Biol Chem. 1964 Oct;239:3474–3482. [PubMed] [Google Scholar]
- Held W. A., West K., Gallagher J. F. Importance of initiation factor preparations in the translation of reovirus and globin mRNAs lacking a 5'-terminal 7-methylguanosine. J Biol Chem. 1977 Dec 10;252(23):8489–8497. [PubMed] [Google Scholar]
- Hershfield M. S., Snyder F. F., Seegmiller J. E. Adenine and adenosine are toxic to human lymphoblast mutants defective in purine salvage enzymes. Science. 1977 Sep 23;197(4310):1284–1287. doi: 10.1126/science.197600. [DOI] [PubMed] [Google Scholar]
- Hildesheim J., Hildesheim R., Lederer E., Yon J. Etude de l'inhibition d'une t-ARN N 2 -guanine méthyl transférase de foie de lapin par des analogues de la S-adénosyl homocystéine. Biochimie. 1972;54(8):989–995. doi: 10.1016/s0300-9084(72)80049-x. [DOI] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Green H. Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J Cell Sci. 1973 Sep;13(2):429–439. doi: 10.1242/jcs.13.2.429. [DOI] [PubMed] [Google Scholar]
- Jacquemont B., Huppert J. Inhibition of viral RNA methylation in herpes simplex virus type 1-infected cells by 5' S-isobutyl-adenosine. J Virol. 1977 Apr;22(1):160–167. doi: 10.1128/jvi.22.1.160-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T., Rabson A. R., Nurse G. T., Lane A. B. Deficiency of adenosine deaminase not associated with severe combined immunodeficiency. J Pediatr. 1976 Nov;89(5):732–736. doi: 10.1016/s0022-3476(76)80792-5. [DOI] [PubMed] [Google Scholar]
- KLENOW H. On the effect of some adenine derivatives on the incorporation in vitro of isotopically labelled compounds into the nucleic acids of Ehrlich ascites tumor cells. Biochim Biophys Acta. 1959 Oct;35:412–421. doi: 10.1016/0006-3002(59)90391-9. [DOI] [PubMed] [Google Scholar]
- Kappler J. W. The kinetics of DNA methylation in cultures of a mouse adrenal cell line. J Cell Physiol. 1970 Feb;75(1):21–31. doi: 10.1002/jcp.1040750104. [DOI] [PubMed] [Google Scholar]
- Kerr S. J., Borek E. The tRNA methyltransferases. Adv Enzymol Relat Areas Mol Biol. 1972;36:1–27. doi: 10.1002/9780470122815.ch1. [DOI] [PubMed] [Google Scholar]
- Kerr S. J. Competing methyltransferase systems. J Biol Chem. 1972 Jul 10;247(13):4248–4252. [PubMed] [Google Scholar]
- Kim S., Paik W. K. Purification and properties of protein methylaase II. J Biol Chem. 1970 Apr 10;245(7):1806–1813. [PubMed] [Google Scholar]
- Kim S., Paik W. K. Studies on the origin of epsilon-N-methyl-L-lysine in protein. J Biol Chem. 1965 Dec;240(12):4629–4634. [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Martin D. V., Jr Role of S-adenosylhomocysteine in adenosinemediated toxicity in cultured mouse T lymphoma cells. Cell. 1977 Dec;12(4):931–938. doi: 10.1016/0092-8674(77)90157-x. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., DUNN D. B. The occurrence and distribution of thymine and three methylated-adenine bases in ribonucleic acids from several sources. Biochem J. 1958 Dec;70(4):642–651. doi: 10.1042/bj0700642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Hodge L. D. Gene expression in synchronized lymphocytes: studies on the control of synthesis of immunoglobulin polypeptides. J Cell Physiol. 1971 Apr;77(2):265–276. doi: 10.1002/jcp.1040770215. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Buell D. N., Creech C., Hirshaut Y., Silverberg H. Further characterization of the WI-L1 and WI-L2 lymphoblastoid lines. J Natl Cancer Inst. 1971 Mar;46(3):647–654. [PubMed] [Google Scholar]
- Liss M., Maxam A. M., Cuprak L. J. Methylation of protein by calf spleen methylase. A new protein methylation reaction. J Biol Chem. 1969 Mar 25;244(6):1617–1622. [PubMed] [Google Scholar]
