Biological studies of acute leukaemias are hampered by the technical difficulties involved in maintaining the viability and proliferation of patient derived blasts in vitro. This is particularly true for studies trying to elucidate the nature and biology of leukaemia propagating cells, the definition of which relies on functional assays of self-renewal/clonal expansion. Furthermore, the lack of critical niche interactions raises concerns over the validity of biological data generated purely in an in vitro setting, for example when using derived cell line approaches (1) or patient-derived cells with compromised viability. Developments in immunocompromised mouse models, notably NOD/scid and more recently the NOD/LtSz-scid IL-2Rγ null (NSG) (2) and similar mice, have provided an opportunity to study leukaemia propagating cells in a bone marrow, albeit xenogeneic, microenvironment (3-5). Alongside this, there is a need to develop techniques to allow the labelling and tracking of leukaemic blasts in such xenotransplantation assays.
We have developed a protocol for the transduction of patient-derived lymphoblastic leukaemic blasts using a lentiviral vector encoding both enhanced green fluorescent protein (EGFP), allowing in vitro analysis and cell sorting, and firefly Luciferase (fLuc), for in vivo bioluminescent imaging - pSLIEW (Figure S1). SLIEW virus was produced by transfection of HEK293T cells by calcium phosphate co-precipitation of equimolar amounts of the packaging vector pCMVΔ8.91, the envelope vector pMD2.G and the pSLIEW transfer vector. Patient-derived material was collected as part of initial diagnostic investigation with written informed consent in accordance with local review board approval (see Supplementary Methods). In the final protocol (used for transductions 9 and 10, see Table S1), blasts were resuspended at 1 × 106 cells/ml in SFEM medium (Stem Cell Technologies, Grenoble, France) supplemented with 10% v/v fetal calf serum, 20 ng/ml recombinant IL-3, 10 ng/ml recombinant human IL-7 and 4 μg/ml polybrene. Cells were seeded into a 48 well plate at 5 × 105 cells/well. Viral transduction was performed by spinfection at 900 x g for 50 minutes at 34°C (Figure 1A). Following incubation overnight (37°C, 5%CO2), 350 μl of medium were removed and replaced with 850 μl fresh medium, supplemented as above. Transduction efficiency was assessed on day 3 or 4 post transduction by multicolour flow cytometry including CD19 PE, CD34 APC and CD20 PerCP Cy5.5, using a FACS Canto II (Becton Dickinson, Oxford, UK).
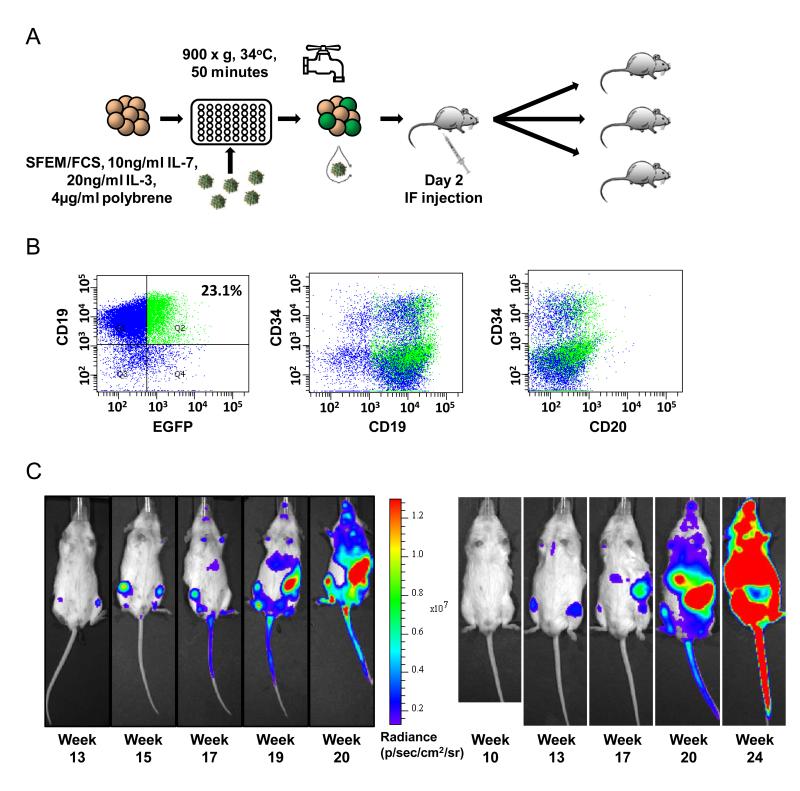
Figure 1. Transduction of patient derived leukaemic blasts.
A) Schema of transduction protocol, demonstrating spinfection, washing and intrafemoral transplantation. B) Transduction of L826. Lymphoid cells have been gated using forward and sideward scatter. The quadrant Q2 is determined by an untransduced sample to include ≤1% CD19+ cells. Population Q2 is then projected onto the central and right plots as green events, demonstrating unbiased transduction of all populations present. In this example, the majority of CD19+ blasts are CD20-/low. C) Bioluminescent imaging of serially transplanted L4951 blasts. Primary recipient M6 (left hand panels) was harvested at week 20 and bone marrow transplanted into secondary recipient M14 (right hand panels), demonstrating bilateral femoral engraftment initially, followed by dissemination to spleen, liver and vertebrae.
Multicolour flow cytometry demonstrated the unbiased transduction of all identified populations defined by the precursor B-cell surface markers CD19, CD20 and CD34 (Figure 1B). During the development of the protocol, a total of 10 transductions (7 primary samples, 3 “primografted” samples which had been passaged through mice) were performed on 5 different patient-derived specimens with a mean transduction efficiency of 21% (range 2-42%) (Table S1). The two final transductions were performed using viral stocks which had previously been titrated using the B-lymphoblastic leukaemia cell line SEM (6) and shown in that system to have 5.6 × 107-1.2 × 108 transducing units/ml. These experiments showed the relative transduction efficiency in L826 to be 0.5-3.4% and L839 to be 1.6-11%, compared to that in SEM.
In order to define a safe time point for transplantation, we analysed the persistence of infectious lentiviral particles in harvested supernatant. Surprisingly, substantial transduction of 293T cells was achieved with supernatant harvested at up to 4 days following spinfection (Figure S2), presumably resulting from the high viral titres required. We therefore introduced a stringent washing procedure to remove infectious particles following transduction (see Supplementary Methods).
In order to assess the engraftment potential of transduced cells, as well as the maintenance of transcription from the SFFV promoter, unsorted transduced cells were intrafemorally transplanted into NSG mice in two separate experiments. Engraftment was seen in 3/3 mice transplanted with 4.0 × 104 transduced primografted blasts from a Philadelphia chromosome-positive sample L4951 (total cell dose 1 × 105 cells per mouse) and 1/1 mice transplanted with 2 × 104 transduced cells from the standard risk sample L578 (total cell dose 1 × 106 cells). Bioluminescent imaging using the IVIS Spectrum (Caliper Life Sciences, Hopkington, MA, USA) demonstrated development of localised femoral engraftment, followed by dissemination of fLuc+ cells to alternative sites of haematopoiesis including the contralateral femur, vertebrae and cranium as well as the spleen and liver (Figure 1C). For sample L4951, bone marrow aspirates also showed engraftment of human CD19+ cells with expression of EGFP (Figure 2A). Furthermore, it was possible to quantify engraftment kinetics by analysis of bioluminescent images (Figure 2B). Heavily engrafted mice were killed and found to have infiltrated bone marrow and grossly enlarged spleens (mean 0.6g, range 0.41-0.8g; normal spleen weight approximately 0.02-0.05g) (Figure 2A). Despite the low level of transduction of L578, three dimensional reconstructions were capable of localising fLuc+ blasts to the spleen, femurs and brain/meninges (Figure S3).
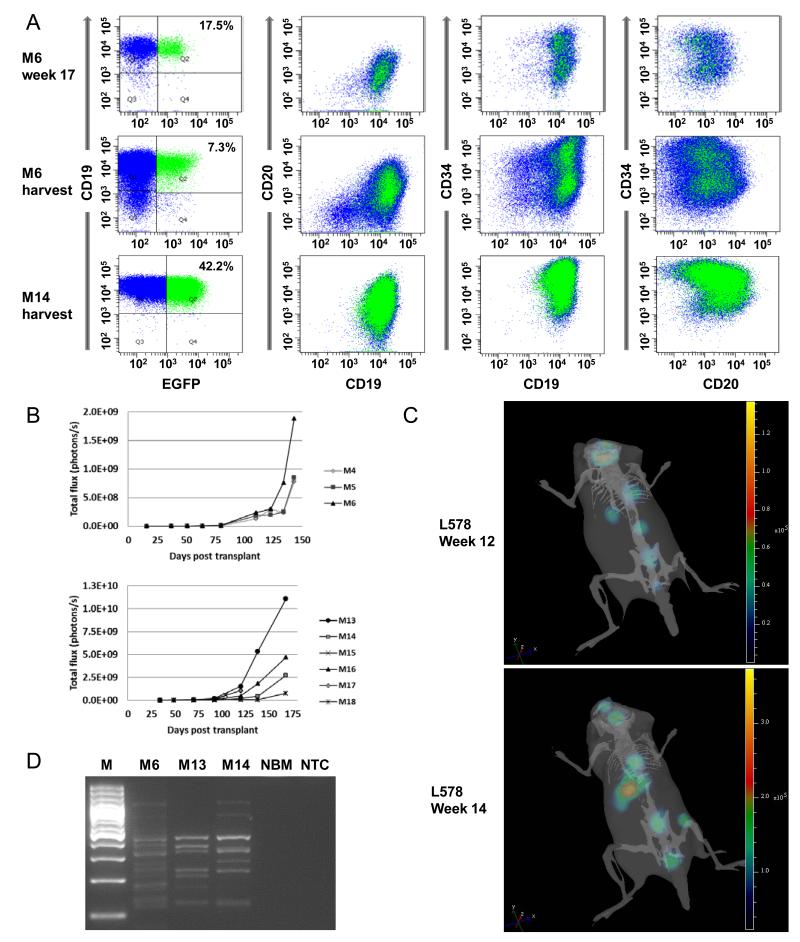
Figure 2. Monitoring of leukaemic engraftment.