- MURRAY K. THE OCCURRENCE OF EPSILON-N-METHYL LYSINE IN HISTONES. Biochemistry. 1964 Jan;3:10–15. doi: 10.1021/bi00889a003. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Salim M. The methylated nucleotide sequences in HELA cell ribosomal RNA and its precursors. J Mol Biol. 1974 Sep 5;88(1):133–152. doi: 10.1016/0022-2836(74)90299-x. [DOI] [PubMed] [Google Scholar]
- Meuwissen H. J., Pollara B., Pickering R. J. Combined immunodeficiency disease associated with adenosine deaminase deficiency. Report on a workshop held in Albany, New York, October 1, 1973. J Pediatr. 1975 Feb;86(2):169–181. doi: 10.1016/s0022-3476(75)80463-x. [DOI] [PubMed] [Google Scholar]
- Morrison D. A., Guild W. R. Transformation and deoxyribonucleic acid size: extent of degradation on entry varies with size of donor. J Bacteriol. 1972 Dec;112(3):1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea R. F., Viveros O. H., Axelrod J., Aswanikaumar S., Schiffmann E., Corcoran B. A. Raipid stimulation of protein carboxymethylation in leukocytes by a chemotatic peptide. Nature. 1978 Mar 30;272(5652):462–464. doi: 10.1038/272462a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Studies on inhibitors of mammalian tRNA methylases. FEBS Lett. 1971 Jul 15;16(1):13–16. doi: 10.1016/0014-5793(71)80672-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Litwack M., Marmor J. Modified bases and transfer RNA function. Cancer Res. 1971 May;31(5):675–678. [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3928–3932. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Schmalstieg F. C., Nelson J. A., Mills G. C., Monahan T. M., Goldman A. S., Goldblum R. M. Increased purine nucleotides in adenosine deaminase-deficient lymphocytes. J Pediatr. 1977 Jul;91(1):48–51. doi: 10.1016/s0022-3476(77)80442-3. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Panayi G. S., Corrigall V. A role for purine metabolism in the immune response: Adenosine-deaminase activity and deoxyadenosine catabolism. Lancet. 1978 Jan 14;1(8055):60–63. doi: 10.1016/s0140-6736(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Singer J., Stellwagen R. H., Roberts-Ems J., Riggs A. D. 5-Methylcytosine content of rat hepatoma DNA substituted with bromodeoxyuridine. J Biol Chem. 1977 Aug 10;252(15):5509–5513. [PubMed] [Google Scholar]
- Snyder F. F., Hershfield M. S., Seegmiller J. E. Cytotoxic and metabolic effects of adenosine and adenine on human lymphoblasts. Cancer Res. 1978 Aug;38(8):2357–2362. [PubMed] [Google Scholar]
- Snyder F. F., Hershfield M. S., Seegmiller J. E. Purine toxicity in human lymphoblasts. Adv Exp Med Biol. 1977;76A:30–39. doi: 10.1007/978-1-4613-4223-6_4. [DOI] [PubMed] [Google Scholar]
- Ullman B., Cohen A., Martin D. W. Characterization of a cell culture model for the study of adenosine deaminase- and purine nucleoside phosphorylase-deficient immunologic disease. Cell. 1976 Oct;9(2):205–211. doi: 10.1016/0092-8674(76)90111-2. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Soeiro R., Warner J. R., Darnell J. E., Jr The effects of methionine deprivation on ribosome synthesis in HeLa cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1527–1534. doi: 10.1073/pnas.58.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolberg G., Zimmerman T. P., Hiemstra K., Winston M., Chu L. C. Adenosine inhibition of lymphocyte-mediated cytolysis: possible role of cyclic adenosine monophosphate. Science. 1975 Mar 14;187(4180):957–959. doi: 10.1126/science.167434. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Schlessinger D. Nuclear metabolism of ribosomal RNA in growing, methionine-limited, and ethionine-treated HeLa cells. Biochemistry. 1977 Jun 14;16(12):2783–2791. doi: 10.1021/bi00631a031. [DOI] [PubMed] [Google Scholar]
- Zappia V., Zydek-Cwick R., Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969 Aug 25;244(16):4499–4509. [PubMed] [Google Scholar]