A) Bone marrow engraftment of leukaemic blasts from patient L4951 was demonstrated in the primary recipient M6, both by bone marrow aspirates week 17 (top dot plots) and at harvest, week 20 (middle dot plots). Bone marrow was again analysed at harvest of the secondary recipient M14 at week 24. All these time points show a persistence of the unbiased transduction of populations defined by CD19, CD20, CD34. Engraftment falls to 7% following the primary transplant, but then rises to 42% following the secondary transplant. B) Quantification of engraftment determined by calculating the total luminescent signal from each mouse. C) Serial 3 dimensional reconstructions of engraftment of L578 in M35. At week 12, focal areas of engraftment are seen within the vertebrae, spleen and skull/brain. At week 14, strong engraftment is present within the spleen, with focal engraftment still visible in the vertebrae and the site of transplantation (right femur). The skeleton projected for orientation is representative and not derived directly from imaging of this animal. D) To investigate whether the increase in the proportion of cells expressing EGFP in secondary recipients of bone marrow from M6 resulted from clonal dominance, the clonality of engrafted bone marrow was analysed by ligation-mediated PCR. The presence of multiple bands, stable between two generations of recipient, demonstrates multiple integration sites and therefore multiple clones of transduced blasts. M – Marker (GeneRuler 100bp Plus, Fermentas, St Leon-Rot, Germany), NBM – Normal murine bone marrow, NTC – Non-target control.
To further confirm the validity of the xenotransplantation model, the liver, spleen, kidneys and brain from the first generation recipient of L578 were analysed histopathologically. This analysis showed widespread infiltration with leukaemic lymphoblasts expressing CD19, CD10, CD79a and TdT, characteristic of B-lymphoblastic leukaemia, as well as EGFP (Figure S4). Finally, flow cytometric analysis of individual tissues from three secondary mice engrafted with L578 demonstrated no organ specific differential engraftment of transduced blasts (Figure S5).
Flow cytometric analysis of the primary transplant of Philadelphia chromosome-positive patient L4951, harvested from mice M4-M6 at week 20, showed that expression of EGFP in both bone marrow and spleen had decreased from 40% at initial transplantation to 5.3-10%. Harvested bone marrow and spleen from M4 and M6 was re-transplanted fresh into a second generation of NSG mice. A total of 105 cells, corresponding to 5.4-10 × 104 EGFP+ cells, were transplanted into each of twelve recipient mice. In contrast, EGFP expression following the primary engraftment of transduced blasts from the standard risk patient L578 was relatively stable (2% EGPF expression at transplantation) with harvested bone marrow and spleen demonstrating 1.6% and 1.9% EGFP expression respectively. In this instance, 105 total bone marrow cells were retransplanted into each of three recipient mice, corresponding to 1.6 × 103 EGFP+ cells.
Secondary recipients of blasts harvested from primary recipients (M4 and M6) of sample L4951 demonstrated good engraftment with 11 of 12 mice (92%) of mice engrafting. Leukaemic cells from mice transplanted with material from M4 showed a further decrease in EGFP expression to between 0.2 and 1.8%. Somewhat to our surprise, however, cells from M6 which had been transplanted with 7.3% (bone marrow) or 8.0% (spleen) cells expressing EGFP showed an expansion so that across all recipients, between 17% and 42% of cells were now EGFP+. Again, EGFP expression was seen in all immunophenotypic populations (Figure 2A). For the standard risk sample L578, all three secondary recipients of bone marrow harvested from the primary recipient showed good engraftment. At harvest, week 17, bone marrow expression of EGFP, which had been 1.6% at secondary transplant, varied between 0.3-2.9%.
In one final transplantation experiment, cells recovered from the second generation of mice transplanted with L4951 were sorted for CD19+EGFP+ (FACS Aria II, Becton Dickinson) and transplanted into 13 mice at a total cell dose of 1.5 × 104 cells/mouse. Three mice died prior to engraftment. Engraftment was seen in all 10 (100%) of the remaining mice.
Serial 3 dimensional reconstructions of M35, a secondary recipient of L578, suggested an intriguing pattern of clonal seeding of secondary sites of engraftment, identified only in this specimen with a low level of transduction of 2% (Figure 2C). Furthermore, we had observed a substantial increase in the proportion of EGFP+ blasts from L4951 between secondary transplant and harvest. To assess whether these findings represented a pattern of mono-/oligoclonal engraftment, we analysed the integration sites of the provirus using ligation-mediated PCR directed at the 3′LTR (7, 8). The substantial number of bands produced demonstrated diverse SLIEW integration sites, confirming the propagation of a polyclonal disease (Figure 2D).
We have developed a protocol for the lentiviral transduction of patient derived leukaemic blasts. The unbiased transduction of different leukaemic populations, combined with the serial engraftment of EGFP+ blasts confirms the transduction of the leukaemia propagating cell compartment, even in one specimen with a low level of initial transduction. This is not unexpected as, unlike the stem cell hierarchy which is found in AML (9-11), leukaemia propagating potential is present across blast populations defined by immunophenotypic markers of precursor B-cells (4, 5, 12) and is more common than in AML (13). The lentiviral/xenotransplantation assay described makes possible the in vivo monitoring of leukaemia propagating cells. Not only will this assay contribute to our understanding of the biology of these cells, it also has the potential to be adapted for a number of different purposes including the monitoring of novel drug therapies, competitive transplantation of sorted populations and potentially the in vivo assessment of candidate stemness genes using either inhibitor or RNAi-based approaches.
Supplementary Material
Acknowledgements
We thank Christopher Baum and Olga Kustikova for their help with the ligation mediated PCR, and Martin Stanulla and Martin Schrappe for the provision of sample L4951. SB is funded by a Medical Research Council (MRC) Clinical Research Training Fellowship. The IVIS Spectrum was funded by a grant from the Wellcome Trust, grant 087961. Additional funding came from a programme grant by CR-UK (grant number C27943/A12788), project funding from Leukaemia Lymphoma Research (LLR) and core infrastructure support from the North of England Children’s Cancer Research Fund (NECCR).
Footnotes
Supplementary information is available at Leukemia’s website
References
- 1.Meacham CE, Ho EE, Dubrovsky E, Gertler FB, Hemann MT. In vivo RNAi screening identifies regulators of actin dynamics as key determinants of lymphoma progression. Nat Genet. 2009;41(10):1133–7. doi: 10.1038/ng.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 3.Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008;319(5861):336–9. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 4.Kong Y, Yoshida S, Saito Y, Doi T, Nagatoshi Y, Fukata M, et al. CD34+CD38+CD19+ as well as CD34+CD38-CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22(6):1207–13. doi: 10.1038/leu.2008.83. [DOI] [PubMed] [Google Scholar]
- 5.le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008;14(1):47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greil J, Gramatzki M, Burger R, Marschalek R, Peltner M, Trautmann U, et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. British journal of haematology. 1994;86(2):275–83. doi: 10.1111/j.1365-2141.1994.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 7.Kustikova OS, Modlich U, Fehse B. Retroviral insertion site analysis in dominant haematopoietic clones. Methods Mol Biol. 2009;506:373–90. doi: 10.1007/978-1-59745-409-4_25. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Hoffmann G, Wissler M, Lemke N, Mussig A, Glimm H, et al. Detection and direct genomic sequencing of multiple rare unknown flanking DNA in highly complex samples. Human gene therapy. 2001;12(7):743–9. doi: 10.1089/104303401750148649. [DOI] [PubMed] [Google Scholar]
- 9.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 10.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–43. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 11.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 12.Rehe K, Wilson K, Bomken S, McNeill H, Stanulla M, Den Boer ML, et al. In acute lymphoblastic leukaemia, stemness is frequent and ubiquitous. Blood. 2010;116(21) Abstract 92. [Google Scholar]
- 13.Morisot S, Wayne AS, Bohana-Kashtan O, Kaplan IM, Gocke CD, Hildreth R, et al. High frequencies of leukemia stem cells in poor-outcome childhood precursor-B acute lymphoblastic leukemias. Leukemia. 2010;24(11):1859–66. doi: 10.1038/leu.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.